Iron and Kidney Disease
Changes in iron distribution have been documented in both acute kidney injury (AKI) and chronic kidney diseases (CKD). Kidney iron and urine iron increase in CKD due to glomerular dysfunction [1, 2, 3] which leaks transferrin and other serum proteins into the filtrate, which are thought to induce secondary tubular damage [4]. Iron appears in the urine from hemoglobin and myoglobin extravasation [5] and from damaged tubular cells as a result of chemotherapy (cis-platin [6]; doxorubicin [7]), ischemia-reperfusion injury [8, 9] and transplant ischemia [10] as well as other forms of AKI [9, 11, 12, 13, 14, 15]. In each case, “catalytic iron” is found in the urine and blood, and oxidized lipids are well documented. Preloading animals with iron [14] worsened these diseases and conversely chelating iron with desferroxamine (DFO), blunted the damage [6, 16, 13, 17, 18, 19]. Iron catalyzed damage is thought to be one of the earliest events in kidney dysfunction and is likely to be important in other organs as well, including the heart [20] where the siderophore chelator, carboxymycobactin was said to be protective. “Ferriuria” may also stimulate urinary tract infections which could result in endotoxemia and profound ischemic damage. These data raise many questions concerning the sources, mechanisms of trafficking and recycling of iron in tissue damage. It is also possible that iron release is not regulated whatsoever.
In normal conditions, transferrin (Tf) regulates iron delivery in circulation by solubilizing ferric iron (which is otherwise entirely insoluble above pH4; Ksp:10−10M-10−18M) [21] and delivering it to cells via the TfR1. Yet, whether Tf-TfR1 participates in all forms of iron transport in all extracellular spaces including the recovery of iron in tissue damage remains to be seen. In fact, non-transferrin mechanisms must be active under specific circumstances, as demonstrated by a number of examples, including the hypomorphic alleles of Tfhpx/hpx which are born iron overloaded [22, 23, 24, 25, 26], Tfr1 knockouts [27] which can still initiate organogenesis, and GFP-Tfr1−/− + Tfr1+/+ embryonic chimeras [28, 29, 30] which demonstrate GFP-Tfr1−/− cells maturing essentially into every component of the kidney, without the ability to capture iron from Tf. In sum, while the mechanisms of Tf-iron chelation and donation are established, there are specific instances which suggest non-transferrin molecules must play a role, but most of these remain to be discovered.
In this brief review, we have examined the biology of the gene lipocalin2, known in tissue damage studies as “NGAL” and for its capture of siderophores, as “Siderocalin (Scn)”. NGAL-Scn protein has been intensely studied by clinical scientists, but few labs have evaluated its basic biology.
NGAL-Scn: Baseline Expression
Biological fluids contain very low levels NGAL-Scn protein at steady state level. Serum contains approximately 20ng/ml NGAL-Scn which is probably derived from neutrophils and from limited expression in liver, spleen and kidney [31]. Renal clearance is a major regulator of this steady state level, because circulating NGAL-Scn undergoes glomerular filtration due to its low molecular weight (23-25KDa) and positive charge (pI>7.4). Theoretically, the kidney processes 3.4-4mg NGAL-Scn per day (20ng/ml × 120-140ml/min glomerular filtration rate, GFR) as a result of filtration [32] followed by capture by the proximal tubule, where it was degraded to a 14KDa fragment in lysosomes. Endocytosis of NGAL-Scn from the apical (luminal) membrane is the most likely pathway of NGAL-Scn traffic because it appeared in the urine when the apical megalin receptor was deleted. Biacore based binding studies confirmed a direct interaction between NGAL-Scn and megalin fragments [33], consistent with capture of NGAL-Scn from the glomerular filtrate rather than from the basolateral (blood) side.
Similar to serum, urine contains approximately 20ng/ml NGAL-Scn at steady state. The origin of this protein is not clear, but some of it may derive from serum NGAL-Scn (“sNGAL-Scn”) that bypasses capture in the proximal tubule (approximately 1/200 molecules). Alternatively, NGAL-Scn might derive from neutrophils or even from bladder epithelia. However, given the data discussed below, it is most likely that urinary NGAL-Scn (“uNGAL-Scn”) derives from a low level of expression in the native kidney at steady state. Indeed, weak in situ RNA reactivity can be seen in the collecting ducts of some kidneys.
NGAL-Scn as the Biomarker
NGAL-Scn came to the attention of clinical scientists when it was found to be markedly upregulated by tissue damage [34]. Hence, NGAL-Scn joins a large number of stress induced proteins, but only in a few cases have in-depth studies demonstrated a quantitative relationship between damage and the expression of the protein. The following “proof of concept studies” demonstrate a tight linkage between stimuli which initiate kidney damage and the expression of NGAL-Scn protein. Much of this work was completed in the urinary pool, but we suspect similar data will emerge in ongoing evaluations of serum.
(1) Does NGAL-Scn originate from injured nephrons or from bystander nephrons?
Whole organ studies, including reciprocal cross-transplants between NGAL knockout and wild type mice have demonstrated that NGAL-Scn derives from damaged kidneys. For example, when chimeric mice (consisting of wild type kidneys placed in NGAL knockout bodies) were treated with ischemia, uNGAL-Scn and sNGAL-Scn were expressed [35]. On the other hand, there was much less uNGAL-Scn when the NGAL knockout kidneys were placed in wild type mice and subjected to ischemia (the urinary level essentially remained in the normal range).
A similar conclusion comes from human transplantation studies: human uNGAL-Scn and sNGAL-Scn were proportional to the rate of recovery from delayed graft function after transplantation, meaning that the greater the dysfunction of the allograft, the greater the expression of NGAL-Scn [36, 37], implying that NGAL-Scn derived from the damaged nephron. Likewise, a chimeric mouse consisting of TLR4 kidneys placed in null hosts resulted in the brisk expression of NGAL-Scn in response to LPS, but TLR4 deleted kidneys placed in wild type hosts failed to generate the protein (Paragas, Unpublished). The most likely interpretation of both of these experiments is cell- or “nephron”-autonomic responses to damage ie that NGAL-Scn derives from the damaged nephron and in the case of LPS from kidney epithelia which expressed TLR4. Cell autonomy was conclusively shown in cells isolated from the medulla of the kidney: NGAL-Scn was expressed in response to typical stimuli which trigger renal disease ie depletion of ATP [38], exposure to H2O2 [39] or the exposure to bacteria [33]. These data confirm that autonomous responses of cells or nephrons generate the NGAL-Scn protein.
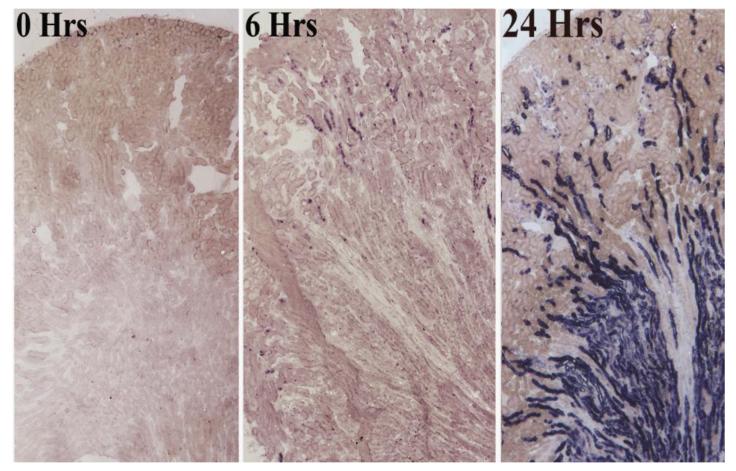
Microscopic analysis provided additional confirmation that damaged nephron generates NGAL-Scn. The induction of ischemia in one part of the kidney resulted in NGAL-Scn RNA expression in nephrons throughout that domain, but not in the adjacent non-ischemic domain. A specific temporal pattern was also seen: tubular expression extended in a cortical-medullary pattern after the injury (Figure 1). In situ hybridization using a paraffin technique localized NGAL-Scn RNA in tubules with morphological evidence of damage including intra-tubular casts. When a selected group of chronic kidney diseases were evaluated, the location of NGAL-Scn RNA also corresponded to sites of tubular disease. An animal model of HIV Associated Nephropathy [40] demonstrated NGAL-Scn expression in medullary microcysts in late stages of renal failure. In fact, NGAL-Scn was expressed at levels far in excess of any other gene that has been previously associated with hypoxia, ischemia and renal damage. Similarly, lining epithelia of Polycystic Kidney Disease [41, 42] expressed NGAL-Scn at levels higher than most other kidney expressed markers of tissue damage. These data show that the damaged tubule generates uNGAL-Scn and sNGAL-Scn.
Figure 1.
In situ hybridization of NGAL-Scn in the mouse kidney after ischemia reperfusion injury. Note the expression of NGAL-Scn in short cortical tubules by 6 hrs and throughout the TALH and Collecting Ducts at a later time point.
(2) Does the transcriptional response of the kidney parallel NGAL-Scn in serum or urine, and is this response a reflection of the intensity of the inciting stimulus?
To investigate the quantitative relationships between the expression of NGAL-Scn in the kidney and the amount of NGAL-Scn protein in the urine, we created a bioluminescent mouse by placing Luciferase2-mCherry reporters in the lipocalin2 locus [33]. Luciferase2 expression, which we designated “kidney NGAL-Scn” was proportional to the dose of ischemia or the dose of LPS. In addition, kidney luminescence paralleled the amount of NGAL-Scn protein appearing in the urine in a dose-responsive fashion. Related findings come from human studies of unilateral vs bilateral urinary obstruction [43], as well as studies of patients entering Emergency Departments where acute kidney injury was quantified by the graded “R-I-F-L” [44, 45] or “AKIN” stages [46]. In these cases, NGAL-Scn responded in a quantitative and dose dependent fashion (bilateral renal obstruction induced twice the level of unilateral disease; higher RIFL or AKIN designations induced progressively greater amounts of NGAL-Scn). Moreover the kinetics of NGAL-Luc2 and uNGAL-Scn protein were nearly identical in the onset of expression, the peak of expression and in the termination of expression. Taken together, these data show that dose of damage generates a graded NGAL-Scn response in kidney and, in turn, in urine.
Mori et al suggested that the disruption of the megalin dependent endocytic system in the damaged kidney could theoretically result in a failure to capture sNGAL-Scn from the filtrate, and consequently enhance the appearance of uNGAL-Scn [47]. While still reflecting nephron damage, this result would differ mechanistically from data obtained with the NGAL-Luc2-mC mouse. The former reflects damage to a segment of the nephron (the proximal tubule) that does not normally synthesize NGAL-Scn, while the latter reflects distal tubular synthesis and secretion into the urinary tract. We suspect that proximal tubule bypass is most likely active in chronic kidney diseases, but perhaps is self limited in AKI where the GFR falls in the damaged nephron (by definition). In addition, coordination between NGAL-Luc2 and uNGAL-Scn and the data from the cross-transplantation experiments (above) argue in favor of intra-renal synthesis and secretion as a dominant, but perhaps not the sole mechanism of uNGAL-Scn accumulation (escape from the proximal tubule being the second mechanism).
(3) Does the appearance of NGAL-Scn temporally correlate with the inciting stimulus and is its expression reversible?
Kidney Luc2-mCherry and uNGAL-Scn both demonstrated rapid and reversible responses to ischemia. Data in mice were similar to the responses of children undergoing surgery and neonates suffering clearly timed stimuli such as sepsis [42, 48, 49]; both Luc2-mCherry and NGAL-Scn protein appeared in blood and urine within 3 hrs after initiation of the stimulus and reversed if the stimulus was removed. The rapidity of the transcription and secretion into the urine differs from other urinary proteins but the specific cellular mechanisms that govern NGAL-Scn secretion have not been characterized.
The reversibility of NGAL-Scn expression can be used to follow the actions of medications which suppress AKI including HCO3 and fenoldapam [50, 51] in humans, CO donors and PARP inhibitors in mice [52], and antibiotics in cell models.
(4) Does the NGAL-Scn predict clinical outcomes?
Many studies have shown that the presence of either serum or urine NGAL-Scn anticipates a severe course for the patient in hospital, including the need for dialysis and the possibility of death (NGAL-Scn at the time of presentation to the hospital presages 3-20 fold increased probability). In a metaanalysis, Haase, Bellomo showed that NGAL-Scn+ patients had more severe illnesses [53] than did the NGAL-Scn-patients and Hall et al [54] showed that NGAL-Scn predicted poor outcomes. Ralib, Endre et al found that the daily amount of NGAL-Scn excretion correlated with the severity of outcomes [44] and NGAL-Scn at the time of discharge from the hospital presaged return to the hospital with clinically significant events [55]. In fact, patients who would otherwise have been overlooked in the Emergency Department because of apparently normal kidney function tests are at risk for rapidly progressive renal failure in the case that NGAL-Scn is expressed.
(5) What is the role of NGAL-Scn in renal injuries?
As described in detail below, the canonical role of NGAL-Scn is to sequester bacterial derived iron-binding catecholate siderophores, particularly enterochelin (Ent). Crystallography and fluorescence quenching techniques demonstrated that NGAL-Scn binds Ent with high affinity extending over a broad range of biological pHs [72]. In vivo, the deletion of the NGAL-Scn gene led to increased mortality when the knockout mice were subjected to lab strains of bacteria [77]. Data relevant to the kidney is also now emerging. NGAL-Scn knockout mice can not defend themselves against urogenital infections (Paragas et al. Unpublished). Hence, NGAL-Scn is a directed response to infectious damage and consequently might also serve as a prophylactic in aseptic injury. On the other hand, NGAL-Scn may have a related function in aseptic injury such as scavenging iron with the metabolite catechol (discussed below and Future Directions). It is also possible that NGAL-Scn plays a role which is not related to iron chelation per se. We initially isolated this protein by chromatography and a bioassay which demonstrated the growth and development of embryonic kidney cells [56]. Recent data from knockout mice also suggested a growth effect, because when damaged by chronic kidney disease, the knockout mice demonstrated reduced levels of epithelial proliferation [57]. Further analysis of these phenotypes may be achieved using experimental forms of NGAL-Scn which can traffic into the kidney tubule to inhibit bacterial growth, or to scavenge iron, or to induce epithelial growth.
(6) Are characteristics of NGAL-Scn conserved across different populations and animal models?
In all models of human and mouse kidney diseases, the NGAL-Scn ‘monomer’ or full length gene product is found in serum and in urine. In addition, the majority of the NGAL-Scn protein induced by acute renal failure in humans and in mice was a monomer. However, human NGAL-Scn contains an unpaired cysteine and can form not only monomers, but also dimers and hetero-multimers. These ‘high molecular forms” are found in chronic kidney diseases but not in acute kidney injury; their presence suggests either different cellular origins of the protein or variations in the metabolism of extracellular NGAL-Scn. For example, the dimer of human NGAL-Scn may arise from neutrophils [58] due to long storage of the protein in the oxidizing environment of a neutrophil granule compared to the rapid secretion of NGAL-Scn from epithelia. High molecular weight NGAL-Scn species also include cross linkage to transport proteins such as polymeric Ig receptors. These data are important because they suggest that the synthesis, trafficking and metabolism of “biomarkers” should be studied in clinical samples especially when the protein is known to be subject to proteolytic processing including Kim1 and Cyr61 and when the subject has chronic kidney disease.
Hence, while the expression and functions of NGAL-Scn are likely to be conserved across man and mouse, gene expression (the monomer) should be separately analyzed from protein metabolism (additional post-translational forms). These data are crucial in the analysis of chronic tissue injury because the specific form that is associated with epithelial injury (ie tubulointerstitial disease) must be the target of future epidemiological investigations [44].
Significance of NGAL-Scn Expression Data in Clinical Medicine
The intrinsic characteristics of NGAL-Scn listed above suggest that its measurement should be instituted in the diagnosis of tissue injury in human and in animal models [59, 60]. In addition, the dose dependent and reversible characteristics of NGAL-Scn expression mean that it is useful to identify therapies that reverse the illness. In contrast, the rise in serum creatinine (sCr), a measure of functional deficiency rather than tissue damage, might be quite delayed in elderly patients with reduced muscle mass (creatinine derives from muscle). Also sCr levels may be elevated even in the absence of nephron damage as a result of normal functional changes, a condition which is classically called “prerenal AKI” [61, 62, 63, 64, 65, 66]. Similar problems apply to the evaluation of chronic kidney disease. While uNGAL-Scn correlates with the severity of tubulo-interstitial disease [44], which is the most important pathologic finding linked to CKD prognosis [67, 68, 69, 70], sCr does not correlate well. Moreover there is a poor relationship between renal clearance [71] and sCr [72, 73], and in 20% of patients with CKD [74] the relationship of [sCr]−1 and disease progression does not hold at all [75]. Taken together these data illustrate that the diagnosis of renal tissue injury requires a single measurement of NGAL-Scn for its analysis, whereas multiple measurements of sCr separated in time are required to measure and characterize functional deficiencies.
NGAL-Scn Binds Co-factors which Bind Iron
1. Siderophores
A breakthrough in the understanding of the prominence of NGAL-Scn in kidney tissue injury was found in bacterially cloned NGAL-Scn by R. Strong and colleagues. NGAL-Scn is a member of the lipocalin superfamily which encodes a series of proteins that bind and transport small organic ligands within their internal calyx (Figure 2). The lipocalin super family proteins bind a number of ligands: Retinoids bind retinal binding protein, purpurin and rat epididymal RBP; pheromones bind major urinary binding proteins; colorants bind astaxanthin, and heme binds nitrophorins and α1-microglobulin. Goetz et al [76, 77] cloned NGAL-Scn in Gram− bacteria and identified a bacterial compound called enterochelin (Ent) bound within the protein’s calyx. Ent is an organic molecule that bacteria utilize to capture iron from the extracellular media. It is composed of three-2,3 dihydroxybenzoates (catechol-carboxylic acids) linked by a tri-serine lactone backbone [78] so that its 6 hydroxyl groups form a ‘hexadentate’ co-ordinate of ferric iron. R. Strong identified 3 positively charged amino acids in the base of the calyx which provided 3 sites of cation-π and Coulombic interactions with Ent’s catecholate rings, positioning their 6 hydroxyl groups to interact with iron. The amino acids are critical because their mutation diminished Ent:Fe binding. Complexes of NGAL-Scn with Ent:Fe (NGAL-Scn:Ent:Fe) could form in the bloodstream [79] followed by their rapid clearance by macrophages in liver and spleen and by filtration and tubular destruction in the kidney. Fe delivery to the proximal tubule by NGAL-Scn:Ent:Fe could be visualized using 55Fe and radioautography. The pathway depended on megalin, since modification of megalin recognition resulted in the appearance of Fe in urine [75]. Hence, the binding of Ent to NGAL-Scn not only sequestered the siderophore but resulted in its clearance in the kidney. Because sNGAL-Scn levels can rise 100 fold to >2000ng/ml, as much as 300-400mg NGAL-Scn/24 hours, containing nearly 1mg of iron can theoretically traffic to the kidney for clearance and recycling, provided Ent is fully saturated and glomerular filtration is maintained.
Figure 2.
Bacterial Enterochelin is composed of a triserine lactone backbone which coordinates three catecholate groups to form a hexadentate site that ligates a single iron atom. Iron-enterochelin complex binds the calyx of the lipocalin. The active site of NGAL-Scn is composed of three positively charged amino acids (R81, K125 and K134, where R is arginine and K is lysine). The binding is pH insensitive (Molecular models courtesy of R. K. Strong, Fred Hutchinson Cancer Research Center, Seattle)
The interaction of Ent with NGAL-Scn directly blocked the capture of iron by bacteria: NGAL-Scn delayed bacterial growth, but an oversupply of iron or the expression of siderophores [80] which do not recognize NGAL-Scn, rescued bacterial growth. Consistently, the deletion of NGAL-Scn by homologous recombination accelerated the growth of Ent dependent bacteria, resulting in sepsis and heightened mortality [81, 82]. Together, these data describe an interaction of mammalian NGAL-Scn protein with a bacterial product (Ent), and the subsequent clearance of Ngal-Scn:Ent:Fe by the proximal tubule.
It is likely that observations from systemic sepsis will also apply to the abundant NGAL-Scn expression within the urinary system, with a number of important caveats. uNGAL-Scn was significantly elevated by community acquired Gram− urinary infections according to a post-hoc analysis of patients in Emergency Departments [42] and other published cohorts [83, 84]. Uropathogenic E. coli were the overwhelming culprits (70-95% of the cases) and most of these bacteria relied on catecholate-siderophores to capture iron [85, 86] suggesting that NGAL-Scn should play a critical role in urinary defense. However, NGAL-Scn has been best characterized with E. coli sHB101 and H9049 which depend solely on Ent, whereas urinary bacteria potentially have multiple mechanisms of iron capture such as Salmochelin (Iro), Salmonella iron transport (Sit), Aerobactin (iutA), Hemin uptake (Chu), which are NGAL-Scn resistant, meaning that direct tests of urinary inhibition are required. In addition, the sources of uNGAL-Scn could be more complex than kidney ischemia, because both bladder and kidney epithelia may be involved in the response to urinary bacteria [87]. There are also many apparent differences between known urinary antimicrobial peptides and NGAL-Scn which require further analysis. For example, unlike the well known antimicrobial proteins [88, 89, 90] the cathelicidins (hCAMP, mCRAMP), the defensins (HBD1 [91, 92]), Tamm-Horsfall [93, 94, 95, 96, 97] and Lactoferrin [92], which are expressed constitutively, uNGAL-Scn is not likely to play a large role at steady state because it is expressed only at very low levels (20ng/ml). In addition, while many of these proteins are modulated only to a small degree by infections (cathelicidin by 3-8 fold; THP and lactoferrin are not stimulated by infections [84, 98, 99, 100, 101] and the AMPs and lactoferrin remain in the low ng/ml range even after stimulation), NGAL-Scn was intensively upregulated by significant infections (Figure 3) [102, 103, 104, 105, 106] as well as aseptic stimuli. Consequently the mechanisms of expression and induction apparently differ between NGAL-Scn and other antimicrobial peptides even though NGAL-Scn originates from the same cells as many of these proteins. Also additional analysis of the molecular characteristics of the NGAL-Scn:Ent interaction is required to determine whether Ent can be chelated in the harsh environment of the distal urinary tract (pH4.5; osmolarity 1200mOsm/L; high concentrations of urea). It is known that similar to other urinary defenses (Defensin HB1 is active at pH5.5; lactoferrin is active at pH 3.0) NGAL-Scn:Ent:Fe binding is unusually pH insensitive.
Figure 3.
NGAL-Scn-Luciferase2 (B) and NGAL-Scn RNA (C) expression detected after a dose of Lipid A. Note the kidney (white circle) and diffuse expression of NGAL-Scn-Luc2 (B) after the introduction of LipidA. (A) The same mouse before treatment. (C) Intercalated cells of the collecting ducts (alternating blue cells) respond to Lipid A and express NGAL-Scn RNA.
In sum, human and mouse studies have shown that NGAL-Scn is intensively upregulated by significant infections that impact the kidney (200-20,000ng/ml, Figure 3). For example, patients entering Emergency Rooms demonstrated a linkage between spontaneous, community-acquired sepsis and uNGAL-Scn expression [62]. In neonates suspected of sepsis only those neonates who were truly infected (i.e. blood cultures later identified a pathogen) expressed high levels of uNGAL-Scn, but those neonates who were not infected (i.e. blood cultures were sterile or pathogen free) did not express uNGAL-Scn [77, 102, 107]. Consistently, cecal ligation and puncture models produced a 531 fold increase in kidney NGAL-Scn (Paragas and Barasch unpublished). Together, these data imply that the release of NGAL-Scn into the urinary system is a major response of the kidney to systemic infection and potentially local urogenital infection as well.
2. Extended Function: Catechol:Iron?
Structural analyses by Strong and co-workers supported the idea that NGAL-Scn might bind additional ligands. First, Ent failed to fill the NGAL-Scn calyx, indicating that additional ligands are possible [73]. Second, NGAL-Scn had at least one additional ligand, a siderophore from mycobacteria called carboxymycobactin, which is structurally dissimilar to Ent. Third, a related member of the lipocalin superfamily, lipocalin1, can accommodate a variety of siderophores [108]. Fourth, while Ent is not synthesized by mammalian cells, it is a composite of well known functional groups such as hydroxybenzoates and hydroxybenzenes which are found in mammalian serum and urine. These data infer that NGAL-Scn may bind additional ligands, other than Ent and carboxymycobactin, and that these ligands may derive from a variety of sources. Nonetheless, to date, few candidates have been proposed.
Given the extensive expression of NGAL-Scn in rodent and human urine subjected to either septic or aseptic renal damage, we used urine as a source of ligands. First, a paper chromatography assay demonstrated that while 55Fe3+ precipitated in water, the addition of low molecular weight filtered urine or candidate urinary compounds solubilized 55Fe3+. Also, their addition to a mixture of NGAL-Scn and 55Fe3+ resulted in stable complexes similar to the addition of Ent. The urinary compounds that contained the dihydroxybenzene (catechol) functional group demonstrated saturation of NGAL-Scn (as measured by iron retention), and competition with a 50 fold excess of Ent:Fe3+ suggested that these compounds potentially occupy the same sites in the NGAL-Scn calyx as Ent. Simple catechol (1,2-dihydroxybenzene), pyrogallol (1,2,3-trihydroxybenzene), and 3- or 4-methyl catechol were the most active compounds and were stable during 20hrs of H-NMR inspection. Using fluorescence quenching and other spectroscopic techniques [72] and structural studies, Strong and Raymond found that the catechols could add to the calyx in a stepwise fashion (bis-catechol:Fe3+ first, followed by the recruitment of a third catechol to form tris-catechol:Fe3+) resulting in hexadentate coordination of iron. Most interestingly, catechol had a poor affinity for NGAL-Scn but in the presence of iron, the complex formed in the calyx at much higher affinity (2.1nM and 0.4nM). The catechols located at the same site where Ent bound (between the side-chains of residues K125 and K134), explaining their competition [109]. These data contrast with two other publications suggesting that norepinephrine (a 3,4 dihydroxycatechol) [110] and the polyphenol 2,5 dihydroxybenzoic acid can serve as NGAL-Scn ligands for iron [111]. Norepinephrine might have some NGAL-Scn iron binding activity (50nM) but it is very reduced compared to 2,3 dihydroxy-catechols, and 2,5 dihydroxybenzoic acid lacks the iron-coordinated ortho-diol needed for strong chelation at neutral pH.
NGAL-Scn:Catechol:Fe complex could form in mice from its component parts and quench the reactivity of catechol mediated Fe3+ reduction, and H202 mediated oxidation. The complex was stable for travel to the kidney and liver and bone marrow. This was particularly evident in the kidney, where the complex could be visualized at the apical membrane of the proximal tubule by radioautography. Its iron was likely to be recovered during the process of endocytosis in acidic endosomes of the proximal cell, because unlike NGAL-Scn:Ent, some catechols are pH sensitive (catechol>pyrogallol) [112]. This can be modeled in kidney cell lines expressing megalin where iron capture can be measured. In contrast to these data, transferrin-55Fe demonstrated much less targeting of the kidney.
Catechol was first identified by von Euler. While difficult to measure because of oxidation upon storage, catechol (up to 150μM) [113, 114, 115, 116, 117] and 4-methylcatechol (30μM), and pyrogallol (500μM) [109, 118] are known to be abundant. The majority of the catechols are sulfated and deactivated and this may take place in the kidney before excretion [119] (free catechols constitute 3-5% of total or 1-5 μM) [120]. However, the exact origin and metabolism of these molecules requires further testing, but dietary plant quinic, shikimic [121] and 3,4-dihydroxybenzoic acids [117, 122] and related hydroxybenzenes [109, 114] or tyrosine metabolism to phenol may be their sources. It appears that enteric microorganisms participate at one or more steps of these pathways because sterilization of the gut reduced catechol excretion [123].
Summary and Future Experiments
NGAL-Scn is a rapid response gene whose expression is driven in a dose dependent manner by stimuli which generally induce tissue damage [42, 62, 75, 124]. Its abundance at both the RNA and at the protein level have intrigued clinical scientists who have confirmed that its expression and its abundance reflects toxic stimulation just hours before gene expression. The rapidity and the intensity of its expression have been useful for identifying patients at particular risk for the onset or progression of kidney dysfunction, and for serious medical complications (dialysis, death). These basic clinical observations have been reproduced in many patient populations and animal models. Given that many stimuli activate NGAL-Scn, the most important next experiment is to determine the mechanism of signaling from clinically relevant stimuli to cells of the distal kidney. These experiments may come from further patient observations of nephrotoxins which target specific domains, or from segment specific methods of nephron damage as well as studies of the NGAL-Scn promoter.
There are also many unknowns concerning the trafficking of NGAL-Scn. A serum pool terminates at the proximal tubule megalin receptor, but an additional receptor 24p3R has been proposed to participate in NGAL-Scn capture as well [125, 126]. It will be exciting to see if its expression is important for recycling of NGAL-Scn ligands at the level of the proximal tubule or whether 24p3R is a highly unusual component of a protein recovery system in the distal nephron. Our data also suggest that the urinary pool derives mostly from tubular cells of TALH and Collecting Ducts but protein secretion from the nephron is not well studied. How NGAL-Scn traffics apically or basolaterally or both is relevant for our understanding of the clinical data as well as to identify cellular targeting mechanisms in nephron cells. These mechanisms are of particular interest in the intercalated cell which undergo reversals of protein polarity to achieve acid-base homeostasis [127].
Most importantly, there has not been an adequate explanation for the abundant expression of serum NGAL-Scn in different kidney diseases. Among the many possible explanations (see below), bacteriostasis has the greatest support from structure-function data and from NGAL-Scn knockout data. However what happens to the siderophore complex upon clearance in the proximal tubule remains to be seen. One paper indicates that Ent itself induces cytokine expression [128], but it is not known whether trafficking of NGAL-Scn:Ent to the proximal tubule has this property; this type of signaling would be a novel mechanism of inducing a renal response to systemic infection.
Bacteriostasis is also the most likely role of urinary NGAL-Scn, but it remains to be seen if typical urinary pathogens are sensitive to NGAL-Scn. Also, while systemic sepsis can activate NGAL-Scn expression through TLR expression, the mechanism of NGAL-Scn induction in the urogenital system is not fully known, and may involve novel TLR11 [129] or other receptors.
Given that kidney NGAL-Scn is abundantly expressed in aseptic injury, it has also remained unclear whether NGAL-Scn plays a “prophylactic” role limiting the eventuality that the damaged kidney might be colonized by ascending bacteria or whether NGAL-Scn has additional activities, such chelation of catechol or its family members when iron is released from damaged cells. Catechol can mobilize Fe3+ from other proteins [130] and the catechol:Fe complex may then participate in iron recycling that stimulates the Fenton reaction [131, 132]. In contrast, ligation of catechol:iron within NGAL-Scn calyx may permit transport and excretion of catechol:iron in a less reactive form. Consequently, targeting of the kidney by both catechol and NGAL-Scn might clear catechol and iron, as well as Ent and iron [75], removing potential iron donors for microbes both at the level of filtration and in the distal nephron. This idea is in principle similar to the proposal of Jones and Cerami et al [133] who found low molecular weight iron carriers in mammalian organs can donate iron to microbes. Further testing of these speculative hypotheses should include isolation of NGAL-Scn complexes from urine or serum to examine occupancy of the calyx, measurement of redox damage in NGAL-Scn knockout mice and oral antibiotic treatment and dietary restrictions to decrease catechol metabolites. It should also be possible to create forms of NGAL-Scn that retarget iron from the proximal tubule to the urine as a test of the role of NGAL-Scn as an iron scavenger in the damaged nephron.
Finally, it should be pointed out that many groups have shown that NGAL-Scn is a growth regulator. We initially utilized the development of rat kidney progenitors to purify growth factors, identifying NGAL-Scn. A number of other groups have also ascribed epithelial growth to NGAL-Scn [134] including in kidney epithelia [135]. In contrast, there are reports that the withdrawal of growth factors from hematopoeitic cells resulted in NGAL-Scn expression followed by apoptosis [136]. A similar correlation was documented in the involuting breast and the post-partum uterus [137, 138, 139] where NGAL-Scn was expressed during the reabsorption phase. Likewise, some authors have found that NGAL-Scn is stimulated by pro-apoptotic factors [140] and that it directly induces apoptosis [132, 141] in myeloid and early erythroid progenitors and in the kidney glomerulus [142], while other authors believe that NGAL-Scn, expressed during apoptosis, has a survival activity, because knockdown of NGAL-Scn expression enhanced apoptosis [136]. Taken together these data suggest that NGAL-Scn has growth activity that is active in the kidney, but its mechanism remains unclear. Resolution of these findings must await confirmation of the proposed receptor 24p3R or identification of additional novel receptors and their signaling mechanisms as clues to growth effects. However, at this time, siderophore chelation and potentially iron scavenging and trafficking remain the most investigated activities of NGAL-Scn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wang H, Nishiya K, Ito H, Hosokawa T, Hashimoto K, Moriki T. Iron deposition in renal biopsy specimens from patients with kidney diseases. Am J Kidney Dis. 2001;38:1038–44. doi: 10.1053/ajkd.2001.28593. [DOI] [PubMed] [Google Scholar]
- [2].Prinsen BH, de Sain-van der Velden MG, Kaysen GA, Straver HW, van Rijn HJ, Stellaard F, Berger R, Rabelink TJ. Transferrin synthesis is increased in nephrotic patients insufficiently to replace urinary losses. J Am Soc Nephrol. 2001;12:1017–25. doi: 10.1681/ASN.V1251017. [DOI] [PubMed] [Google Scholar]
- [3].Harris DC, Tay C, Nankivell BJ. Lysosomal iron accumulation and tubular damage in rat puromycin nephrosis and ageing. Clin Exp Pharmacol Physiol. 1994;21:73–81. doi: 10.1111/j.1440-1681.1994.tb02472.x. [DOI] [PubMed] [Google Scholar]
- [4].Alfrey AC. Toxicity of tubule fluid iron in the nephrotic syndrome. Am J Physiol. 1992;263:F637–641. doi: 10.1152/ajprenal.1992.263.4.F637. [DOI] [PubMed] [Google Scholar]
- [5].Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int. 1996;49:362–369. doi: 10.1038/ki.1996.53. [DOI] [PubMed] [Google Scholar]
- [6].Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- [7].Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001;43:211–218. doi: 10.1006/phrs.2000.0769. [DOI] [PubMed] [Google Scholar]
- [8].Paller MS, Jacob HS. Cytochrome P-450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc Natl Acad Sci USA. 1994;91:7002–7006. doi: 10.1073/pnas.91.15.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baliga R, Ueda N, Shah SV. Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem J. 1993;291:901–905. doi: 10.1042/bj2910901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baron P, Gomez-Marin O, Casas C, Heil J, Will N, Condie R, Burke B, Najarian JS, Sutherland DE. Renal preservation after warm ischemia using oxygen free radical scavengers to prevent reperfusion injury. J Surg Res. 1991;51:60–65. doi: 10.1016/0022-4804(91)90070-3. [DOI] [PubMed] [Google Scholar]
- [11].Cooper MA, Buddington B, Miller NL, Alfrey AC. Urinary iron speciation in nephrotic syndrome. J Kidney Dis. 1995;25:314–9. doi: 10.1016/0272-6386(95)90014-4. [DOI] [PubMed] [Google Scholar]
- [12].Zager RA. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J Clin Invest. 1992;90:711–9. doi: 10.1172/JCI115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988;34:474–80. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- [14].Wu ZL, Paller MS. Iron loading enhances susceptibility to renal ischemia in rats. Renal Fail. 1994;16:471–80. doi: 10.3109/08860229409045078. [DOI] [PubMed] [Google Scholar]
- [15].Gonzalez-Michaca L, Farrugia G, Croatt AJ, Alam J, Nath KA. Heme: a determinant of life and death in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F370–7. doi: 10.1152/ajprenal.00300.2003. [DOI] [PubMed] [Google Scholar]
- [16].Walker PD, Shah SV. Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest. 1988;81:334–341. doi: 10.1172/JCI113325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Paller MS, Hedlund BE. Extracellular iron chelators protect kidney cells from hypoxia/reoxygenation. Free Radic Biol Med. 1994;17:597–603. doi: 10.1016/0891-5849(94)90099-x. [DOI] [PubMed] [Google Scholar]
- [18].de Vries B, Walter SJ, von Bonsdorff L, Wolfs TG, van Heurn LW, Parkkinen J, Buurman WA. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation. 2004;77:669–675. doi: 10.1097/01.tp.0000115002.28575.e7. [DOI] [PubMed] [Google Scholar]
- [19].Zager RA, Burkhart KM, Conrad DS, Gmur DJ. Iron, heme oxygenase, and glutathione: effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995;48:1624–1634. doi: 10.1038/ki.1995.457. [DOI] [PubMed] [Google Scholar]
- [20].Horwitz LD, Sherman NA, Kong Y, Pike AW, Gobin J, Fennessey PV, Horwitz MA. Lipophilic siderophores of Mycobacterium tuberculosis prevent cardiac reperfusion injury. Proc Natl Acad Sci USA. 1998;95:5263–5268. doi: 10.1073/pnas.95.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chipperfield JR, Ratledge C. Salicylic acid is not a bacterial siderophore: a theoretical study. Biometals. 2000;13:165–8. doi: 10.1023/a:1009227206890. [DOI] [PubMed] [Google Scholar]
- [22].Trenor CC, Campagna DR, Sellers VM, Andrews NC, Fleming MD. The molecular defect in hypo-transferrinemic mice. Blood. 2000;96:1113–8. [PubMed] [Google Scholar]
- [23].Buys SS, Martin CB, Eldridge M, Kushner JP, Kaplan J. Iron absorption in hypotransferrinemic mice. Blood. 1991;78:3288–90. [PubMed] [Google Scholar]
- [24].Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J Lab Clin Med. 1987;110:690–705. [PubMed] [Google Scholar]
- [25].Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypo-transferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci U S A. 1987;84:3457–61. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thompson K, Molina RM, Brain JD, Wessling-Resnick M. Belgrade rats display liver iron loading. J Nutr. 2006;136:3010–4. doi: 10.1093/jn/136.12.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–9. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- [28].Ned RM, Swat W, Andrews NC. Transferrin receptor 1 is differentially required in lymphocyte development. Blood. 2003;102:3711–8. doi: 10.1182/blood-2003-04-1086. [DOI] [PubMed] [Google Scholar]
- [29].Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, Williams D, Lin CS, Schmidt-Ott KM, Andrews NC, Barasch J. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009 Jan;16(1):35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Macedo MF, de Sousa M, Ned RM, Mascarenhas C, Andrews NC, Correia-Neves M. Transferrin is required for early T-cell differentiation. Immunology. 2004;112:543–9. doi: 10.1111/j.1365-2567.2004.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Satoh N, Sugawara A, Barasch J, Nakao K. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009 Feb;75(3):285–94. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- [32].Axelsson L, Bergenfeldt M, Ohlsson K. Studies of the release and turnover of a human neutrophil lipocalin. Scandinavian Journal of Clinical & Laboratory Investigation. 1995;55:577–588. doi: 10.3109/00365519509110257. [DOI] [PubMed] [Google Scholar]
- [33].Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–7. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- [34].Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- [35].Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–22. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21:189–97. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P, Urine P. NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–45. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- [38].Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- [39].Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, Kuwahara Y, Fukumoto M, Shokrgozar MA. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H(2)O(2) toxicity. Arch. Med. Res. 2008;39:560–6. doi: 10.1016/j.arcmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [40].Paragas N, Nickolas TL, Wyatt C, Forster CS, Sise M, Morgello S, Jagla B, Buchen C, Stella P, Sanna-Cherchi S, Carnevali ML, Mattei S, Bovino A, Argentiero L, Magnano A, Devarajan P, Schmidt-Ott KM, Allegri L, Klotman P, D’Agati V, Gharavi AG, Barasch J. Urinary NGAL marks cystic disease in HIV-associated nephropathy. J Am Soc Nephrol. 2009;20:1687–92. doi: 10.1681/ASN.2009010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parikh CR, Dahl NK, Chapman AB, Bost JE, Edelstein CL, Comer DM, Zeltner R, Tian X, Grantham JJ, Somlo S. Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int. 2012 Jan 18; doi: 10.1038/ki.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sise ME, Forster C, Singer E, Sola-Del Valle D, Hahn B, Schmidt-Ott KM, Barasch J, Nickolas TL. Urine neutrophil gelatinase-associated lipocalin identifies unilateral and bilateral urinary tract obstruction. Nephrol Dial Transplant. 2011;26:4132–5. doi: 10.1093/ndt/gfr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O’Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Luft FC, Barasch J. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–55. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, Frei U, Dragun D, Haase M. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24:3349–54. doi: 10.1093/ndt/gfp234. [DOI] [PubMed] [Google Scholar]
- [46].Ralib A, Pickering JW, Shaw GM, Devarajan P, Edelstein CL, Bonventre JV, Endre ZH. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23:322–33. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Satoh N, Sugawara A, Barasch J, Nakao K. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–94. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- [48].Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- [49].Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, Lorenz JM, Barasch JM. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010;67:636–40. doi: 10.1203/PDR.0b013e3181da75c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haase M, Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Reade MC, Bagshaw SM, Seevanayagam N, Seevanayagam S, Doolan L, Buxton B, Dragun D. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med. 2009;37:39–47. doi: 10.1097/CCM.0b013e318193216f. [DOI] [PubMed] [Google Scholar]
- [51].Ricci Z, Luciano R, Favia I, Garisto C, Muraca M, Morelli S, Di Chiara L, Cogo P, Picardo S. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocalin and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15:R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Stone DH, Al-Badawi H, Conrad MF, Stoner MC, Entabi F, Cambria RP, Watkins MT. PJ34, a poly-ADP-ribose polymerase inhibitor, modulates renal injury after thoracic aortic ischemia/reperfusion. Surgery. 2005;138:368–74. doi: 10.1016/j.surg.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [53].Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, Han WK, Parikh CR. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6:2740–9. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, Clopton P, van Veldhuisen DJ. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL Evaluation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–51. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002 Nov;10(5):1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- [57].Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120(11):4065–76. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5:2229–35. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- [62].Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–9. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–31. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- [64].Macedo E, Mehta RL. Prerenal failure: from old concepts to new paradigms. Curr Opin Crit Care. 2009;15:467–73. doi: 10.1097/MCC.0b013e328332f6e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–30. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- [66].Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G, “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN) Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. Kidney Int. 1998;53:1209–16. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- [68].Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med. 1988;318:1657–66. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- [69].Eikmans M, Ijpelaar DH, Baelde HJ, de HE, Bruijn JA. The use of extracellular matrix probes and extracellular matrix-related probes for assessing diagnosis and prognosis in renal diseases. Curr Opin Nephrol Hypertens. 2004;13:641–7. doi: 10.1097/00041552-200411000-00010. [DOI] [PubMed] [Google Scholar]
- [70].Vleming LJ, de Fijter JW, Westendorp RG, Daha MR, Bruijn JA, van Es LA. Histomorphometric correlates of renal failure in IgA nephropathy. Clin Nephrol. 1998;49:337–44. [PubMed] [Google Scholar]
- [71].Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, Schneider KA, The Hypertension Detection and Follow-up Program Cooperative Group Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. Hypertension. 1989;13:I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- [72].Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- [73].The Renal Association . The Sixth Annual Report. Feb 8, 2005. UK Renal Registry. 2005. [Google Scholar]
- [74].Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, Gill J. Mc., Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- [75].United States Renal Data System Annual data report: incidence and prevalence of ESRD. American Journal of Kidney Disease. 2003;2003;42:S37–S173. [Google Scholar]
- [76].Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- [77].Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [78].Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A. 2003;100:3584–8. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin2. Proc Natl Acad Sci USA. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- [82].Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Natl Acad Sci USA. 2006;103:1834–9. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yilmaz A, Sevketoglu E, Gedikbasi A, Karyagar S, Kiyak A, Mulazimoglu M, Aydogan G, Ozpacaci T, Hatipoglu S. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr Nephrol. 2009;24:2387–92. doi: 10.1007/s00467-009-1279-6. [DOI] [PubMed] [Google Scholar]
- [84].Decavele AS, Dhondt L, De Buyzere ML, Delanghe JR. Increased urinary neutrophil gelatinase associated lipocalin in urinary tract infections and leukocyturia. Clin Chem Lab Med. 2011;49:999–1003. doi: 10.1515/CCLM.2011.156. [DOI] [PubMed] [Google Scholar]
- [85].Brzuszkiewicz E, Bruggemann H, Liesegang H, Emmerth M, Olschlager T, Nagy G, Albermann K, Wagner C, Buchrieser C, Emody L, Gottschalk G, Hacker J, Dobrindt U. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc Natl Acad Sci USA. 2006;103:12879–12884. doi: 10.1073/pnas.0603038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. The American Journal of Medicine. 2002;113(Suppl 1A):14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- [87].Reigstad CS, Hultgren SJ, Gordon JI. genomic studies of uro-pathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. The Journal of Biological Chemistry. 2007;282:21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- [88].Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- [89].Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- [90].Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- [91].Hiratsuka T, Nakazato M, Ihi T, Minematsu T, Chino N, Nakanishi T, Shimizu A, Kangawa K, Matsukura S. Structural analysis of human beta-defensin-1 and its significance in urinary tract infection. Nephron. 2000;85:34–40. doi: 10.1159/000045627. [DOI] [PubMed] [Google Scholar]
- [92].Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. The Journal of clinical investigation. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- [94].Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney international. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- [95].Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun. 2005;73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Abrink M, Larsson E, Gobl A, Hellman L. Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney International. 2000;57:2004–2010. doi: 10.1046/j.1523-1755.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- [97].Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. P Natl Acad Sci USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Reinhart H, Obedeanu N, Hooton T, Stamm W, Sobel J. Urinary excretion of Tamm-Horsfall protein in women with recurrent urinary tract infections. J Urol. 1990;144:1185–1187. doi: 10.1016/s0022-5347(17)39687-8. [DOI] [PubMed] [Google Scholar]
- [99].Perron GG, Zasloff M, Bell G. Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci. 2006;273:251–256. doi: 10.1098/rspb.2005.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Raffi HS, Bates JJM, Laszik Z, Kumar S. Tamm-Horsfall Protein Acts as a General Host-Defense Factor against Bacterial Cystitis. American Journal of Nephrology. 2005;25:570–578. doi: 10.1159/000088990. [DOI] [PubMed] [Google Scholar]
- [101].Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka LU, Ehren I, Gudmundsson TGH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nature Medicine. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- [102].Parravicini E, Lorenz JM, Nemerofsky SL, O’Rourke M, Barasch J, Bateman D. Reference range of urinary neutrophil gelatinase-associated lipocalin in very low-birth-weight infants: preliminary data. Am J Perinatol. 2009;26:437–440. doi: 10.1055/s-0029-1214242. [DOI] [PubMed] [Google Scholar]
- [103].Kumpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, Raulhaber-Walter R, Kielstein JT. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Critical Care (London, England) 2010;14:R9. doi: 10.1186/cc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, Piccinni P, Ronco C. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Medicine. 2010;36:444–451. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Critical Care Medicine. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D’Amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Medicine. 2010;36:452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- [107].Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, Lorenz JM, Barasch JM. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010;67:636–40. doi: 10.1203/PDR.0b013e3181da75c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–72. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, Viltard M, Williams D, Paragas N, Leete T, Kulkarni R, Li X, Lee B, Kalandadze A, Ratner AJ, Pizarro JC, Schmidt-Ott KM, Landry DW, Raymond KN, Strong RK, Barasch J. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6:602–9. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Miethke M, Skerra A. Neutrophil gelatinase-associated lipocalin expresses antimicrobial activity by interfering with L-norepinephrine-mediated bacterial iron acquisition. Antimicrob Agents Chemother. 2010;54:1580–9. doi: 10.1128/AAC.01158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–17. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sánchez P, Gálvez N, Colacio E, Miñones E, Domínguez-Vera JM. Catechol releases iron(III) from ferritin by direct chelation without iron(II) production. Dalton Trans. 2005;4:811–3. doi: 10.1039/b416669h. [DOI] [PubMed] [Google Scholar]
- [113].Carmella SG, La Voie EJ, Hecht SS. Quantitative analysis of catechol and 4-methylcatechol in human urine. Food Chem Toxicol. 1982;20:587–90. doi: 10.1016/s0278-6915(82)80068-9. [DOI] [PubMed] [Google Scholar]
- [114].Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Rothman N, Smith MT, Zhang L, Li G, Shen M, Yin S, Rappaport SM. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772–81. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- [115].Ong CN, Lee BL. Determination of benzene and its metabolites: application in biological monitoring of environmental and occupational exposure to benzene. J Chromatogr B Biomed Appl. 1994;660:1–22. doi: 10.1016/0378-4347(94)00278-9. Review. [DOI] [PubMed] [Google Scholar]
- [116].Marrubini G, Calleri E, Coccini T, Castoldi AF, Manzo L. Direct Analysis of Phenol, Catechol and Hydroquinone in Human Urine by Coupled-Column HPLC with Fluorimetric Detection. Chromatographia. 2003;62:25–31. [Google Scholar]
- [117].Qu Q, Melikian AA, Li G, Shore R, Chen L, Cohen B, Yin S, Kagan MR, Li H, Meng M, Jin X, Winnik W, Li Y, Mu R, Li K. Validation of biomarkers in humans exposed to benzene: urine metabolites. Am J Ind Med. 2000;37:522–31. doi: 10.1002/(sici)1097-0274(200005)37:5<522::aid-ajim8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [118].Lang R, Mueller C, Hofmann T. Development of a stable isotope dilution analysis with liquid chromatography-tandem mass spectrometry detection for the quantitative analysis of di- and trihydroxybenzenes in foods and model systems. J Agric Food Chem. 2006;54:5755–62. doi: 10.1021/jf061118n. [DOI] [PubMed] [Google Scholar]
- [119].Rennick B, Quebbemann A. A Site of excretion of catechol and catecholamines: renal metabolism of catechol. Am J Physiol. 1970;218:1307–12. doi: 10.1152/ajplegacy.1970.218.5.1307. [DOI] [PubMed] [Google Scholar]
- [120].Bakke OM, Scheline RR. Analysis of simple phenols of interest in metabolism. II. Conjugate hydrolysis and extraction method Anal Biochem. 1969;27:451. doi: 10.1016/0003-2697(69)90059-1. [DOI] [PubMed] [Google Scholar]
- [121].Booth AN, Robbins DJ, Masri MS, DeEds F. Excretion of catechol after ingestion of quinic and shikimic acids. Nature. 1960;187:691. doi: 10.1038/187691a0. [DOI] [PubMed] [Google Scholar]
- [122].Martin AK. The origin of urinary aromatic compounds excreted by ruminants. 3 The metabolism of phenolic compounds to simple phenols. Br J Nutr. 1982;48:497–507. doi: 10.1079/bjn19820135. [DOI] [PubMed] [Google Scholar]
- [123].Smith AA. Origin of urinary pyrocatechol. 1961;4771:167. [Google Scholar]
- [124].Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, Luft FC, Schmidt-Ott KM. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–14. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- [126].Langelueddecke C, Roussa E, Fenton RA, Wolff NA, Lee WK, Thévenod F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J Biol Chem. 2012;287:159–69. doi: 10.1074/jbc.M111.308296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Al-Awqati Q, Gao XB. Differentiation of intercalated cells in the kidney. Physiology (Bethesda) 2011;26:266–72. doi: 10.1152/physiol.00008.2011. [DOI] [PubMed] [Google Scholar]
- [128].Nelson AL, Ratner AJ, Barasch J, Weiser JN. Interleukin-8 secretion in response to aferric enterobactin is potentiated by siderocalin. Infect Immun. 2007;75:3160–8. doi: 10.1128/IAI.01719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- [130].Sánchez P, Gálvez N, Colacio E, Miñones E, Domínguez-Vera JM. Catechol releases iron(III) from ferritin by direct chelation without iron(II) production. Dalton Trans. 2005;4:811–3. doi: 10.1039/b416669h. [DOI] [PubMed] [Google Scholar]
- [131].Rodríguez J, Parra C, Contreras J, Freer J, Baeza J. Dihydroxybenzenes: driven Fenton reactions. Water Sci Technol. 2001;44:251–6. [PubMed] [Google Scholar]
- [132].Iwahashi H, Morishita H, Ishii T, Sugata R, Kido R. Enhancement by catechols of hydroxyl-radical formation in the presence of ferric ions and hydrogen peroxide. J Biochem. 1989;105:429–34. doi: 10.1093/oxfordjournals.jbchem.a122681. [DOI] [PubMed] [Google Scholar]
- [133].Jones RL, Peterson CM, Grady RW, Cerami A. Low molecular weight iron-binding factor from mammalian tissue that potentiates bacterial growth. J Exp Med. 1980;151:418–28. doi: 10.1084/jem.151.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Playford RJ, Belo A, Poulsom R, Fitzgerald AJ, Harris K, Pawluczyk I, Ryon J, Darby T, Nilsen-Hamilton M, Ghosh S, Marchbank T. Effects of mouse and human lipocalin homologues 24p3/lcn2 and neutrophil gelatinase-associated lipocalin on gastrointestinal mucosal integrity and repair. Gastroenterology. 2006;131:809–17. doi: 10.1053/j.gastro.2006.05.051. [DOI] [PubMed] [Google Scholar]
- [135].Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–76. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–34. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- [137].Ryon J, Bendickson L, Nilsen-Hamilton M. High expression in involuting reproductive tissues of uterocalin/24p3, a lipocalin and acute phase protein. Biochem J. 2002;367:271–7. doi: 10.1042/BJ20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bong JJ, Seol MB, Kim HH, Han O, Back K, Baik M. The 24p3 gene is induced during involution of the mammary gland and induces apoptosis of mammary epithelial cells. Mol Cells. 2004;17:29–34. [PubMed] [Google Scholar]
- [139].Liu Q, Ryon J, Nilsen-Hamilton M. Uterocalin: a mouse acute phase protein expressed in the uterus around birth. Mol Reprod Dev. 1997;46:507–14. doi: 10.1002/(SICI)1098-2795(199704)46:4<507::AID-MRD9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [140].Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Miharada KI, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005 Sep 12; doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- [142].Pawar RD, Pitashny M, Gindea S, Tan Tieng A, Levine B, Goilav B, Campbell SR, Xia Y, Qing X, Thomas D, Herlitz L, Berger T, Mak TM, Putterman C. Neutrophil gelatinase associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis. Arthritis Rheum. 2011 Nov 14; doi: 10.1002/art.33485. [DOI] [PMC free article] [PubMed] [Google Scholar]