Abstract
Background
We previously reported similar AIDS-free survival at 3 years in children who were >1 year old initiating antiretroviral therapy (ART) and randomized to early vs. deferred ART in the PREDICT Study. We now report neurodevelopmental outcomes.
Methods
284 HIV-infected Thai and Cambodian children aged 1–12 years with CD4 counts between 15–24% and no AIDS-defining illness were randomized to initiate ART at enrollment (“early”, n=139) or when CD4 count became <15% or a CDC C event developed (“deferred”, n=145). All underwent age-appropriate neurodevelopment testing including Beery Visual Motor Integration (VMI), Purdue Pegboard, Color Trails and Child Behavioral Checklist (CBCL). Thai children (n=170) also completed Wechsler Intelligence Scale (IQ) and Stanford Binet Memory test. We compared week 144 measures by randomized group and to HIV-uninfected children (n=319).
Results
At week 144, the median age was 9 years and 69 (48%) of the deferred arm children had initiated ART. The early arm had a higher CD4 (33% vs. 24%, p<0.001) and a greater percentage of children with viral suppression (91% vs. 40%, p<0.001). Neurodevelopmental scores did not differ by arm and there were no differences in changes between arms across repeated assessments in time-varying multivariate models. HIV-infected children performed worse than uninfected children on IQ, Beery VMI, Binet memory and CBCL
Conclusions
In HIV-infected children surviving beyond one year of age without ART, neurodevelopmental outcomes were similar with ART initiation at CD4 15–24% vs. < 15%; but both groups performed worse than HIV-uninfected children. The window of opportunity for a positive effect of ART initiation on neurodevelopment may remain in infancy.
Keywords: HIV, Children, ART, neurodevelopment, resource-limited settings
HIV afflicts greater than three million children worldwide1 yet our scientific understanding of the effects of timing of antiretroviral therapy (ART) on neurodevelopmental outcomes of these children is limited. While ART decreases rates of encephalopathy, a most severe form of HIV-associated brain insult, chronic neurobehavioral deficits remain prevalent resulting in impairments in cognition, executive and adaptive functioning and school performance. 2–9 Studies from developed and developing countries have reported poorer neurocognitive functioning in HIV-infected children compared to their HIV–uninfected peers with greater impairments observed in children with severe HIV symptoms and immune suppression. These studies are mainly cross-sectional and observational in nature and invariably include children who initiated ART with advanced HIV disease; limiting their ability to adequately assess timing of ART on neurocognitive outcomes. 3–5, 7, 9–11 There are two randomized studies assessing timing of ART on these outcomes. The Children with HIV Early Antiretroviral Therapy (CHER) study performed neurodevelopment testing in a subset of infants and reported superior neurocognitive outcomes in those randomized to early ART vs. deferred ART. 12 Our study, PREDICT, assesses a similar randomized strategy in older Thai and Cambodian children. 13
Thailand and Cambodia are among countries in Asia with the highest HIV prevalence: 1.3% in Thailand and 0.5% in Cambodia. At year end 2009, Thailand had 530,000 adults and 20,000 children living with HIV while these numbers for Cambodia were 63,000 and 1120. 1, 14, 15 Treatment policies in both countries have evolved over time from recommending ART based on moderate to severe HIV symptoms and/or immune suppression in the early 2000s when the PREDICT study was conceived to the current policy that is recommending earlier ART initiation similar to that in developed countries. 14, 16, 17
Treatment guidelines now recommend ART in all infants as soon as their HIV is confirmed, without regard to immunologic status.18, 19 In reality, the number of infected children diagnosed with HIV and started on ART during infancy is troublingly low20–22 This raises the question of how best to care for these older children, where guidelines are more similar to that of adults and based on immunocompromise or symptoms. The PREDICT study was designed to assess timing of ART on AIDS-free survival in children who survived and entered into care after infancy. After 3 years, children who were randomized to starting ART at CD4 15–24% and those who waited until CD4 was below 15% had similarly high rates of AIDS-free survival (98.7% and 97.9%, respectively)13. The current analysis compared cognitive functioning and neurodevelopmental outcomes between randomized arms. We hypothesized that children given early ART would have better cognitive function and neurodevelopmental outcomes at week 144 compared to those deferring ART. We included two HIV-uninfected control groups, one exposed and the other unexposed to HIV. The HIV-exposed group serves as a control group without HIV but with similarly compromised socioeconomic status and exposed in-utero to HIV and its associated immune, virologic and treatment effects whereas the HIV-unexposed group serves as a control group without HIV or HIV-exposure and compromised socioeconomic status. These control groups aid our understanding of HIV and socioeconomic effects on neurodevelopmental outcomes.
MATERIALS AND METHODS
Participants
Between 2006 to 2011 we conducted the PREDICT study, a multicenter randomized, open-label trial of early vs. deferred ART in children aged 1–12 years with CD4 15–24%, no history of AIDS-defining illness and no prior ART (ISRCTN00234091)13 (Figure 1). This neurodevelopmental substudy began two years after starting enrollment, and was conducted between 2008 and 2011. Participants were recruited from 7 sites in Thailand and 2 sites in Cambodia. We enrolled two age- and gender- matched HIV-uninfected control groups from the PREDICT sites: HIV-unexposed and HIV-exposed children. Matching for age was accomplished using four age bands: 2–5 years, 5–8 years, 8–11 years and ≥ 11 years old, based on the age when the HIV-infected child first enrolled in the neurodevelopmental substudy. Written informed consent was obtained from the caregivers prior to enrollment.
Figure 1.
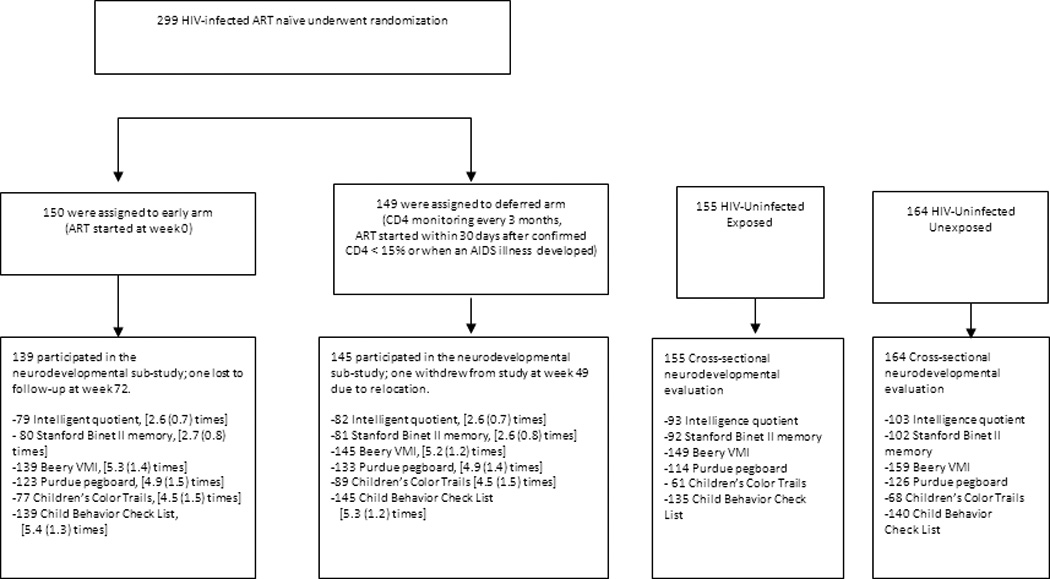
Study design, patient disposition and numbers completing each neurodevelopmental test
Footnote: The number of children who underwent each test in each of the 4 study groups is shown. For the HIV-infected children in the early and deferred ART study arms, the mean (standard deviation) of the number of tests performed during the study period is also shown. The cognitive and behavior assessments include 1) The intelligence tests: Wechsler Intelligence Scale for Children (WISC)-III (age 6 to 17 years) or the Wechsler Preschool and Primary Scale of Intelligence (WPPSI)-III (age 2 to 7.25 years); 2) The Stanford Binet II memory test (Beads/Sentences for age 3.5 to 17 years; Digits/Objects for age 6 to 17 years); 3) Beery Visual Motor Integration Test (VMI) (age 2 to 17 years); 4) Purdue Pegboard (age 5 to 17 years); 5) Children’s Color Trails (age 8 to 17 years); 6) The Child Behavior Checklist (age 2-17 years).
Randomizations
The HIV-infected children were randomly assigned to either initiating ART at CD4 15–24% (early arm) or initiating ART when CD4% was <15% or a CDC category C event developed (deferred arm). In December 2008, the CD4 threshold for children aged 1–3 years was modified to CD4 < 20% based on local treatment guidelines changes. Randomization was stratified by research site and history of nevirapine exposure for preventing mother-to-child HIV transmission (PMTCT), and managed from the trial coordinating center (HIV-NAT in Bangkok, Thailand) using a computerized system.
Procedures
Our primary objective was to determine neurodevelopmental outcomes at week 144 in early vs. deferred ART children. The secondary objective was to compare these outcomes between the HIV-infected children in PREDICT and their HIV-uninfected peers. We assessed a range of behavioral and cognitive abilities which are at risk for HIV-associated impairment using standardized tests that were in use in Thailand and Cambodia and which have good validity and reliability in their original form.. Neuropsychological tests that required attention, tapping memory, psychomotor speed, and processing speed were selected for administration in this study as these cognitive domains are frequently impaired among HIV-infected individuals. Further, measures that tap these cognitive constructs are relatively brief to administer and score and they minimize language responses. Finally, the selected neuropsychological measures allowed straightforward translation of instructions as well as appropriate methods to ensure data fidelity through staff training and efficient monitoring of accuracy in test administration and scoring. HIV-infected children underwent age-appropriate psychomotor and behavioral assessments every 6 months and cognitive tests annually. The Cambodian sites performed only the psychomotor and behavioral measures; the Intelligence and Memory tests were not administered. The cognitive tests were either the Thai-version of the Wechsler Intelligence Scale for Children (WISC)-III (age 6 to 17 years) or the Wechsler Preschool and Primary Scale of Intelligence (WPPSI)-III (age 2 to 7.25 years), and the Stanford Binet II memory test (Beads/Sentences for age 3.5 to 17 years; Digits/Objects for age 6 to 17 years). The psychomotor assessments were Beery Visual Motor Integration (VMI) (age 2 to 17 years), Purdue Pegboard (age 5 to 17 years) and Children’s Color Trails (age 8 to 17 years). Thai versions of WISC-III, WPPSI-III and Stanford Binet II memory tests were available and widely used in Thailand, and have been validated by the Thai Psychologist Society. The assessments were completed by psychologists at all Thai sites. For the fine motor tests (Berry VMI, Purdue Pegboard and Color Trails), the English instructions were translated into Thai and Khmer by bi-lingual translators. Trained nurses were certified to administer these tests by experienced Thai and US neuropsychologists after correctly performing and scoring a minimum of 10 subjects per test. External quality assurance review by a US neuropsychologist was performed several times over the course of the study. The Child Behavior Checklist (CBCL) was completed by primary caregivers to assess behavioral problems at all sites (age 2 to 17 years), using the preschool (18 months to less than 6 years) and school-aged (age 6–18 years) forms. The English version of CBCL was translated into Thai and Khmer, and then back translated into English to ensure accurate translation. Between 2010–2011, the HIV-uninfected controls (155 born to HIV-infected mothers (“exposed”) and 164 born to HIV-uninfected mothers (“unexposed”) underwent a one-time neurodevelopmental assessment using the same tests. HIV-uninfected/exposed children were recruited from siblings of HIV-infected children and from children delivered to HIV-infected mothers at the study sites. HIV-uninfected/unexposed children were recruited from well-child clinics in the same hospitals. In the HIV-infected children, first-line ART consisted of zidovudine, lamivudine and nevirapine. A protease inhibitor (lopinavir/ritonavir or nelfinavir) was substituted for nevirapine in children with prior exposure to nevirapine as part of PMTCT (nelfinavir was not used after September 2007).16 The protocol received approval from the Thai and Cambodian National, and local Institutional Review Boards.
Statistical analysis
Analyses were conducted with Stata 11.2 (Stata Corporation, College Station, Tx, USA). All analyses used available data that was considered valid by the psychologist who conducted the test. Apart from Perdue Pegboard and CBCL, the outcome variables for statistical comparison in primary analysis were the neurodevelopment scaled scores, standardized against US norms. Internal, external and total problem age and gender adjusted T scores were used as the outcome variable in the CBCL models. For the Purdue Pegboard, regression models compared the number of successful peg placements adjusting for age and gender since standardized scores were not available. The unadjusted mean difference in each standardized neurodevelopmental test score between the early and deferred arms were compared at week 144. For comparisons between the HIV-infected and uninfected groups, early and deferred data were combined because there was no difference between arms, and mean differences were adjusted for socio-demographic characteristics. In addition to comparing the mean CBCL internalized, externalized and total problem T scores, we assessed the proportion of children with CBCL syndrome-based subscale T scores in the borderline/clinical ranges (T ≥ 65) and assessed whether this was different in school-age children aged 6–11 years versus those aged ≥12 years. We then made comparisons of the mean full scale IQ in children with borderline/clinical syndrome T scale scores against those with scores in the normal range, within these age groups. Three sensitivity analyses were conducted. First, we compared the change in neurodevelopmental scores from the first to the last evaluation by treatment arm, and amongst children in the deferred arm who initiated treatment versus those who did not. Second, changes in neurodevelopmental scores for 39 early and 32 deferred children who had an assessment at the time of randomization were compared. Third, we used a multivariable random effects regression panel model to compare the neurodevelopmental scores at each study visit between the early and the deferred arms over the entire duration of follow-up. In univariate analyses we assessed the contribution of age, gender, CD4% and log10 HIV RNA at each visit, the educational level of the caregiver, income level, and whether the child lived with family. Factors significant in univariate analysis at P < 0.2 were included in the multivariable models. Years from the first to last test, the first score, whether the child was on ART at the time of the test and treatment arm were adjusted for in each model.
RESULTS
Demographic characteristics
Between March 2006 and September 2008, 455 children were screened and 300 HIV-infected children (180 Thai and 120 Cambodian) enrolled in the main PREDICT study. Of these, 284 HIV-infected children participated in this neurodevelopmental substudy (139 in the early arm and 145 in the deferred arm). Three hundred and nineteen age-matched HIV-uninfected children including 155 HIV-exposed and 164 HIV-unexposed were enrolled as controls. The number of children that underwent each test and number of repeat tests are also shown in Figure 1. The numbers of assessments prior to week 144 were not different between the early and the deferred groups while the HIV-uninfected groups had only a single assessment. The HIV-infected early and deferred arms were similar in age (median age 9 years), gender (58% female), ethnicity (60% Thai) and socioeconomic status at entry and study week 144 (Table 1). Only 48% of the deferred arm children initiated ART and had a lower median duration of ART. At week 144, the early arm had higher CD4%, CD4 count and HIV RNA <50 copies/ml as well as CD4 nadir (all p < 0.001). ART regimens were zidovudine/lamivudine/nevirapine (n=141), zidovudine/lamivudine/lopinavir (n=25) and others (n=42). The HIV-uninfected controls were two years younger than the infected children at week 144. The gender and ethnicity of the infected and uninfected children were similar. The HIV-exposed, uninfected group more closely resembled the HIV-infected children for caregiver’s education and income (Table 1).
Table 1.
Demographics, socioeconomics and HIV-related characteristics
| HIV-infected (at week 144 of PREDICT) |
HIV-uninfected | |||
|---|---|---|---|---|
| Early ART (n=139) |
Deferred ART (n=145) |
HIV exposed (n=155) |
HIV unexposed (n=164) |
|
| Demographic and socioeconomic characteristics | ||||
| Median age | 9 years | 9 years | 7 years** | 7 years** |
| Age, n | ||||
| < 5 years | 14 | 13 | 40** | 38** |
| 5 to 12 years | 106 | 106 | 96 | 112 |
| > 12 years | 19 | 26 | 19 | 14 |
| Female | 51% | 64% | 57% | 58% |
| Thai | 60% | 59% | 63% | 66% |
| Father or mother as primary caregiver |
65% | 64% | 93%** | 87%** |
| Caregiver education; high school/bachelor |
43% | 43% | 34% | 65%** |
| Low/very low income | 59% | 66% | 58% | 35%** |
| HIV-related characteristics | ||||
| CDC N:A:B | 1:61:38% | 1:63:36% | ||
| Time on ART (weeks) | 144 weeks | 48% on ART for a median of 81 weeks |
||
| Median current CD4% | 33% | 24%** | ||
| Median current CD4 count (cells/mm3) |
978 | 653** | ||
| Median %CD4 nadir | 18% | 15%** | ||
| %HIV RNA < 50 copies/ml | 91% | 40%** | ||
Footnote: p value < 0.001
Characteristics at time of primary neurocognitive endpoint (week 144 of PREDICT) are shown here for the HIV-infected children in the randomized early and deferred antiretroviral therapy (ART) arms. The two HIV-uninfected groups had a cross-sectional neurodevelopmental assessment, and their characteristics at time of enrollment are included.
The significant p values for the demographic and socioeconomic characteristics denote significant differences between each of the HIV-uninfected groups with the combined HIV-infected groups. Exceptions are caregiver’s education and low/very low income in which only the HIV-unexposed uninfected group is significantly different to the other 3 groups. For HIV-related characteristics, the p value denotes significant differences between the early and deferred HIV-infected arm.
Cognitive and Intellectual function
For all cognitive tests, the test scores and mean difference in test scores did not differ by study arms (Table 2 and Figure 2). Specifically, the mean (SD) scores did not significantly differ between the early and deferred arms for full scale IQ, verbal IQ, performance IQ and processing speed quotient. However, all mean scores were lower than those of HIV-uninfected controls (p < 0.001). The Stanford Binet II memory mean (SD) scores for beads, sentences and digits did not differ by randomized arms though the deferred arm had a marginally higher score for objects (mean 52 vs. 50, p = 0.04). The sentence and digit memory scores were lower in the HIV-infected children compared to uninfected controls (p ≤ 0.01) (Fig., Supplemental Digital Content 1, http://links.lww.com/INF/B428).
Table 2.
Mean differences (95%CI) in neurodevelopmental test score at week 144 between deferred and early treatment arms, and between all HIV-infected PREDICT children versus the HIV-exposed/uninfected and HIV-unexposed/uninfected controls
| Deferred VS early | Predict VS exposed* |
Predict VS unexposed* |
||||
|---|---|---|---|---|---|---|
| Diff 95%CI | P | Diff 95%CI | P | Diff 95%CI | P | |
| Intelligence quotient (IQ) | ||||||
| Full Scale IQ | −0.78 (−5.02,3.46) | 0.72 | −9.03(−13.04,−5.01) | <0.001 | −12.45(−16.91,−7.98) | <0.001 |
| Verbal IQ | −0.15 (−3.95,3.65) | 0.94 | −9.25(−12.91,−5.58) | <0.001 | −14.85(−19.06,−10.64) | <0.001 |
| Performance IQ | −0.42 (−5.12,4.27) | 0.86 | −6.71(−11.15,−2.28) | 0.003 | −9.04(−13.95,−4.13) | <0.001 |
| Processing speed quotient | −0.5 (−5.57,4.56) | 0.84 | −10.15(−15.17,−5.13) | <0.001 | −12.99(−18.48,−7.50) | <0.001 |
| Binet memory | ||||||
| Beads | −2.04 (−4.68,0.6) | 0.13 | −0.71(−3.27,1.86) | 0.59 | −2.18(−4.93,0.58) | 0.12 |
| Objects | 2.27 (0.17,4.38) | 0.04 | 0.03(−2.03,2.09) | 0.98 | −0.68(−3.04,1.67) | 0.569 |
| Digits | −0.31 (−2.35,1.73) | 0.77 | −2.70(−4.78,−0.63) | 0.01 | −4.08(−6.39,−1.76) | 0.001 |
| Sentences | −0.29 (−1.84,1.25) | 0.71 | −2.39(−3.93,−0.85) | 0.002 | −4.03(−5.96,−2.11) | <0.001 |
| Fine motor | ||||||
| Beery visual motor integration |
1.01 (−2.3,4.32) | 0.55 | −8.41(−11.25,−5.57) | <0.001 | −10.56(−13.72,−7.39) | <0.001 |
| Color Trail 1 standard score |
1.1 (−3.99,6.2) | 0.67 | 0.66(−4.54,5.85) | 0.80 | −0.81(−6.46,4.83) | 0.78 |
| Color Trail 2 standard score |
2.33 (−2.26,6.91) | 0.32 | −0.15(−4.74,4.45) | 0.95 | 2.49(−2.73,7.71) | 0.35 |
| Purdue pegboard dominant hand pin placements† |
0.12 (−0.39,0.63) | 0.64 | 0.98(0.49,1.46) | <0.001 | 0.74(0.24,1.24) | 0.004 |
| Purdue pegboard non- dominant hand pin placements† |
0.24 (−0.29,0.76) | 0.37 | 0.79(0.30,1.28) | 0.002 | 0.48(−0.01,0.97) | 0.06 |
| Behaviour | ||||||
| CBCL total problem T score |
0.91 (−1.33,3.14) | 0.42 | 0.94(−1.10,2.99) | 0.37 | 2.35(0.21,4.49) | 0.03 |
| CBCL internalized problem T score |
0.99 (−1.18,3.16) | 0.37 | 1.92(−0.13,3.98) | 0.066 | 0.85(−1.20,2.89) | 0.42 |
| CBCL externalized problem T score |
0.01 (−2.17,2.2) | 0.99 | 2.44(0.47,4.41) | 0.015 | 3.25(1.20,5.30) | 0.002 |
Footnote: CBCL: Child behaviour checklist
Significant differences are between each of the HIV-uninfected groups relative to the combined early and deferred groups. Comparisons with HIV-uninfected control groups are adjusted for differences between groups (parent as caregiver, educational level of caregiver and income).
Purdue Pegboard pin placements are raw scores adjusted for age and gender.
Figure 2.
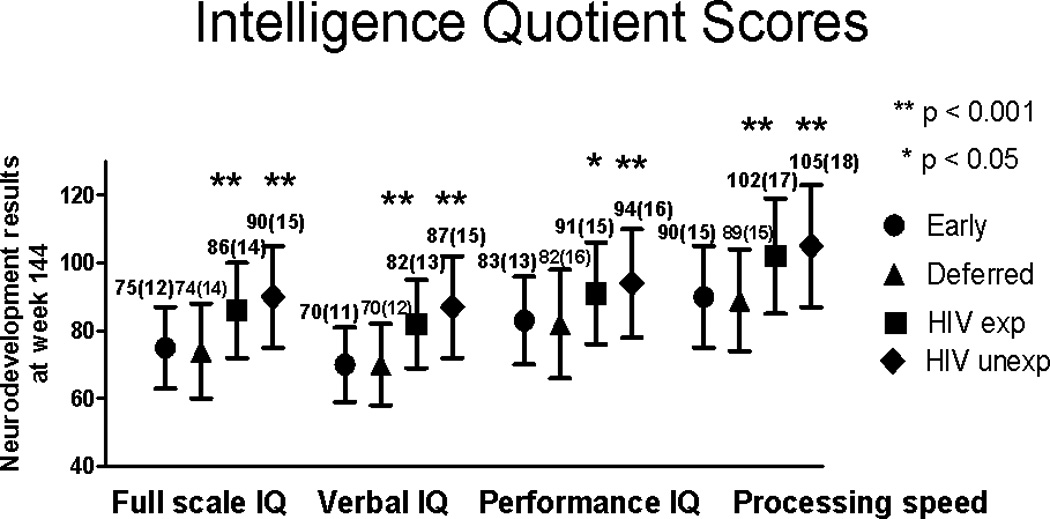
Intelligence quotient (IQ) comparison between the HIV-infected children (early vs. deferred antiretroviral therapy arms) at week 144 of PREDICT and the HIV-uninfected (HIV-exposed and –unexposed) control group children.
Footnote: Mean (standard deviation) scores are shown. P values for the comparison between PREDICT children and the uninfected HIV-exposed and HIV-unexposed controls have been adjusted for parent as caregiver, educational level of caregiver and income.
The intelligence quotient (IQ) tests included Wechsler Intelligence Scale for Children (WISC)-III (age 6 to 17 years) or the Wechsler Preschool and Primary Scale of Intelligence (WPPSI)-III (age 2 to 7.25 years).
The number of children in each group: HIV-infected/early antiretroviral therapy arm (n=79), HIV-infected/deferred antiretroviral therapy arm (n=82), HIV-uninfected/exposed (n=93) and HIV-uninfected/unexposed (n=103).
P value represents significant differences between each of the HIV-uninfected groups relative to the combined early and deferred groups.
The mean (SD) Beery VMI score did not differ by early vs. deferred arm, but were lower than that from the HIV-uninfected control groups (p < 0.001) (Table 2 and Figure, Supplemental Digital Content 2, http://links.lww.com/INF/B429). The mean (SD) Color Trails 1 and 2 scores were not different across the four groups. For Color Trails 1, the mean (SD) scores were 79 (17) for the early arm, 80 (14) for the deferred arm, 78 (17) for the HIV-uninfected/exposed and 83 (17) for the HIV-uninfected/unexposed children (p > 0.05). For the Color Trails 2, the mean (SD) scores were 86 (15) for the early arm, 88 (13) for the deferred arm, 88 (14) for the HIV-uninfected/exposed and 87 (16) for the HIV-uninfected/unexposed children (p > 0.05). The mean (SD) scores for Purdue Pegboard-dominant hand were the same in the early and deferred arms (14 [3] in both) and the mean score for the non-dominant hand was not significantly different in the deferred compared to the early arm (13 [3] vs. 12 [3], respectively). For both of these scores, the HIV-infected children performed better than the HIV-uninfected controls (Table 2, p <0.05).
The early and deferred children had similar mean (SD) T scores on the CBCL, with total problem scores of 51 (10) vs. 52 (9), externalized problem scores of 53 (9) vs. 53 (10), and internalized problem scores of 52 (10) vs. 53 (9), but they had higher total and externalized problem scores when compared to the HIV-uninfected/exposed [total score 50 (9) and externalized score 51 (8), p < 0.05)] and the HIV-uninfected/unexposed [total score 49 (10) and externalized score 50 (9), p < 0.05] groups. The externalized, internalized and total problem T scores > 60 typically suggest behavioral problems in the borderline-clinical range. This cutoff was met on total score for 16%, 20%, 16%, 15% (P=0.6) in early arm, deferred arm, HIV-uninfected/exposed and uninfected/unexposed, respectively [22%, 19%, 13%, 11% (p=0.04) for externalized and 22%, 19% 16%, 17% (p=0.6) for internalized scores].
For HIV-infected children, the proportion of 188 children aged 6–11 with CBCL Syndrome scale T scores in the borderline/clinical problem range was not significantly different to 42 children aged ≥ 12. However, although no significant differences in full scale IQ were noted in the younger children with borderline/clinical problem syndrome T scores, older children with borderline/clinical problem T scores in several syndrome scales had significantly lower full scale IQs in several domains. The mean difference in full scale IQ in children with abnormal vs. normal scores (95%CI; p) were −12.4 (−24.7 to −0.2; p = 0.048) for anxious/depressed and aggressive behavior and thought problems, and −15.3 (−28.0 to −2.6; p = 0.03) for attention problems. For the HIV-uninfected children, no significant differences in full scale IQ were observed between children with or without borderline/clinical problems in the 6–11 or 12–18 year age brackets; however, power to make comparisons in the older age group was limited due to smaller sample size.
Sensitivity analyses showed no differences in neurodevelopmental outcomes at week 144 among three groups of HIV-infected children: early arm (n=139), deferred arm children who did not start ART (n=75) and deferred arm children who did start ART (n=64). The change in scores from first to last test also did not differ by arm (data not shown). In multivariable random effects models of change from first test over total study follow-up, no differences were noted between the treatment arms (Table, Supplemental Digital Content 3, http://links.lww.com/INF/B430) and p-values for differences in neurodevelopmental scores in the deferred versus early arms over all follow up). Due to delay in starting the neurodevelopmental substudy, only 39 early and 32 deferred children had assessment at the time of randomization. Among these 71 children, no differences were noted in change of scores for all tests from baseline to week 144.
DISCUSSION
This study demonstrated that regardless of whether children age ≥ 1 year started ART at CD4 15–24% or deferred ART until CD4 was below 15%, the IQ, memory, psychomotor, and behavioral outcomes did not differ at three years after study entry, despite the early ART arm achieving higher CD4 levels and viral suppression. Nevertheless, the HIV-infected children performed worse on cognitive and neurodevelopmental tests compared to their HIV-uninfected (HIV-exposed and unexposed) peers. Our data suggest that cognitive and developmental deficits in HIV-infected children occur earlier than one year of life and they do not improve with initiation of ART.
These data complement those from the CHER study which showed better neurodevelopment in infants aged 10–15 months treated with ART before 3 months of age compared to those who deferred ART until CD4 criteria were reached (CD4 ~ 25%). Moreover, infants who started early on ART performed similarly to HIV-unexposed, uninfected infants.12 Other studies suggest that HIV-infected children who initiated treatment a few years later, during preschool, did not achieve the same levels of cognition as their healthy peers.11 School aged, ART-naive Ugandan HIV-infected children with similarly high CD4 levels as children in this study also demonstrated poorer cognition compared to those who were uninfected.23 These data, together with ours, suggest that CNS insult occurs very early in the life of infected infants and thus that the optimal window of opportunity for neuroprotection through ART initiation remains early in infancy.
Our children responded well to ART with a significant rise in CD4 and fall in HIV RNA; however, their neurocognitive scores changed little over time. Additionally, the changes were similar between children who initiated and did not initiate ART. Detailed analysis using random effects models of change showed no difference between the early and deferred arms for all tests over the entire study duration. This provides evidence for an irreversible but non-progressive HIV brain insult even in the presence of successful ART when ART is initiated in older children.3, 5, 10 Improvement in cognition in children following ART has been observed by some2, 6, 24 but not others3. It is hypothesized to be related to a decrease of inflammation and HIV viral burden in the CNS as well as possibly some general health improvements. Difference may also be due to severity of disease upon ART initiation. In our work, children never developed advanced immune compromise, by study design. Consistent among studies, some degree of chronic neurocognitive impairment generally persists.2, 6, 10, 24, 25 The statistical significant findings of better Purdue Pegboard scores in the HIV-infected vs. –uninfected are clinically insignificant, and could be due to data variability and/or learning/practice effects from repeat testing in the HIV-infected children. Aside from Purdue Pegboard where we used age and gender adjusted raw scores for analysis, we used US norms to obtain scaled scores for the other neurodevelopment tests due to the lack of Thai and Cambodian norms. By using the same method for all four groups of children, we partially mitigate the bias for interpretation. As the obtained serial scores for the HIV-infected children did not significantly change during the course of the study, it suggests that the outcome comparison of this group with HIV-uninfected children who were two years younger is valid.
Cognitive function is critical for maturing perinatally HIV-infected children to assimilate into society.4, 26 The HIV-infected children in this study demonstrated impairments in a wide range of abilities including intelligence, memory and eye-hand coordination, which could place them at a disadvantage in the current fast-pace and competitive world.27 Furthermore poor cognition and memory is associated with learning and behavioral problems.25 Compared to the HIV-uninfected controls, our HIV-infected children had more interruptive and hyperactive behaviors. In addition, HIV-infected adolescents with borderline/clinical ratings of anxious/depression, thought problems, attention problems and aggressive behaviors had significantly poorer full scale IQ scores. These behavioral consequences are likely to critically impact their transition into adulthood.4, 7, 27 Additionally, HIV preferentially affects the pre-frontal cortex of the brain, an area critical for executive function and ability to perform complex tasks.4, 5 Deficits associated with damage to these areas may only be identified in late adolescents and young adulthood when the functions associated with the pre-frontal lobes become critical for optimal functioning, also known as growing into a deficit.28 Low socioeconomic status could further impedes their success, as this alone can adversely affect cognition.29 Although the HIV-uninfected children had similarly low income and primary caregivers with low education as those infected with HIV, they exhibited superior neurodevelopmental outcomes which suggests a dominant negative effect of HIV infection on the brain. The contribution of family, social and economic factors on neurodevelopment is however complex and our study did not perform in depth evaluation of important factors such as resilience, child-caregiver relationship, social and family support, stigma, life stresses and mental health. These issues warrant future investigations.
Governments and public health systems face challenges with scaling up HIV diagnostics and treatment for HIV-infected infants. In 2010, the World Health Organization issued a recommendation, based on the CHER results30, that HIV diagnosis for HIV-exposed and ART for HIV-infected infants should be instituted during the first few months of life18. Each year, around 400,000 infants are newly infected with HIV, most living in sub-Saharan Africa1. Due to limited infrastructure and resources, fewer than half of these infants are being identified and treated in the first year of life.20–22 This missed opportunity for early ART has led to a crisis of information regarding how to approach aging-up HIV-infected children who are clinically asymptomatic without ART after 2 years of age. Our data suggest that with close CD4 monitoring, deferring ART to CD4 < 15% in older children who are well did not lead to worse AIDS-free survival13, cognition or neurodevelopment. However, deferring ART was associated with lower CD4 and higher viral replication which could potentially lead to neuro-inflammation and -damage.
The randomized design of our study is a major strength; however, there are some limitations. The lack of baseline neurodevelopmental assessment in a substantial number of children limits our ability to measure changes in neurodevelopmental scores from prior to treatment initiation. The random effects model estimating changes over time utilizing all data points however does allow us to evaluate longitudinal growth in neurocognitive function. Our population includes mainly older children, limiting relevance to younger children who may be at greater risk for HIV-associated brain injury.8, 9, 24 However, our population is unique in that it generally lacks maternal illicit drug use which often confounds reports from developed countries.31 We partially controlled for non-HIV confounders with comparative data from HIV-uninfected both exposed and unexposed to HIV.
In conclusion, in HIV-infected children surviving beyond age one year without ART, neurodevelopmental outcomes were similar with ART initiation at CD4 15–24% vs. < 15%; but both groups performed worse than HIV-unifected children. The results suggest that the window of opportunity to prevent neurobehavioral deficits remains in the first year of life. We continue to follow these children beyond 3 years following randomization into the early vs. deferred arms and have incorporated neuroimaging to capture effects of ART timing on brain growth and integrity. As children may age into their deficits, it will be important to determine how HIV and ART timing influence long-term neuropsychological, behavioral and psychiatric outcomes to enable identification of interventions to improve outcomes. These data may pivotally inform this issue and impact on future treatment guidelines.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank the families and children who participated in the trial. We thank Ms. June Piraporn Ohata for her help in preparing the manuscript for submission. Representatives of the National Institutes of Health were involved in the design of the study, data interpretation and writing of this report. The pharmaceutical sponsors had no role in the study design, and data analysis/interpretation. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication
Sources of support:
The work was supported by a grant from the National Institute of Allergy and Infectious Diseases of the National Institute of Health through the Comprehensive International Program of Research on AIDS Network (U19 AI53741); co-funded by the Eunice Shriver Kennedy National Institute of Child Health and Human Development and the National Institute of Mental Health. The antiretroviral drug supported by ViiV Health Care/GlaxoSmithKline, Boehringer-Ingelheim, Merck, Abbott and Roche. The views in this report do not necessary reflect the views of the National Institutes of Health or U.S. Department of Health and Human Services. KR has received support through grants HR1161A from the National Research University (NRU) Project of CHE and the Ratchadaphiseksomphot Endowment Fund, Thailand; the Professional Researcher Strengthen Grant from the National Science and Technology Development Agency (NSTDA), BIOTEC; and the Senior researcher scholar from Thai Research Fund (TRF), Thailand.
Appendix
The following investigators, clinical centers, and committees participated in the Pediatric Randomized of Early versus Deferred Initiation in Cambodia and Thailand (PREDICT trial).
Steering committee: Praphan Phanuphak, David A. Cooper, John Kaldor, Mean Chhi Vun, Saphonn Vonthanak, Kiat Ruxrungtham.
Primary endpoint review committee: Carlo Giaquinto, Mark Cotton, Rangsima Lolekha.
Clinical events review committee: Virat Sirisanthana, Kulkanya Chokephaibulkit, and Piyarat Suntarattiwong.
Data safety monitoring board members: Paul Volberding, Shrikant Bangdiwala, Kruy Lim, N.M. Samuel, David Schoenfeld, Annette Sohn, Suniti Solomon, Panpit Suwangool, Ruotao Wang, Fujie Zhang, Laurie Zoloth, Dennis O. Dixon.
National Institutes of Health: Lawrence Fox, Akinlolu O. Ojumu, Jane E. Bupp, Michael Ussery, Neal T. Wetherall, Pim Brouwers, Lynne M. Mofenson, Macaya Douoguih, Eva Petrakova.
Advisors: Matthew Law, William T. Shearer, Victor Valcour, Rober Paul, Kovit Pattanapanyasat, Natthaga Sakulploy, Janet M. McNicholl, Chutima Saisaengjan, Kattiya Rattanadilok.
Pharmaceuticals: ViiV Health Care/GlaxoSmithKline; Wendy Snowden, Navdeep K. Thoofer, Boehringer-Ingelheim; Manuel Distel, Abbott; Annette S. Meints, Adawan Methasate, Roche; Matei Popescu, Aeumporn Srigritsanapol, Merck; Suchai Kitsiripornchai.
Clinical research site
CIP TH001: HIV Netherlands Australia Thailand (HIV-NAT) Research Collaboration, Thai Red Cross AIDS Research Center, Bangkok, Thailand; Kiat Ruxrungtham, Jintanat Ananworanich, Thanyawee Puthanakit, Chitsanu Pancharoen, Torsak Bunupuradah, Jasper van der Lugt, Wasana Prasitsuebsai, Stephen Kerr, Theshinee Chuenyam, Sasiwimol Ubolyam, Apicha Mahanontharit, Tulathip Suwanlerk, Jintana Intasan, Thidarat Jupimai, Tawan Hirunyanulux, Praneet Pinklow, Kanchana Pruksakaew, Oratai Butterworth, Chulalak Sriheara, Anuntaya Uanithirat, Sunate Posyauattanakul, Thipsiri Prungsin, Pitch Boonrak, Waraporn Sakornjun, Tanakorn Apornpong, Jiratchaya Sophonphan, Ormrudee Rit-im, Nuchapong Noumtong, Noppong Hirunwadee, Chowalit Phadungphon, Wanchai Thongsee, Orathai Chaiya, Augchara Suwannawat, Threepol Sattong, Niti Wongthai, Kesdao Nantapisan, Umpaporn Methanggool, Narumon Suebsri, Taksin Panpuy, Pattiya Jootakarn, Kanitta Santikul, Apiwan Niyomlaksakul, Chayapa Phasomsup.
CIP TH003: Bamrasnaradura Infectious Diseases Institute, Nonthaburi, Thailand; Jurai Wongsawat, Rujanee Sunthornkachit, Visal Moolasart, Natawan Siripongpreeda, Supeda Thongyen, Piyawadee Chathaisong, Vilaiwan Prommool, Duangmanee Suwannamass, Simakan Waradejwinyoo, Nareopak Boonyarittipat, Thaniya Chiewcharn, Sirirat Likanonsakul, Chatiya Athichathana, Boonchuay Eampokalap, Wattana Sanchiem, Jarunsri Pantoe, Pintorn Prashyanusorn.
CIP TH004: Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; Pope Kosalaraksa, Pagakrong Lumbiganon, Chulapan Engchanil, Piangjit Tharnprisan, Chanasda Sopharak, Viraphong Lulitanond, Samrit Khahmahpahte, Ratthanant Kaewmart, Prajuab Chaimanee, Mathurot Sala, Thaniita Udompanit, Ratchadaporn Wisai, Somjai Rattanamanee, Yingrit Chantarasuk, Sompong Sarvok, Yotsombat Changtrakun, Soontorn Kunhasura, Sudthanom Kamollert, Jintana Singkhornard, Peerada Unprai, Chonnikarn Udomsri
CIP TH005: Queen Savang Vadhana Memorial Hospital, Chonburi, Thailand; Wicharn Luesomboon, Pairuch Eiamapichart, Tanate Jadwattanakul, Isara Limpet-ngam, Daovadee Naraporn, Pornpen Mathajittiphun, Chatchadha Sirimaskul, Woranun Klaihong, Pipat Sittisak, Tippawan Wongwian, Kansiri Charoenthammachoke, Pornchai Yodpo.
CIP TH007: Nakornping Hospital, Chiang Mai, Thailand; Suparat Kanjanavanit, Maneerat Ananthanavanich, Penpak Sornchai, Thida Namwong, Duangrat Chutima, Suchitra Tangmankhongworakun, Pacharaporn Yingyong, Juree Kasinrerk, Montanee Raksasang, Pimporn Kongdong, Siripim Khampangkome, Suphanphilat Thong Ngao, Sangwan Paengta, Kasinee Junsom, Ruttana Khuankaew, Parichat Moolsombat, Duanpen Khuttiwung, Chanannat Chanrin, Maetee Wongwerapan, Wanwisa Pansakul.
CIP TH009: Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; Rawiwan Hansudewechakul, Yaowalak Jariyapongpaiboon, Chulapong Chanta, Areerat Khonponoi, Chaniporn Yodsuwan, Warunee Srisuk, Pojjavitt Ussawawuthipong, Yupawan Thaweesombat, Polawat Tongsuk, Chaiporn Kumluang, Ruengrit Jinasen, Noodchanee Maneerat, Kajorndej Surapanichadul, Pornpinit Donkaew, Apsornsri Thanapaisan, Phornpatchara Sririindharatorn, Arpaporn Hanpa.
CIP TH010: National Pediatric Hospital, Phnom Penh, Cambodia; Saphonn Vonthanak, Ung Vibol, Sam Sophan, Pich Boren, Kea Chettra, Lim Phary, Toun Roeun, Tieng Sunly, Mom Chandara, Chuop Sokheng, Khin Sokoeun, Tuey Sotharin.
CIP TH011: Social Health Clinic, Phnom Penh, Cambodia; Saphonn Vonthanak, Ung Vibol, Vannary Bun, Somanythd ChhayMeng, Kea Chettra, Sam Phan, Wuddhika In vong, Khuon Dyna.
CIPTH012: Prapokklao Hospital, Chantaburi, Thailand; Chaiwat Ngampiyaskul, Naowarat Srisawat, Wanna Chamjamrat, Sayamol Wattanayothin, Pornphan Prasertphan, Tanyamon Wongcheeree, Pisut Greetanukroh, Chataporn Imubumroong, Pathanee Teirsonsern, Nittaya Khantilapapan, Petcharat Deenarn, Bhensiri Charoenvikkai.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work has been presented in part at the 19th Conference on Retroviruses and Opportunistic. March 5 – 8, 2012, Seattle (Abstract 24)
Conflict of interest of authors
TP has received speaker honoraria from Abbott. JA has received consultation or speaker honoraria from ViiV Health Care and Abbott. KR has been received consultation or speaker honoraria from ViiV Health Care and Abbott. Other authors reported no conflict of interest.
REFERENCES
- 1.Global report: UNAIDS report on the global AIDS epidemic 2010. [Accessed December 10, 2011]. [Google Scholar]
- 2.Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART) J Pediatr. 2005;146:402–407. doi: 10.1016/j.jpeds.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Puthanakit T, Aurpibul L, Louthrenoo O, et al. Poor cognitive functioning of school-aged children in thailand with perinatally acquired HIV infection taking antiretroviral therapy. AIDS Patient Care STDS. 2010;24:141–146. doi: 10.1089/apc.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachman S, Chernoff M, Williams P, et al. Human Immunodeficiency Virus Disease Severity, Psychiatric Symptoms, and Functional Outcomes in Perinatally Infected Youth. Arch Pediatr Adolesc Med. 2012 doi: 10.1001/archpediatrics.2011.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith R, Chernoff M, Williams PL, et al. Impact of HIV Severity on Cognitive and Adaptive Functioning During Childhood and Adolescence. Pediatr Infect Dis J. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomaidis L, Bertou G, Critselis E, et al. Cognitive and psychosocial development of HIV pediatric patients receiving highly active anti-retroviral therapy: a case-control study. BMC Pediatr. 2010;10:99. doi: 10.1186/1471-2431-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood SM, Shah SS, Steenhoff AP, Rutstein RM. The impact of AIDS diagnoses on long-term neurocognitive and psychiatric outcomes of surviving adolescents with perinatally acquired HIV. AIDS. 2009;23:1859–1865. doi: 10.1097/QAD.0b013e32832d924f. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LG, Sharer LR, Oleske JM, et al. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78:678–687. [PubMed] [Google Scholar]
- 9.Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Jeremy RJ, Kim S, Nozyce M, et al. Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005;115:380–387. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- 11.Lowick S, Sawry S, Meyers T. Neurodevelopmental delay among HIV-infected preschool children receiving antiretroviral therapy and healthy preschool children in Soweto, South Africa. Psychol Health Med. 2012 doi: 10.1080/13548506.2011.648201. [DOI] [PubMed] [Google Scholar]
- 12.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012 doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthanakit T, Saphonn V, Ananworanich J, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis. 2012 doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthanakit T, Tangsathapornpongb A, Ananworanich J, et al. for the Thai National HIV Guidelines Working Group. Thai National guidelines for the use of antiretroviral therapy in Pediatric HIV infection in 2010. Asian Biomedicine. 2010;4:505–513. [Google Scholar]
- 15.UNAIDS. [Accessed October 5, 2012];Ministry of Health. National Center for HIV/AIDS, Dermatology and STD. Report of a Consensus Workshop. HIV Estimates and Projections for Cambodia 2006 – 2012. Surveillance Unit Phnom Penh, 25–29 June 2007. Available at: http://www.unaids.org/en/dataanalysis/knowyourepidemic/countryreportsonhivestimates/cambodia_hiv_estimation_report_2006_en.pdf.
- 16. [Accessed December 10, 2011];DHHS Pediatric Panel Notice on Nelfinavir FDA-Pfizer Letter. September 11, 2007. Available at: http://aidsinfo.nih.gov/contentfiles/PedNFVnotice1.pdf.
- 17.NCHADS. [Accessed October 10, 2012];National Guidelines for the use of Pediatric Antiretroviral Therapy in Cambodia 3rd Edition June 27, 2011. Available at: http://wwwmedicam-cambodia.org/publication/details.asp?publication_id=52&pub_language=English.
- 18.Geneva: World Health Organization 2010. [Accessed April 20, 2012];Antiretroviral therapy for HIV infection in infants and children: towards universal access-recommendations for a public health approach. Available at: www.who.int. [PubMed]
- 19. [Accessed April 20, 2012];Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. August 11, 2011. Available at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 20.Nyandiko WM, Otieno-Nyunya B, Musick B, et al. Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr. 2010;54:42–50. doi: 10.1097/QAI.0b013e3181d8ad51. [DOI] [PubMed] [Google Scholar]
- 21.Mirkuzie AH, Hinderaker SG, Sisay MM, Moland KM, Morkve O. Current status of medication adherence and infant follow up in the prevention of mother to child HIV transmission programme in Addis Ababa: a cohort study. J Int AIDS Soc. 2011;14:50. doi: 10.1186/1758-2652-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuwagaba-Biribonwoha H, Werq-Semo B, Abdallah A, et al. Introducing a multi-site program for early diagnosis of HIV infection among HIV-exposed infants in Tanzania. BMC Pediatr. 2010;10:44. doi: 10.1186/1471-2431-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54:1001–1009. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–e693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- 25.Nozyce ML, Lee SS, Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 26.Dowshen N, D'Angelo L. Health care transition for youth living with HIV/AIDS. Pediatrics. 2011;128:762–771. doi: 10.1542/peds.2011-0068. [DOI] [PubMed] [Google Scholar]
- 27.Kapetanovic S, Wiegand RE, Dominguez K, et al. Associations of medically documented psychiatric diagnoses and risky health behaviors in highly active antiretroviral therapy-experienced perinatally HIV-infected youth. AIDS Patient Care STDS. 2011;25:493–501. doi: 10.1089/apc.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012;129:e254–e261. doi: 10.1542/peds.2011-0311. [DOI] [PubMed] [Google Scholar]
- 29.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lester P, Stein JA, Bursch B, et al. Family-based processes associated with adolescent distress, substance use and risky sexual behavior in families affected by maternal HIV. J Clin Child Adolesc Psychol. 2010;39:328–340. doi: 10.1080/15374411003691677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


