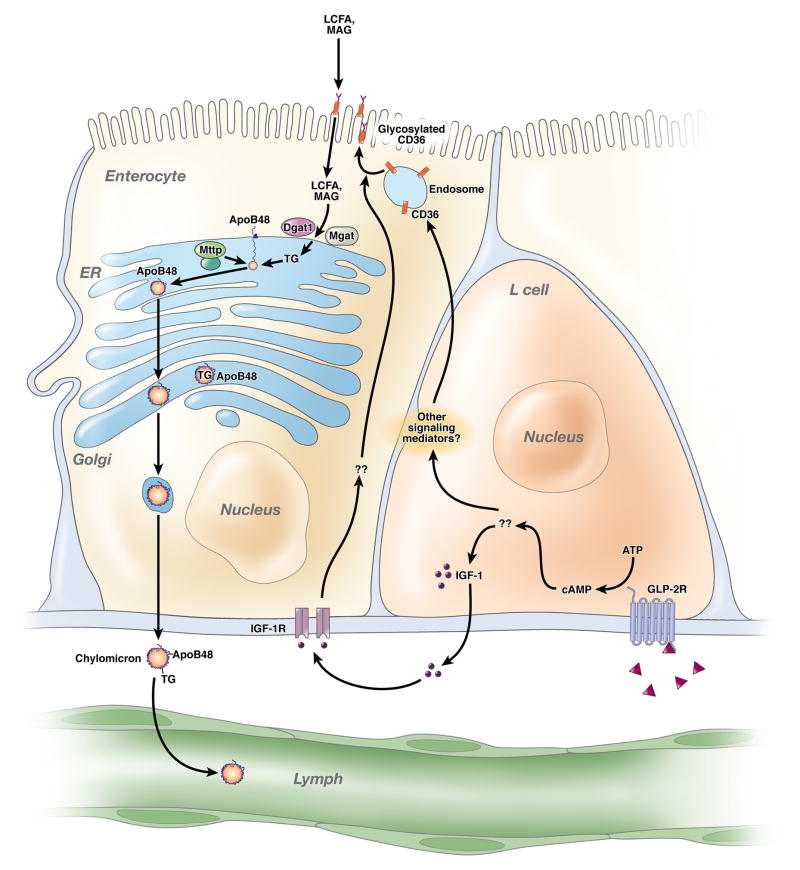
Intestinal lipid transport is a highly evolved and astonishingly efficient process whose genetic basis and metabolic regulation continues to provide surprises. The essence of the process (Figure 1) involves uptake of micellarized dietary long chain fatty acids (FA) and monoglyceride across the microvillus membrane, transport through apical cytoplasmic domains, and enzymatic reassembly into complex lipid droplets within membrane profiles of the endoplasmic reticulum (ER) 1. These lipid droplets then fuse with a requisite structural protein, apolipoprotein B48 (apoB48), in a process involving physical interaction of the nascent apoB48 protein at its amino terminus with a resident ER chaperone, microsomal triglyceride transfer protein (Mttp), thereby initiating formation of a primordial lipoprotein particle 1. Further addition of neutral lipid within the ER allows progressive enlargement of the lipoprotein particle, which is surrounded by a single molecule of apoB48 1. The maturing lipoprotein particles (prechylomicrons) undergo vectorial vesicular transport through Golgi membranes 2 where they undergo terminal modifications and are secreted as mature chylomicron (CM) particles into the lacteal for transport into the mesenteric lymph. Among the continuing mysteries is the nature and identity of the microvillus membrane FA carrier(s) and the role of paracrine and endocrine influences on the dynamics of intestinal lipoprotein assembly and secretion.
Figure 1. GLP2 enhances intestinal lipid absorption, but how?
Glucagon like peptide 2 (triangles) binds its receptor (GLP-2R) on intestinal L cells, generating mediators including insulin-like growth factor 1 (IGF-1) which signals via IGF-1 receptors (IGF-1R) on epithelial cells. These various signals promote CD36 glycosylation, in turn altering epithelial long chain fatty acid (LCFA) and monoacylglycerol (MAG) processing into triglyceride (TG) via diacylglycerol acyltransferase 1 (Dgat1) and monoacylglycerol acyltransferase (Mgat). The rate-limiting step in chylomicron formation is fusion of nascent apolipoprotein B48 (apoB48) with the luminal endoplasmic reticulum (ER) chaperone microsomal triglyceride transfer protein (Mttp). GLP-2 mediated enhancement of intestinal lipid transport was attenuated in Cd36 −/− mice, but further work is required to define the mechanisms involved.
One of the leading candidates proposed to mediate facilitated FA uptake across the microvillus membrane is the multi-ligand scavenger receptor CD36, which is expressed on the plasma membrane of many tissues including adipose tissue, skeletal muscle, and liver as well as on the apical membrane of enterocytes lining the small intestine 3. Just how CD36 participates in the regulation of intestinal lipoprotein assembly and secretion is the focus of considerable investigation (discussed below), but studies in the current issue of Gastroenterology by Adeli and colleagues 4 add further insight by demonstrating that hamsters and mice treated with the intestinotrophic hormone glucagon-like peptide 2 (GLP-2) manifest increased CM secretion and increased accumulation of post-prandial serum lipids within 30 minutes of GLP-2 treatment. GLP-2 induced augmentation of lipid trafficking was not observed in Cd36 −/− mice, implying that GLP-2 stimulates lipid uptake and secretion via a pathway requiring CD36. New questions concerning intestinal lipid metabolism are raised by the current work, including the identity of the receptor(s) and other trans-acting factors required for signal transduction following acute GLP-2 administration, as well as the biochemical role of CD36 in modulating intestinal CM formation and secretion and the intracellular compartment(s) involved.
GLP-2 is a 33 residue peptide produced by posttranslational proteolytic cleavage of proglucagon from intestinal L cells [Reviewed in 5]. In addition to its intestinotrophic actions, GLP-2 stimulates glucagon secretion, reduces gastric acid secretion and increases post-prandial serum triglyceride (TG) when administered to humans 6. Adeli and colleagues found increased serum TG and apoB48 levels after GLP-2 treatment of hamsters and increased apoB48 secretion from isolated jejunal segments 4. However, the signaling pathways by which GLP-2 treatment induces alterations in intestinal CM production remain obscure. Enterocytes do not express GLP-2 receptor (GLP-2R) and the trophic effects of chronic GLP-2 administration likely occur through liganding of GLP-2R on nearby cells, including enteroendocrine L cells, resulting in increased production of growth factors such as IGF-1 (Figure 1). Recent studies in Glp2r−/− mice suggest that the downstream effects GLP-2 on intestinal growth are mediated by ErbB signaling 7, while GLP-2 mediated increases in intestinal blood flow—which occur within minutes of GLP-2 administration—are blocked by inhibitors of nitric oxide production 8. Future studies will presumably address whether similar signaling pathways mediate the acute effects of GLP-2 on intestinal lipid metabolism.
Adeli and colleagues observed that serum TG and apoB48 levels increase in parallel following GLP-2 treatment, which most likely reflects an increase in CM number rather than a change in the size or lipid content of CM particles 4. Interestingly, GLP-2 did not augment CM production through enhanced ApoB48 synthesis or increased Mttp mRNA expression, proteins required for the rate-limiting step in intestinal lipoprotein synthesis and secretion 4. Instead, augmented intestinal lipid secretion appears to correlate with increased glycosylation of CD36, a modification shown to alter CD36 localization 9, although these conclusions are tempered by the finding that apical intestinal membranes from GLP-2 treated and control animals contained comparable amounts of glycosylated CD36 4 (Figure 3E). The observation that the GLP-2 induced increase in post-prandial TG was lost in Cd36 −/− mice suggests a role for this gene in modulating intestinal lipid secretion.
Although there is general consensus that CD36 plays a role in the uptake and intracellular metabolism of long chain FA and cholesterol in the small intestine 3, 10–12, it is still unclear exactly how CD36 might regulate intestinal CM production. Genetic, biochemical and functional evidence all suggest that Mttp-dependent fusion of lipid droplets with apoB48 in the ER represents the crucial restriction point in intestinal CM formation 13. Mttp activity, not FA availability, is generally believed to be limiting in this process. Adeli and colleagues suggest that glycosylated CD36 facilitates lipidation of apoB48 by increasing FA transport, although the cellular compartment(s) involved in such facilitated lipidation (ER or Golgi) remains to be defined. While further study is needed to confirm the significance of intestinal CD36 glycosylation, it has been demonstrated that CD36 translocates from an intracellular compartment to the plasma membrane in skeletal and cardiac muscle, increasing long chain FA uptake 14, 15.
Previous studies in Cd36 −/− mice demonstrated impaired intestinal CM secretion, particularly in response to bolus lipid administration, and showed that the effects of CD36 deletion are regionally selective, preferentially involving the proximal small intestine 11, 12. Other work has expanded our understanding of the functional role of intestinal CD36 in the facilitated transport of dietary very long chain FA by demonstrating that the transport efficiency of FAs with chain lengths greater than C20 was significantly decreased in Cd36 −/− mice, and transport of C24:0 FA was virtually eliminated 10. The functional interpretation and physiological impact of these findings, however, is balanced against observations that net fat absorption (both cholesterol and FA, as revealed by fecal output or dual isotope labeling studies) is unimpaired in Cd36 −/− mice, suggesting that recruitment of more distal regions of the intestine may compensate for the subtle loss of function resulting from CD36 deletion in the proximal small intestine 11, 12, 16. To make matters even more confusing, recent studies demonstrated that CM secretion into mesenteric lymph of Cd36 −/− mice was actually increased rather than decreased 17. Taken together, the findings suggest that intestinal CD36, while not required for lipid absorption, may play a facilitative role in the proximal intestinal absorption of long and very long chain FAs, and perhaps provide a mechanism to acutely upregulate intestinal lipid secretion.
Among the factors contributing to the ambiguity surrounding the role of CD36 in promoting intestinal CM production is the systemic role of CD36 in regulating FA uptake into adipose tissue, liver, and skeletal muscle. Cd36 −/− mice display increased fasting serum FA levels that cause accumulation of chylomicron remnants, evidenced by elevated TG levels and increased abundance of apoB48, despite normal lipase activity 3, 17. Elevations of both fasting TG and apoB48 were noted in Cd36 −/− mice by Adeli and colleagues 4, complicating interpretation of the effects of GLP-2, since the background influence of reduced hepatic CM clearance is difficult to discern from acute augmentation of intestinal CM production. Unambiguous resolution of the role of CD36 in regulating CM production will thus require examination of an intestine- or even duodenum-specific Cd36 knockout mouse. Beyond its local role in modulating enterocyte FA transport, other data suggest that CD36 participates in orosensory lipid detection 18 and in mediating the satiety response to lipid feeding through production of oleoylethanolamide 19. These findings have broad implications for intestinal lipid biology and imply that CD36 plays a potential role in dietary lipid preferences and feeding behavior.
Finally, it is worth pointing out that the loss-of-function phenotypes encountered in Cd36 −/− mice have a relevant counterpart in human CD36 deficiency 17, 20. CD36 deficient patients were identified by the absence of CD36 on monocytes or platelets and were found to have elevated serum TG levels with low levels of high density lipoprotein and elevated systolic blood pressure, collectively features of the metabolic syndrome 17. In addition, there was increased accumulation of chylomicron remnants (TG and apoB48) in these patients following an oral fat bolus and, as noted above, no distinction could be made regarding increased CM secretion versus delayed clearance 17. However, regardless of whether one or both of these mechanisms is in play, there is emerging evidence that chylomicron remnants likely play an important role in atherosclerotic cardiovascular disease 21. Against this background, studies of the regulation of intestinal CM production are timely, relevant and important. These most recent findings suggest that there is yet more to learn about the genetic regulation of intestinal lipid transport.
Acknowledgments
Grant awards HL-38180, DK-56260 and DK-52574 to NOD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–94. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqi SA, Siddiqi S, Mahan J, Peggs K, Gorelick FS, Mansbach CM., 2nd The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J Biol Chem. 2006;281:20974–82. doi: 10.1074/jbc.M601401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–7. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-like Peptide-2 Increases Intestinal Lipid Absorption and Chylomicron Production via CD36. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130:44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 8.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–47. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 9.Hoosdally SJ, Andress EJ, Wooding C, Martin CA, Linton KJ. The Human Scavenger Receptor CD36: GLYCOSYLATION STATUS AND ITS ROLE IN TRAFFICKING AND FUNCTION. J Biol Chem. 2009;284:16277–88. doi: 10.1074/jbc.M109.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem. 2008;283:13108–15. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 12.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem. 2006;281:4075–86. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 14.Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–34. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 15.Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–8. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DV, Drover VA, Knopfel M, Dhanasekaran P, Hauser H, Phillips MC. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J Lipid Res. 2009 doi: 10.1194/jlr.M900036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, Koseki M, Matsuura F, Nishida M, Kawamoto T, Ishigami M, Hori M, Shimomura I, Yamashita S. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res. 2009;50:999–1011. doi: 10.1194/jlr.P700032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V, Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–8. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita S, Hirano K, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]
- 21.Cohn JS. Are we ready for a prospective study to investigate the role of chylomicrons in cardiovascular disease? Atheroscler Suppl. 2008;9:15–8. doi: 10.1016/j.atherosclerosissup.2008.05.003. [DOI] [PubMed] [Google Scholar]