Abstract
Although leukemia is the most common childhood cancer diagnosis, the subtype, acute myeloid leukemia (AML), is less common and fewer etiologic studies exist. This review summarizes the major risk factors for AML. We searched the literature using PubMed for articles on childhood AML and reviewed 180 articles. While few risk factors are definitive, we identify several with consistent evidence of a possible effect. Thorough analysis of genetic and epigenetic factors is missing from this literature and methodological issues are unresolved. Future studies should more closely examine causal mechanisms, improve exposure measurement, and include analysis using genetic and epigenetic factors.
Keywords: acute myeloid leukemia, children, epidemiology
Introduction
Leukemia is the most common of pediatric cancers accounting for about 30% of diagnoses[1]. There are two main subtypes; acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). AML is less common, accounting for approximately 18% of childhood leukemia diagnoses[2]. The etiology of the two subtypes is likely quite different based on both cell lineage and epidemiological studies of incidence and risk factors. Few risk factors have been conclusively determined for childhood AML.
AML develops through a transformation of hematopoietic progenitor cells that leads to a block in differentiation, increasing the number of progenitor cells and decreasing the number of mature blood cells[3,4]. Evidence for multiple mutations required for AML development has been demonstrated from backtracking studies that have found mutations associated with childhood AML present at birth, yet development of leukemia takes place sometimes years later[5,6].
Many studies have included an analysis of risk factors for childhood AML, but few have definitively emerged. Only in utero exposure to ionizing radiation is considered an established cause of pediatric de novo AML[7]. Evidence about risk factors for AML is limited due to small sample sizes and improper grouping of AML with ALL. This review will synthesize what is known about childhood AML, identify gaps in current knowledge and suggest directions for future studies.
Descriptive Epidemiology
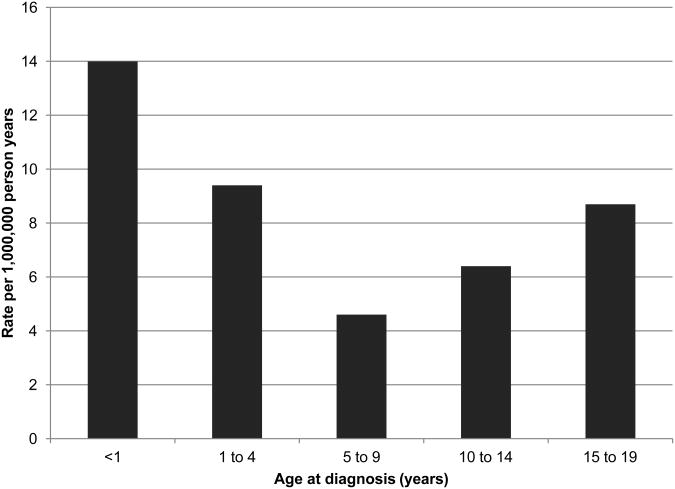
The incidence of childhood AML in the United States was estimated at 7.7 cases per million children aged 0-14 in 2005-2009 with some indication of an increase in incidence over time[8]. Incidence peaks in infants less than one year of age with a rate of 18.4 per million, declining to 4.3 per million for ages 5-9 years and increasing up to 7.7 per million for children ages 10 to 14 years (Figure 1)[8]. Little variation is seen by racial/ethnic groups in the US, with the exception of a possible increased rate in Hawaiians[9,10] and potentially higher incidence of a specific subtype of AML, acute promyelocytic leukemia (APL), in Hispanic/Latino children[11,12]. In a report using data from the Surveillance, Epidemiology, and End Results (SEER) Program, Asian and Pacific Islanders had the highest rate of childhood AML (8.4 per million), followed by Hispanics (8.1 per million), Caucasians (7.5), and African Americans (6.6)[2].
Figure 1. Incidence rates for AML by age at diagnosis, U.S. SEER 1992-2004a.
a Adapted from data presented in Linabery and Ross[2].
Worldwide incidence of childhood AML varies with annual standardized incidence rates ranging from 2 per million in Kuwait to 14.4 per million for the Maori in New Zealand[10]. Differences in rates could either be due to differences in incidence or differences in ascertainment methods between registries. Countries with available data observed rates between 5-8 per million. Increased incidence in Maori, Hawaiians, and Pacific Islanders in general could suggest shared genetic predisposition[13].
Survival
The five year survival rate for children <15 years of age at diagnosis was estimated at 64.3% in the US (2002-2008)[8]. AML subtypes have very different prognoses with five year survival expectations ranging from 22% to 90% [14]. Higher survival rates are seen in children with APL (due to the sensitivity to all-trans-retinoic acid and arsenic trioxide) and in children with other specific mutations[14]. Lower survival is seen in children with mutations such as FLT3-ITD, monosomy 7, del 5q, and poor disease response.
Methods
We searched Medline to identify articles that studied childhood AML etiology. The search string was (“childhood leukemia” or “pediatric leukemia” or “childhood leukaemia” or “paediatric leukaemia”) AND (etiology OR aetiology OR risk factors OR epidemiology OR case-control), yielding 1,983 articles (through 6/30/2012). Articles were considered if they reported etiologic analysis results for childhood AML separately from childhood ALL or all leukemias. References of selected articles were also explored for additional studies. Overall, 180 articles were identified that performed etiologic analysis of childhood AML or acute non-lymphoblastic leukemia either in an original study or a meta-analysis. Only selected risk factors are presented here. If meta-analysis was available for particular exposure, only those results were reported.
Results
Genetic factors
The most common genetic factor for development of AML is trisomy 21. Children with Down syndrome (DS) have an increased risk of childhood AML and a 500-fold increased risk of developing a specific subtype of AML, acute megakaryocytic leukemia[15]. A small proportion of childhood AML cases are associated with other genetic syndromes including Fanconi anemia[16], Bloom syndrome[17], ataxia telangiectasia[18], Shwachman-Diamond syndrome[19], Noonan syndrome[20], severe congenital neutropenia[21], familial monosomy 7[22], familial platelet disorder[23], and dyskeratosis congenita[24].
Two studies found a significant association between family history of hematologic cancers in first or second degree relatives and childhood AML[25,26]. An association between any cancer in first or second degree relatives and childhood AML was found in one study, but not replicated in others[26-28].
The majority of genetic studies on childhood AML involve examining variation in single nucleotide polymorphisms (SNPs). No genome-wide association studies have been performed for predisposition to childhood AML. Previous studies have been based on candidate genes, typically examining genes involved with xenobiotic metabolism or folate metabolism. While some positive results have been found, additional confirmation is needed.
The glutathione S-transferase (GST) genes are involved in the metabolism of various carcinogens in tobacco smoke including benzene. Two genes have been examined in childhood AML (GSTT1 and GSTM1). Null genotypes (no enzyme activity in homozygotes) in these two genes are quite prevalent with 50% of non-Hispanic whites null for GSTM1 and 20% null for GSTT1[29]. While studies involving the GSTT1 null genotype found no significant association, the GSTM1 null genotype has been associated with a small increased risk[30-33]. The NAD(P)H:quinone oxidoreductase 1 (NQO1) gene plays a role in preventing toxicity due to benzene exposure and a SNP (rs1800566) results in complete reduction in enzymatic activity for homozygotes[34]. In a meta-analysis of three original studies of childhood AML, no statistically significant association was observed[35]. Many genes in the cytochrome P450 (CYP) family also contribute to detoxification, and CYP1A1, CYP2D6, and CYP2E1 have been investigated in relation to childhood AML. In three studies of a SNP (rs4646903) in CYP1A1, no association was found[30-32]. One study found a possible association between a SNP (rs2031920) in CYP2E1 (OR = 4.9; 95% CI 1.6, 15.2, for heterozygotes vs. homozygous wild type with no cases or controls homozygous for the variant allele), while the association with SNPs in CYP2D6 (rs35742686 and rs3892097) was not significant[30].
Four studies have explored the relationship between SNPs (rs1801133 and rs1801131) in the methylenetetrahydrofolate reductase (MTHFR) gene and childhood leukemia; three finding no association[31,36,37] and one finding possible association with rs1901133 (OR = 2.2; 95% CI 1.0, 4.8)[38]. One examined other genes in this pathway along with maternal genotype. In affected children, mutations in the 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) gene (rs1805087) (OR = 2.74; 95% CI 1.07, 7.01) and the thymidylate synthetase (TS) gene (1494 del 6) (OR = 2.04; 95% CI 1.03, 4.03) were both significantly associated with increased risk of childhood AML [36], while no associations were observed with maternal polymorphisms in these genes.
Parental factors
Parental age
Differing classification, use of continuous variables, and correlation between maternal and paternal age make studies of parental age challenging to synthesize, but overall the data supports an increased risk with older maternal age. Three studies found a statistically significant association with advanced maternal age, one being a very large pooled study with 804 cases that included data from the other two (OR = 1.08; 95% CI 1.01, 1.16, for a five year increase in age)[39-41]. An additional study found a significant effect of maternal age in children with DS and AML for women aged ≥ 35 years compared with women <30 years old (OR =2.63; 95% CI 1.26, 5.49).[42] Others found positive but non-significant associations[43-51], a negative but non-significant associations[52-54] and null associations[55]. When examining large studies with over 200 cases of childhood AML, almost all found a positive association[39,43,45,48] (one did not report the direction of the effect[56]), but only in the large pooled study was this association statistically significant[39].
Only one study found a significant relationship between paternal age and childhood AML, but this finding was only significant when not adjusted for maternal age[39]. Other studies found non-significant positive associations with older paternal age[43,45,47,48,51], a non-significant inverse associations with older paternal age[52-54], or an unspecified non-significant difference[56]. For studies with over 200 cases, results were mixed as well. Overall, the evidence is inconclusive for an association between older paternal age and childhood AML.
Prior fetal loss
Overall, there appears to be an increased risk of AML with prior fetal loss. However, most studies examining prior fetal loss were small, including less than 100 cases. Four studies indicated significant associations between prior fetal loss and childhood AML, including the largest study with over 200 cases of childhood AML[47,52,57,58]. Within those studies, one used number of miscarriages and found an increase risk for 2 or more compared to none (OR = 11.3; 95% CI 2.3, 56)[57], two looked at any fetal loss compared to none (OR = 2.7; 95% CI 1.0, 6.8 ; OR =2.0; 95% CI 1.3, 2.9)[52,58] and one split early and late fetal loss (<20 weeks, >20 weeks) and found an association only with early fetal loss (OR = 2.3; 95% CI 1.0, 5.2; 2 or more vs. none)[47]. In contrast, one study found a significant protective effect of prior fetal loss (OR = 0.6; 95% CI 0.4, 1.0; any vs. none)[50]. Among other studies with non-significant results, two were positive [44,59], three were negative [48,53,55] , and the rest were null or had mixed findings[40,42,46,60].
Birth order
Some evidence of an increased risk of childhood AML with increasing birth order exists, although this could be due in part to a maternal age effect. Three studies found significant associations and had relatively high sample sizes with over 100 cases in each study[48,49,59]. One found a significant linear trend for increasing birth order in infants (p=0.04)[59], another reported a significant association for 3 or more previous live births compared to 1 or 2 (OR = 1.9; 95% CI 1.0, 3.3), [48] and the third found an association for 3 or more previous live births compared to none (OR = 1.6; 95% CI 1.0, 2.8)[49]. Studies with non-significant associations have suggested an increased risk with increasing birth order or prior live births[40,43,53-55,61], but others found inconsistent results or possible negative associations[42,44,45,51,52,56,62,63].
Periconceptional and prenatal exposures
Alcohol
Meta-analysis results support a positive association for any alcohol during pregnancy with a combined OR of 1.56 (95% CI 1.13, 2.15) overall and 2.68 (95% CI 1.85, 3.89) for those diagnosed with AML under 5 years of age[64]. Analysis of specific beverages singled out wine (OR = 1.67; 95% CI 1.21, 2.32)[64]. Several studies have attempted to quantify the number of drinks, but dose response patterns have been inconsistent between studies.
Tobacco
A meta-analysis found no association between maternal smoking and childhood AML (OR = 0.99; 95% CI 0.90, 1.09)[65]. The only prospective study of maternal smoking and childhood leukemia did not find a significant effect overall, but did find an increased risk for women smoking 10 or more cigarettes per days compared to non-smokers (OR = 2.28; 95% CI 1.05, 4.94)[66]. Most studies examining the risk between paternal smoking and childhood AML have been null[50,67-78], although a few studies have indicated a possible increase in risk[74,77,78]. Differing time periods and smoking patterns are likely important, but have not been well studied.
Dietary DNA topoisomerase II inhibitors
Two studies have shown a possible association between maternal dietary intake of DNA topoisomerase II inhibitors and infant AML, although both were relatively small studies with less than 100 cases. A 10-fold increased risk of AML in infants whose mothers consumed medium or high levels of DNA topoisomerase II inhibitors (beans, fresh vegetables, canned vegetables, fruit, soy, regular coffee, black tea, green tea, cocoa, and wine)[79] was reported in an initial test of this hypothesis. The larger second study examined the relationship within the subgroup of infants with an MLL translocation, and again found some indication of a higher risk for increasing levels in the AML MLL+ subgroup (OR = 3.2; 95% CI 0.9, 11.9, highest versus lowest quartile)[80].
Medications
Antibiotic use during pregnancy has been examined either as a group or by specific drugs. One study in infants found that maternal use of metronidazole, used to treat vaginal infections, was associated with a possible increased risk (OR = 4.66; 95% CI 0.89, 24.24)[81], while another study in infants found no association with maternal use of any specific antibiotic during pregnancy, although a positive, non-significant association was observed for metronidazole[82]. Other studies have been mixed with one finding a significant association for any antibiotic use (OR = 3.20; 95% CI 1.72, 5.96)[83] and two others finding no relationship[84,85]. All studies were similar in size with 70-100 cases of AML and most used maternal interviews or questionnaires.
While it is possible that the underlying condition may be the “true” etiologic risk factor rather than the use of antibiotics, four studies found no significant association between maternal infections and childhood AML for urinary tract or lower genital tract infections[62,86-88], although both studies based on medical records found a non-significant elevated OR of 4.0 (95% CI 0.85, 18.8)[88] and 1.34 (95% CI 0.32, 5.56)[62] for risk of childhood AML[88]. An additional study found no association between viral infections during pregnancy and childhood AML[85]. Current data is limited, but could suggest some relationship with either metronidazole use or urinary tract or lower genital tract infections.
Benzene
Evidence of an association with benzene has been mixed likely due to the heterogeneity of sources and issues of measurement. Sources of benzene include cigarette smoke, car exhaust, industrial emissions as well as from building materials, paints and adhesives. Sources of parental benzene exposure can be divided into direct exposure (e.g. occupational or household product use) and inferred exposure (e.g. occupation title or traffic density). Only one study indicated an increased risk for direct paternal occupational exposure to petroleum products (OR = 2.4; 95% CI 1.3, 4.1 for over 1000 days of exposure vs. none) and solvents (OR = 2.0; 95% CI 1.2, 3.8, over 1000 days of exposure vs. none), the relationship was similar for time periods before and during the index pregnancy, but was reduced for exposures after the index pregnancy[89]. Others have found no association with paternal occupational or household exposure to benzene[53,90]. One study found an association for direct maternal occupational exposure to benzene or gasoline (OR = 4.0; 95% CI 1.8, 8.3 and OR = 2.1; 95% CI 1.1, 4.3, respectively)[53]. Others indicated non-significant but positive relationships for occupational or household exposure benzene, hydrocarbons, petroleum products, paints, or solvents[85,89-91].
Apart from parental smoking, few studies have examined inferred parental exposures. One study found a significant association with paternal employment as a mechanic[89]. Another study found a significant effect of proximity to repair garages and gas stations during pregnancy[92]. A third study found a significant association with either maternal or paternal employment in tire production (OR = 7.6; 95% CI 1.7, 34.2 and OR = 10.1; 95% CI 2.2, 46.0, respectively)[93]. A recent review of the association between benzene and childhood AML found no consistent pattern of increased risk of childhood AML for any individual exposure which might suggest higher levels of benzene[94].
Pesticides
In two meta-analyses, which included different studies, both found an increased risk for maternal occupational exposure to pesticides (OR = 2.7; 95% CI 1.1, 6.8 and OR = 2.6; 95% CI 1.4, 5.0) and no association for paternal occupational exposure[95,96]. However, the studies of paternal exposure were more heterogeneous with different time periods and types of exposures.
The relationship between household pesticide use during pregnancy and childhood AML is less clear with two meta-analyses producing different results depending on study inclusion for exposure to any pesticide (OR = 2.3; 95% CI 1.5, 3.5 and OR = 1.4; 95% CI 0.8, 2.6)[97,98]. However, both studies show a significant relationship with insecticides (OR = 3.1; 95% CI 1.5, 6.8 and OR = 1.9; 95% CI 1.3, 2.6)[97,98]. In addition, one study indicated that prenatal exposure to the insecticide propoxur (as measured in meconium) was associated with a specific translocation in cord blood[99] which is seen in about 12% of childhood AML cases[14].
Radiation
Ionizing radiation in utero is a well established cause of childhood leukemia, including AML[7]. This observation led to decreased use of X-rays in pregnant women and, combined with the lower dose now received, is thought to not contribute substantively to the current burden of childhood leukemia. However, in recent case-control studies, while no significant results have been observed, in seven of the nine studies OR estimates were greater than one[49,53,63,85,88,93,100-102].
Child factors
Birth weight
The evidence suggests that there may be an increased risk of childhood AML in children with both low and high birth weight. Two meta-analyses have explored the relationship between AML and birth weight. The first included four studies and indicated a non-significant increased risk for high birth weight (OR = 1.3; 95% CI 0.7, 2.2, for ≥4000 grams vs. <4000 grams)[103]. The more recent analysis, which included nine studies combining different definitions of low and high birth weight, found an increased risk for both high and low birth weight (OR = 1.24; 95% CI 1.16, 1.33; OR = 1.50; 95% CI 1.05, 2.13, respectively) [104]. A recent study, not included in the meta-analyses, suggested that the relationship between birth weight and childhood AML was non-linear, and found increased risks both for low and high birth weight[105].
Breastfeeding
A meta-analysis combining data from eight studies found that long term breast feeding (> 6 months) had a protective effect on the development of childhood AML (OR = 0.85; 95% CI 0.73, 0.98) while short-term breast feeding (6 months or less) also had a protective effect, but did not reach statistical significance (OR = 0.90; 95% CI 0.80, 1.02)[106].
Discussion
Prior studies have uncovered possible associations between various risk factors and childhood AML (Table I). Notable findings have been observed for maternal age, birth weight, prior fetal loss, birth order, maternal exposure to pesticide, and maternal alcohol use in pregnancy. To date, genetic studies have focused on candidate genes in small studies lacking the ability to fully characterize genetic variance and have been unable to examine complex genetic interactions. Future research should develop biomarkers for difficult exposures, explore causal mechanisms rather than risk factors, analyze genetic contributions more fully, and include analysis of specific AML subtypes.
Table I. Summary of risk factors for AML.
| Generally accepted risk factors | Suggestive of increased risk | Suggestive of decreased risk | Limited evidence |
|---|---|---|---|
| Down syndrome | Older maternal age | Long term breastfeeding | Paternal exposure to benzene |
| Fanconi anemia | Increasing birth order | Parental smoking | |
| Familial monosomy 7 | Prior fetal loss | Maternal exposure to benzene | |
| Ataxia telangiectasia | Maternal alcohol use | Maternal use of antibiotics | |
| Shwachman-Diamond syndrome | Maternal exposure to pesticides | Maternal dietary consumption of DNA topoisomerase II inhibitors (infant) | |
| Bloom syndrome | High birth weight | ||
| Ionizing radiation in utero | Low birth weight |
One of the biggest challenges in measuring maternal exposures during pregnancy is accurate measurement. Retrospective case-control studies are prone to both differential recall of exposures and selection bias. Precise measurement of exposures that occurred many years ago is also problematic. Development of biomarkers for these exposures that could be measured in a neonatal blood spots or other relevant samples would provide a more accurate account of exposures during pregnancy. Epigenetic biomarkers of environmental exposures, such as DNA methylation, are starting to be developed. Low level benzene exposures have been linked with a decrease in global DNA methylation levels[107] and in utero exposure to cigarette smoke has been linked to a decrease in global DNA methylation levels[108]. While these studies are not specific enough for biomarkers, other studies are looking at DNA methylation in specific genes. One study found that DNA methylation in a set of genes accounted for 78% of the variation in birth weight[109], so specific epigenetic biomarkers could be possible in the future. This may be important for childhood AML as some studies have suggested fewer genetic alterations, opening a potential larger role for epigenetic disruptions[110,111]. However, it is not currently known if epigenetic alterations are initiating events in childhood AML or a result of downstream effects of genomic changes.
Factors such as maternal age, birth weight, birth order, and prior fetal loss are not causes themselves but potential markers of some unknown factor. We need to move beyond these exposure variables and examine causative mechanisms to better understand the development of childhood AML. Causal mechanisms could involve alterations in DNA methylation or chromatin structure both of which may be affected by maternal age as well as birth order and prior fetal loss[112]. Genetic alterations could also be more likely for children born to older fathers affecting the observed association with maternal age and birth order[113]. Fetal loss may also be associated with genetic alterations or exposure to environmental factors. Birth weight represents a combination of genetics and in utero factors[114]. Maternal factors including diabetes, pre-pregnancy body mass index, and weight gain during pregnancy are related to birth weight[115,116]. Maternal and fetal genetic factors are also important with maternal genetic effects estimated to account for 22% of the variability and fetal genetic effects estimated to account for 31% of the variability in birth weight[114]. Further analysis should include innovative methods to explore the underlying causes of these observed associations.
A thorough examination of genetic variation is necessary for a fuller understanding of childhood AML etiology. Previous research has focused only on candidate genes and no comprehensive genome-wide study has been conducted either in children or their mothers. A next generation sequencing approach could lead to discoveries of genes not anticipated to be involved in childhood AML development a priori and would provide new genetic leads for future studies. It will be important to examine genomes of affected children and their mothers given the large contribution of the maternal genome to the intrauterine environment. Few studies have examined the possibility of maternal genetic effects in childhood AML development and none have yet found significant genetic main effects, although a very small number of genes were examined[36,37]. More complex genetic studies are also necessary to assess the possibility of synergistic interactions between genes and environmental factors both in children and their mothers. Little has been done to examine these types of interactions due to small study sizes. Given the possible relationship between childhood AML and environmental toxicants, these complex effects could contribute significantly to childhood AML etiology. These studies will require collaboration between investigators and cooperative pediatric oncology groups to assemble enough case to produce meaningful results.
In summary, the epidemiological data to date has produced several possible risk factors for childhood AML. Future studies should build on this research by exploring potential causes of observed relationships between maternal age, birth weight, and prior fetal loss; developing innovative measurement tools for complex and difficult to measure exposures such as alcohol, pesticides, and benzene; examining the etiology by AML subgroups; and fully exploring the role of genetic factors including interactions. Additional research in these areas will greatly enhance the knowledge of childhood AML etiology.
Acknowledgments
Dr. Ross was supported in part by NIH K05 CA157439.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflict of interest to report.
References
- 1.Smith MA, Ries L, Gurney J, et al. Leukemia. In: Ries L, Smith M, Gurney J, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. pp. 17–34. [Google Scholar]
- 2.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004) Cancer. 2008;112(2):416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 3.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 4.Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3(2):89–101. doi: 10.1038/nrc989. [DOI] [PubMed] [Google Scholar]
- 5.McHale CM, Wiemels JL, Zhang L, et al. Prenatal origin of childhood acute myeloid leukemias harboring chromosomal rearrangements t(15;17) and inv(16) Blood. 2003;101(11):4640–4641. doi: 10.1182/blood-2003-01-0313. [DOI] [PubMed] [Google Scholar]
- 6.Wiemels JL, Xiao Z, Buffler PA, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99(10):3801–3805. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 8.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2012. [Google Scholar]
- 9.Goodman MT, Yoshizawa CN, Kolonel LN. Incidence trends and ethnic patterns for childhood leukaemia in Hawaii: 1960-1984. Br J Cancer. 1989;60(1):93–97. doi: 10.1038/bjc.1989.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin DM, Kramarova E, Draper GJ, et al., editors. International Incidence of Childhood Cancer. Lyon, France: IARC Scientific Publications No. 144; 1998. [Google Scholar]
- 11.Matasar MJ, Ritchie EK, Consedine N, et al. Incidence rates of acute promyelocytic leukemia among Hispanics, blacks, Asians, and non-Hispanic whites in the United States. Eur J Cancer Prev. 2006;15(4):367–370. doi: 10.1097/00008469-200608000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Mendes WL, Coser VM, Ramos G, et al. The apparent excess of acute promyelocytic leukemia in infant acute leukemias in Brazil. Haematologica. 2004;89(11):ELT16. [PubMed] [Google Scholar]
- 13.Dachs GU, Currie MJ, McKenzie F, et al. Cancer disparities in indigenous Polynesian populations: Maori, Native Hawaiians, and Pacific people. Lancet Oncol. 2008;9(5):473–484. doi: 10.1016/S1470-2045(08)70127-X. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Carroll WL, Meshinchi S, et al. Biology, Risk Stratification, and Therapy of Pediatric Acute Leukemias: An Update. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 william beaumont hospital symposium on molecular pathology. J Mol Diagn. 2009;11(5):371–380. doi: 10.2353/jmoldx.2009.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green AM, Kupfer GM. Fanconi anemia. Hematol Oncol Clin North Am. 2009;23(2):193–214. doi: 10.1016/j.hoc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poppe B, Van Limbergen H, Van Roy N, et al. Chromosomal aberrations in Bloom syndrome patients with myeloid malignancies. Cancer Genet Cytogenet. 2001;128(1):39–42. doi: 10.1016/s0165-4608(01)00392-2. [DOI] [PubMed] [Google Scholar]
- 18.Viniou N, Terpos E, Rombos J, et al. Acute myeloid leukemia in a patient with ataxia-telangiectasia: a case report and review of the literature. Leukemia. 2001;15(10):1668–1670. doi: 10.1038/sj.leu.2402210. [DOI] [PubMed] [Google Scholar]
- 19.Dror Y, Freedman MH. Shwachman-diamond syndrome. Br J Haematol. 2002;118(3):701–713. doi: 10.1046/j.1365-2141.2002.03585.x. [DOI] [PubMed] [Google Scholar]
- 20.Hasle H. Malignant diseases in Noonan syndrome and related disorders. Horm Res. 2009;72(2):8–14. doi: 10.1159/000243773. [DOI] [PubMed] [Google Scholar]
- 21.Freedman MH, Alter BP. Risk of myelodysplastic syndrome and acute myeloid leukemia in congenital neutropenias. Semin Hematol. 2002;39(2):128–133. doi: 10.1053/shem.2002.31912. [DOI] [PubMed] [Google Scholar]
- 22.Chitambar CR, Robinson WA, Glode LM. Familial leukemia and aplastic anemia associated with monosomy 7. Am J Med. 1983;75(5):756–762. doi: 10.1016/0002-9343(83)90404-7. [DOI] [PubMed] [Google Scholar]
- 23.Jongmans MC, Kuiper RP, Carmichael CL, et al. Novel RUNX1 mutations in familial platelet disorder with enhanced risk for acute myeloid leukemia: clues for improved identification of the FPD/AML syndrome. Leukemia. 2010;24(1):242–246. doi: 10.1038/leu.2009.210. [DOI] [PubMed] [Google Scholar]
- 24.Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood. 2009;113(26):6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrillat F, Clavel J, Jaussent I, et al. Family cancer history and risk of childhood acute leukemia (France) Cancer Causes Control. 2001;12(10):935–941. doi: 10.1023/a:1013758114381. [DOI] [PubMed] [Google Scholar]
- 26.Ripert M, Menegaux F, Perel Y, et al. Familial history of cancer and childhood acute leukemia: a French population-based case-control study. Eur J Cancer Prev. 2007;16(5):466–470. doi: 10.1097/01.cej.0000243849.82232.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasle H, Olsen JH. Cancer in relatives of children with myelodysplastic syndrome, acute and chronic myeloid leukaemia. Br J Haematol. 1997;97(1):127–131. doi: 10.1046/j.1365-2141.1997.202664.x. [DOI] [PubMed] [Google Scholar]
- 28.Wen WQ, Shu XO, Sellers T, et al. Family history of cancer and autoimmune disease and risk of leukemia in infancy: a report from the Children's Cancer Group (United States and Canada) Cancer Causes Control. 1998;9(2):161–171. doi: 10.1023/a:1008830210605. [DOI] [PubMed] [Google Scholar]
- 29.Piacentini S, Polimanti R, Porreca F, et al. GSTT1 and GSTM1 gene polymorphisms in European and African populations. Mol Biol Rep. 2011;38(2):1225–1230. doi: 10.1007/s11033-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 30.Aydin-Sayitoglu M, Hatirnaz O, Erensoy N, et al. Role of CYP2D6, CYP1A1, CYP2E1, GSTT1, and GSTM1 genes in the susceptibility to acute leukemias. Am J Hematol. 2006;81(3):162–170. doi: 10.1002/ajh.20434. [DOI] [PubMed] [Google Scholar]
- 31.Balta G, Yuksek N, Ozyurek E, et al. Characterization of MTHFR, GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes in childhood acute leukemia. Am J Hematol. 2003;73(3):154–160. doi: 10.1002/ajh.10339. [DOI] [PubMed] [Google Scholar]
- 32.Clavel J, Bellec S, Rebouissou S, et al. Childhood leukaemia, polymorphisms of metabolism enzyme genes, and interactions with maternal tobacco, coffee and alcohol consumption during pregnancy. Eur J Cancer Prev. 2005;14(6):531–540. doi: 10.1097/00008469-200512000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Davies SM, Robison LL, Buckley JD, et al. Glutathione S-transferase polymorphisms in children with myeloid leukemia: a Children's Cancer Group study. Cancer Epidemiol Biomarkers Prev. 2000;9(6):563–566. [PubMed] [Google Scholar]
- 34.Ross D, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–144. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 35.Guha N, Chang JS, Chokkalingam AP, et al. NQO1 polymorphisms and de novo childhood leukemia: a HuGE review and meta-analysis. Am J Epidemiol. 2008;168(11):1221–1232. doi: 10.1093/aje/kwn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lightfoot TJ, Johnston WT, Painter D, et al. Genetic variation in the folate metabolic pathway and risk of childhood leukemia. Blood. 2010;115(19):3923–3929. doi: 10.1182/blood-2009-10-249722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu CY, Hsu YH, Pan PC, et al. Maternal and offspring genetic variants of AKR1C3 and the risk of childhood leukemia. Carcinogenesis. 2008;29(5):984–990. doi: 10.1093/carcin/bgn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amigou A, Rudant J, Orsi L, et al. Folic acid supplementation, MTHFR and MTRR polymorphisms, and the risk of childhood leukemia: the ESCALE study (SFCE) Cancer Causes Control. 2012 doi: 10.1007/s10552-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 39.Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer: a pooled analysis. Epidemiology. 2009;20(4):475–483. doi: 10.1097/EDE.0b013e3181a5a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson KJ, Soler JT, Puumala SE, et al. Parental and infant characteristics and childhood leukemia in Minnesota. BMC Pediatr. 2008;8:7. doi: 10.1186/1471-2431-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin CC, Baptiste MS, Schymura MJ, et al. Birth weight, maternal weight and childhood leukaemia. Br J Cancer. 2006;94(11):1738–1744. doi: 10.1038/sj.bjc.6603173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puumala SE, Ross JA, Olshan AF, et al. Reproductive history, infertility treatment, and the risk of acute leukemia in children with down syndrome: a report from the Children's Oncology Group. Cancer. 2007;110(9):2067–2074. doi: 10.1002/cncr.23025. [DOI] [PubMed] [Google Scholar]
- 43.Larfors G, Hallbook H, Simonsson B. Parental Age, Family Size, and Offspring's Risk of Childhood and Adult Acute Leukemia. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0178. [DOI] [PubMed] [Google Scholar]
- 44.Cnattingius S, Zack M, Ekbom A, et al. Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 1995;4(5):441–445. [PubMed] [Google Scholar]
- 45.Dockerty JD, Draper G, Vincent T, et al. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;30(6):1428–1437. doi: 10.1093/ije/30.6.1428. [DOI] [PubMed] [Google Scholar]
- 46.Jourdan-Da Silva N, Perel Y, Mechinaud F, et al. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br J Cancer. 2004;90(1):139–145. doi: 10.1038/sj.bjc.6601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podvin D, Kuehn CM, Mueller BA, et al. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. 2006;20(4):312–322. doi: 10.1111/j.1365-3016.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds P, Von Behren J, Elkin EP. Birth characteristics and leukemia in young children. Am J Epidemiol. 2002;155(7):603–613. doi: 10.1093/aje/155.7.603. [DOI] [PubMed] [Google Scholar]
- 49.Roman E, Simpson J, Ansell P, et al. Perinatal and reproductive factors: a report on haematological malignancies from the UKCCS. Eur J Cancer. 2005;41(5):749–759. doi: 10.1016/j.ejca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Schuz J, Kaatsch P, Kaletsch U, et al. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. 1999;28(4):631–639. doi: 10.1093/ije/28.4.631. [DOI] [PubMed] [Google Scholar]
- 51.Wong DI, Dockerty JD. Birth characteristics and the risk of childhood leukaemias and lymphomas in New Zealand: a case-control study. BMC Blood Disord. 2006;6:5. doi: 10.1186/1471-2326-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma X, Metayer C, Does MB, et al. Maternal pregnancy loss, birth characteristics, and childhood leukemia (United States) Cancer Causes Control. 2005;16(9):1075–1083. doi: 10.1007/s10552-005-0356-9. [DOI] [PubMed] [Google Scholar]
- 53.Shu XO, Gao YT, Brinton LA, et al. A population-based case-control study of childhood leukemia in Shanghai. Cancer. 1988;62(3):635–644. doi: 10.1002/1097-0142(19880801)62:3<635::aid-cncr2820620332>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Westergaard T, Andersen PK, Pedersen JB, et al. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. J Natl Cancer Inst. 1997;89(13):939–947. doi: 10.1093/jnci/89.13.939. [DOI] [PubMed] [Google Scholar]
- 55.Zack M, Adami HO, Ericson A. Maternal and perinatal risk factors for childhood leukemia. Cancer Res. 1991;51(14):3696–3701. [PubMed] [Google Scholar]
- 56.Hjalgrim LL, Rostgaard K, Hjalgrim H, et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. J Natl Cancer Inst. 2004;96(20):1549–1556. doi: 10.1093/jnci/djh287. [DOI] [PubMed] [Google Scholar]
- 57.Perrillat F, Clavel J, Jaussent I, et al. Breast-feeding, fetal loss and childhood acute leukaemia. Eur J Pediatr. 2002;161(4):235–237. doi: 10.1007/s00431-001-0906-4. [DOI] [PubMed] [Google Scholar]
- 58.Yeazel MW, Buckley JD, Woods WG, et al. History of maternal fetal loss and increased risk of childhood acute leukemia at an early age. A report from the Childrens Cancer Group. Cancer. 1995;75(7):1718–1727. doi: 10.1002/1097-0142(19950401)75:7<1718::aid-cncr2820750725>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 59.Ross JA, Potter JD, Shu XO, et al. Evaluating the relationships among maternal reproductive history, birth characteristics, and infant leukemia: a report from the Children's Cancer Group. Ann Epidemiol. 1997;7(3):172–179. doi: 10.1016/s1047-2797(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 60.Spector LG, Davies SM, Robison LL, et al. Birth characteristics, maternal reproductive history, and the risk of infant leukemia: a report from the Children's Oncology Group. Cancer Epidemiol Biomarkers Prev. 2007;16(1):128–134. doi: 10.1158/1055-9965.EPI-06-0322. [DOI] [PubMed] [Google Scholar]
- 61.Von Behren J, Spector LG, Mueller BA, et al. Birth order and risk of childhood cancer: A pooled analysis from five US States. Int J Cancer. 2010 doi: 10.1002/ijc.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milne E, Laurvick CL, Blair E, et al. Fetal growth and acute childhood leukemia: looking beyond birth weight. Am J Epidemiol. 2007;166(2):151–159. doi: 10.1093/aje/kwm065. [DOI] [PubMed] [Google Scholar]
- 63.Roman E, Ansell P, Bull D. Leukaemia and non-Hodgkin's lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer. 1997;76(3):406–415. doi: 10.1038/bjc.1997.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latino-Martel P, Chan DS, Druesne-Pecollo N, et al. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1238–1260. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- 65.Klimentopoulou A, Antonopoulos CN, Papadopoulou C, et al. Maternal smoking during pregnancy and risk for childhood leukemia: a nationwide case-control study in Greece and meta-analysis. Pediatr Blood Cancer. 2012;58(3):344–351. doi: 10.1002/pbc.23347. [DOI] [PubMed] [Google Scholar]
- 66.Mucci LA, Granath F, Cnattingius S. Maternal smoking and childhood leukemia and lymphoma risk among 1,440,542 Swedish children. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1528–1533. [PubMed] [Google Scholar]
- 67.Brondum J, Shu XO, Steinbuch M, et al. Parental cigarette smoking and the risk of acute leukemia in children. Cancer. 1999;85(6):1380–1388. [PubMed] [Google Scholar]
- 68.Ji BT, Shu XO, Linet MS, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;89(3):238–244. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 69.MacArthur AC, McBride ML, Spinelli JJ, et al. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes Control. 2008;19(3):283–295. doi: 10.1007/s10552-007-9091-8. [DOI] [PubMed] [Google Scholar]
- 70.Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer. 2003;88(3):373–381. doi: 10.1038/sj.bjc.6600774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Severson RK, Buckley JD, Woods WG, et al. Cigarette smoking and alcohol consumption by parents of children with acute myeloid leukemia: an analysis within morphological subgroups--a report from the Childrens Cancer Group. Cancer Epidemiol Biomarkers Prev. 1993;2(5):433–439. [PubMed] [Google Scholar]
- 72.Shu XO, Ross JA, Pendergrass TW, et al. Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: a Childrens Cancer Group study. J Natl Cancer Inst. 1996;88(1):24–31. doi: 10.1093/jnci/88.1.24. [DOI] [PubMed] [Google Scholar]
- 73.Sorahan T, Lancashire RJ, Hulten MA, et al. Childhood cancer and parental use of tobacco: deaths from 1953 to 1955. Br J Cancer. 1997;75(1):134–138. doi: 10.1038/bjc.1997.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang JS, Selvin S, Metayer C, et al. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163(12):1091–1100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 75.Menegaux F, Ripert M, Hemon D, et al. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: a French population-based case-control study. Paediatr Perinat Epidemiol. 2007;21(4):293–299. doi: 10.1111/j.1365-3016.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 76.Menegaux F, Steffen C, Bellec S, et al. Maternal coffee and alcohol consumption during pregnancy, parental smoking and risk of childhood acute leukaemia. Cancer Detect Prev. 2005;29(6):487–493. doi: 10.1016/j.cdp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Rudant J, Menegaux F, Leverger G, et al. Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE) Cancer Causes Control. 2008;19(10):1277–1290. doi: 10.1007/s10552-008-9199-5. [DOI] [PubMed] [Google Scholar]
- 78.Sorahan T, Prior P, Lancashire RJ, et al. Childhood cancer and parental use of tobacco: deaths from 1971 to 1976. Br J Cancer. 1997;76(11):1525–1531. doi: 10.1038/bjc.1997.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ross JA, Potter JD, Reaman GH, et al. Maternal exposure to potential inhibitors of DNA topoisomerase II and infant leukemia (United States): a report from the Children's Cancer Group. Cancer Causes Control. 1996;7(6):581–590. doi: 10.1007/BF00051700. [DOI] [PubMed] [Google Scholar]
- 80.Spector LG, Xie Y, Robison LL, et al. Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: a report from the children's oncology group. Cancer Epidemiol Biomarkers Prev. 2005;14(3):651–655. doi: 10.1158/1055-9965.EPI-04-0602. [DOI] [PubMed] [Google Scholar]
- 81.Alexander FE, Patheal SL, Biondi A, et al. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61(6):2542–2546. [PubMed] [Google Scholar]
- 82.Ross JA, Xie Y, Davies SM, et al. Prescription medication use during pregnancy and risk of infant leukemia (United States) Cancer Causes Control. 2003;14(5):447–451. doi: 10.1023/a:1024953532355. [DOI] [PubMed] [Google Scholar]
- 83.Kaatsch P, Scheidemann-Wesp U, Schuz J. Maternal use of antibiotics and cancer in the offspring: results of a case-control study in Germany. Cancer Causes Control. 2010;21(8):1335–1345. doi: 10.1007/s10552-010-9561-2. [DOI] [PubMed] [Google Scholar]
- 84.Robison LL, Buckley JD, Daigle AE, et al. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group) Cancer. 1989;63(10):1904–1911. [PubMed] [Google Scholar]
- 85.van Duijn CM, van Steensel-Moll HA, Coebergh JW, et al. Risk factors for childhood acute non-lymphocytic leukemia: an association with maternal alcohol consumption during pregnancy? Cancer Epidemiol Biomarkers Prev. 1994;3(6):457–460. [PubMed] [Google Scholar]
- 86.Canfield KN, Spector LG, Robison LL, et al. Childhood and maternal infections and risk of acute leukaemia in children with Down syndrome: a report from the Children's Oncology Group. Br J Cancer. 2004;91(11):1866–1872. doi: 10.1038/sj.bjc.6602223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ognjanovic S, Puumala S, Spector LG, et al. Maternal health conditions during pregnancy and acute leukemia in children with Down syndrome: A Children's Oncology Group study. Pediatr Blood Cancer. 2009;52(5):602–608. doi: 10.1002/pbc.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naumburg E, Bellocco R, Cnattingius S, et al. Intrauterine exposure to diagnostic X rays and risk of childhood leukemia subtypes. Radiat Res. 2001;156(6):718–723. doi: 10.1667/0033-7587(2001)156[0718:ietdxr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 89.Buckley JD, Robison LL, Swotinsky R, et al. Occupational exposures of parents of children with acute nonlymphocytic leukemia: a report from the Childrens Cancer Study Group. Cancer Res. 1989;49(14):4030–4037. [PubMed] [Google Scholar]
- 90.Scelo G, Metayer C, Zhang L, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environ Health Perspect. 2009;117(1):133–139. doi: 10.1289/ehp.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alderton LE, Spector LG, Blair CK, et al. Child and maternal household chemical exposure and the risk of acute leukemia in children with Down's syndrome: a report from the Children's Oncology Group. Am J Epidemiol. 2006;164(3):212–221. doi: 10.1093/aje/kwj203. [DOI] [PubMed] [Google Scholar]
- 92.Brosselin P, Rudant J, Orsi L, et al. Acute childhood leukaemia and residence next to petrol stations and automotive repair garages: the ESCALE study (SFCE) Occup Environ Med. 2009;66(9):598–606. doi: 10.1136/oem.2008.042432. [DOI] [PubMed] [Google Scholar]
- 93.Magnani C, Pastore G, Luzzatto L, et al. Parental occupation and other environmental factors in the etiology of leukemias and non-Hodgkin's lymphomas in childhood: a case-control study. Tumori. 1990;76(5):413–419. doi: 10.1177/030089169007600501. [DOI] [PubMed] [Google Scholar]
- 94.Pyatt D, Hays S. A review of the potential association between childhood leukemia and benzene. Chem Biol Interact. 2010;184(1-2):151–164. doi: 10.1016/j.cbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Van Maele-Fabry G, Lantin AC, Hoet P, et al. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer Causes Control. 2010;21(6):787–809. doi: 10.1007/s10552-010-9516-7. [DOI] [PubMed] [Google Scholar]
- 96.Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117(10):1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Maele-Fabry G, Lantin AC, Hoet P, et al. Residential exposure to pesticides and childhood leukaemia: a systematic review and meta-analysis. Environ Int. 2011;37(1):280–291. doi: 10.1016/j.envint.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 98.Turner MC, Wigle DT, Krewski D. Residential pesticides and childhood leukemia: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(1):33–41. doi: 10.1289/ehp.0900966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lafiura KM, Bielawski DM, Posecion NC, Jr, et al. Association between prenatal pesticide exposures and the generation of leukemia-associated T(8;21) Pediatr Blood Cancer. 2007;49(5):624–628. doi: 10.1002/pbc.21283. [DOI] [PubMed] [Google Scholar]
- 100.Rajaraman P, Simpson J, Neta G, et al. Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. BMJ. 2011;342:d472. doi: 10.1136/bmj.d472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shu XO, Reaman GH, Lampkin B, et al. Association of paternal diagnostic X-ray exposure with risk of infant leukemia. Investigators of the Childrens Cancer Group. Cancer Epidemiol Biomarkers Prev. 1994;3(8):645–653. [PubMed] [Google Scholar]
- 102.Bartley K, Metayer C, Selvin S, et al. Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol. 2010;39(6):1628–1637. doi: 10.1093/ije/dyq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hjalgrim LL, Westergaard T, Rostgaard K, et al. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158(8):724–735. doi: 10.1093/aje/kwg210. [DOI] [PubMed] [Google Scholar]
- 104.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124(11):2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 105.O'Neill KA, Bunch KJ, Vincent TJ, et al. Immunophenotype and cytogenetic characteristics in the relationship between birth weight and childhood leukemia. Pediatr Blood Cancer. 2012;58(1):7–11. doi: 10.1002/pbc.23209. [DOI] [PubMed] [Google Scholar]
- 106.Kwan M, Buffler P, Abrams B, et al. Breastfeeding and the risk of childhood leukemia: A meta-analysis. Public Health Rep. 2004;119(6):521–535. doi: 10.1016/j.phr.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fustinoni S, Rossella F, Polledri E, et al. Global DNA methylation and low-level exposure to benzene. Med Lav. 2012;103(2):84–95. [PubMed] [Google Scholar]
- 108.Guerrero-Preston R, Goldman LR, Brebi-Mieville P, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5(6):539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turan N, Ghalwash MF, Katari S, et al. DNA methylation differences at growth related genes correlate with birth weight: a molecular signature linked to developmental origins of adult disease? BMC Med Genomics. 2012;5:10. doi: 10.1186/1755-8794-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Radtke I, Mullighan CG, Ishii M, et al. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106(31):12944–12949. doi: 10.1073/pnas.0903142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuhn MW, Radtke I, Bullinger L, et al. High-resolution genomic profiling of adult and pediatric core-binding factor acute myeloid leukemia reveals new recurrent genomic alterations. Blood. 2012;119(10):e67–75. doi: 10.1182/blood-2011-09-380444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Menezo YJ. Paternal and maternal factors in preimplantation embryogenesis: interaction with the biochemical environment. Reprod Biomed Online. 2006;12(5):616–621. doi: 10.1016/s1472-6483(10)61188-1. [DOI] [PubMed] [Google Scholar]
- 113.Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lunde A, Melve KK, Gjessing HK, et al. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165(7):734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 115.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 116.Ricart W, Lopez J, Mozas J, et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia. 2005;48(9):1736–1742. doi: 10.1007/s00125-005-1877-1. [DOI] [PubMed] [Google Scholar]