Abstract
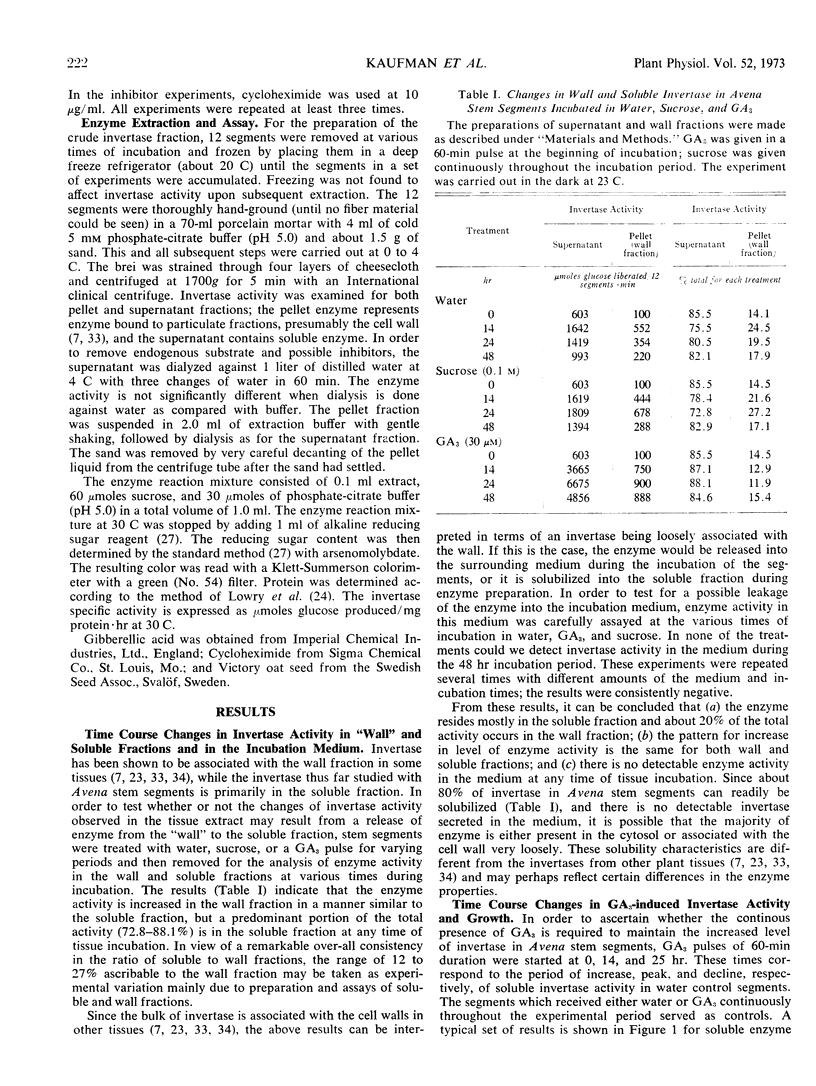
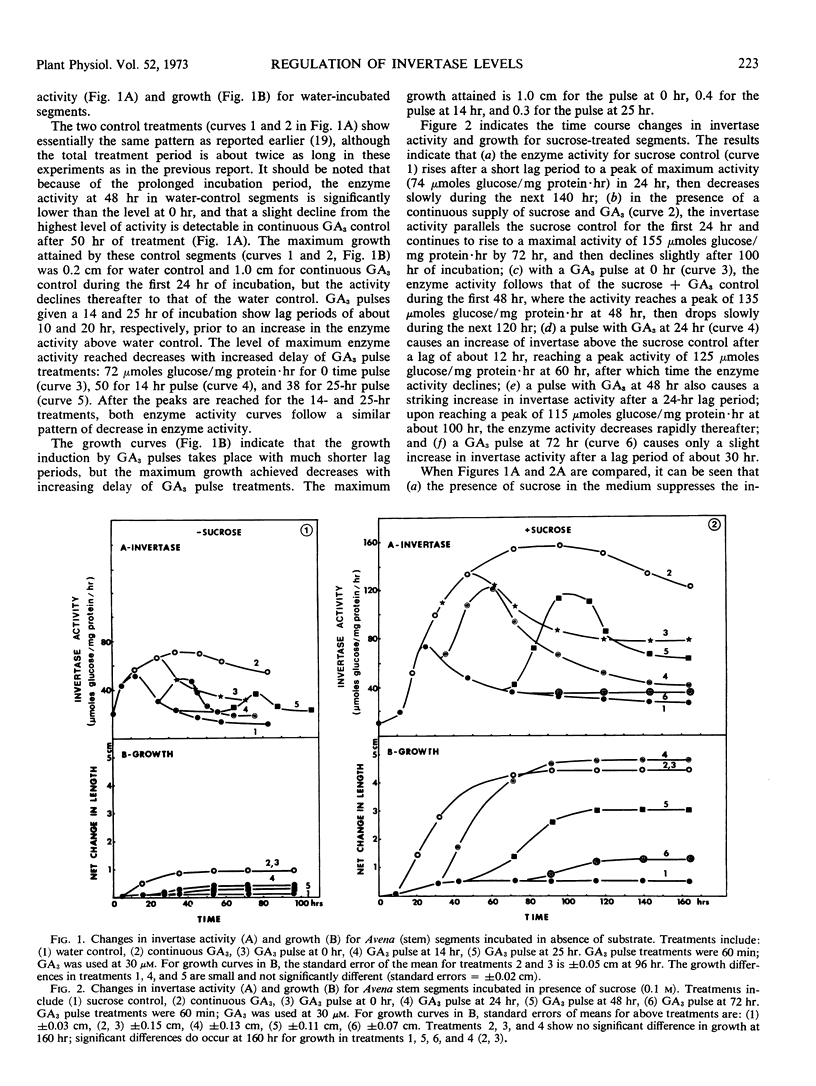
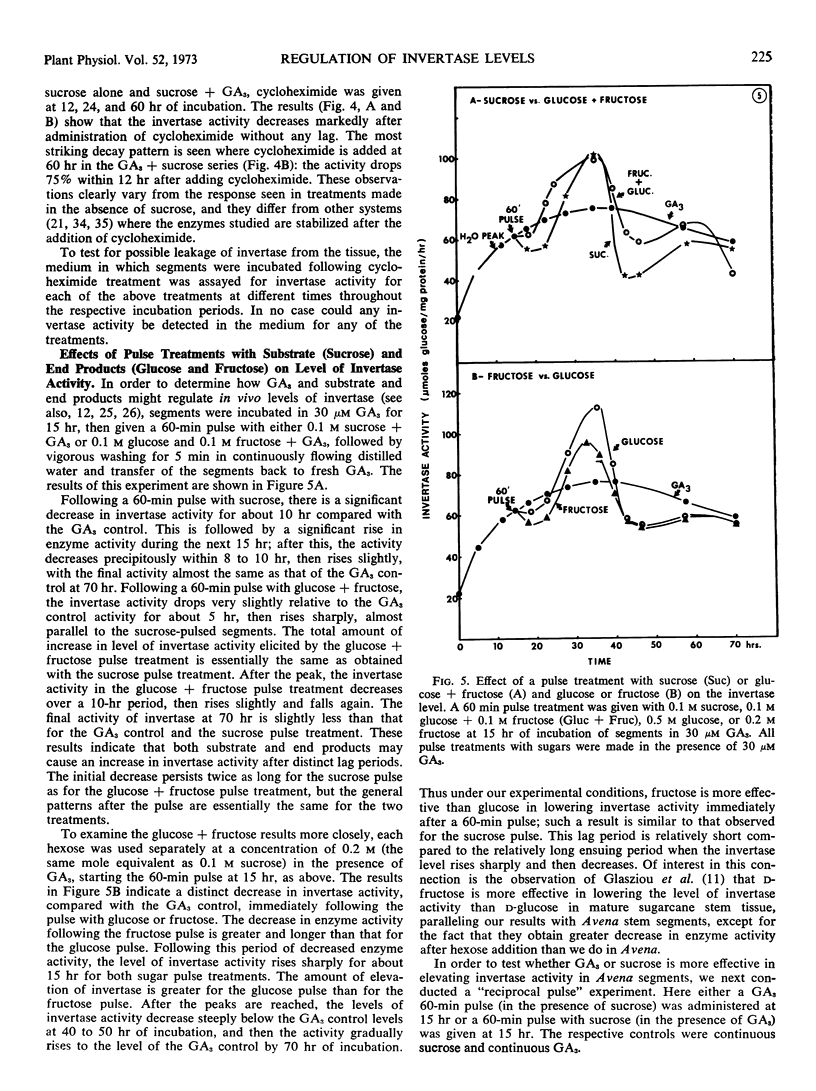
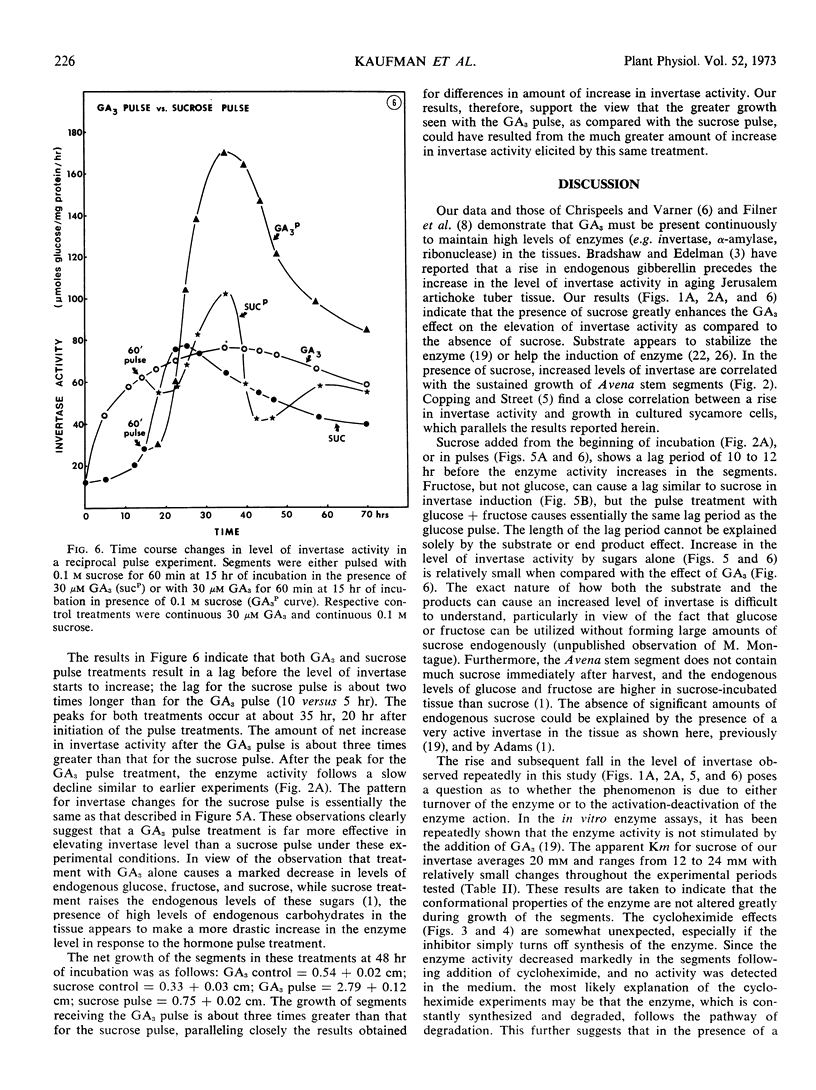
Gibberellic acid and sucrose play significant roles in the increases in invertase and growth in Avena stem segments. About 80% of invertase is readily solubilized, whereas the rest is in the cell wall fraction. The levels of both types of invertase change in a similar manner in the response to gibberellic acid and sucrose treatment. The work described here was carried out with only the soluble enzyme. In response to a treatment, the level of invertase activity typically follows a pattern of increase followed by decrease; the increase in activity is approximately correlated with the active growth phase, whereas the decrease in activity is initiated when growth of the segments slows. A continuous supply of gibberellic acid retards the decline of enzyme activity. When gibberellic acid was pulsed to the segments treated with or without sucrose, the level of invertase activity increased at least twice as high in the presence of sucrose as in its absence, but the lag period is longer with sucrose present. Cycloheximide treatments effectively abolish the gibberellic acid-promoted growth, and the level of enzyme activity drops rapidly. Decay of invertase activity in response to cycloheximide treatment occurs regardless of gibberellic acid or sucrose treatment or both, and it is generally faster when the inhibitor is administered at the peak of enzyme induction than when given at its rising phase. Pulses with sucrose, glucose, fructose, or glucose + fructose elevate the level of invertase significantly with a lag of about 5 to 10 hours. The increase in invertase activity elicited by a sucrose pulse is about one-third that caused by a gibberellic acid pulse given at a comparable time during mid-phase of enzyme induction, and the lag before the enzyme activity increases is nearly twice as long for sucrose as for gibberellic acid. Moreover, the gibberellic acid pulse results in about three times more growth than the sucrose pulse. Our studies support the view that gibberellic acid, as well as substrate (sucrose) and end products (glucose and fructose), play a significant role in regulating invertase levels in Avena stem tissue, and that such regulation provides a mechanism for increasing the level of soluble saccharides needed for gibberellic acid-promoted growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. A., Kaufman P. B., Ikuma H. Effects of gibberellic Acid and sucrose on the growth of oat (Avena) stem segments. Plant Physiol. 1973 Jun;51(6):1102–1108. doi: 10.1104/pp.51.6.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN J., HALL M. A. EFFECT OF GROWTH HORMONES ON THE DEVELOPMENT OF INVERTASE ASSOCIATED WITH CELL WALLS. Nature. 1964 Jan 18;201:296–297. doi: 10.1038/201296b0. [DOI] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Grimes W. J., Jones B. L., Albersheim P. Sucrose synthetase from Phaseolus aureus seedlings. J Biol Chem. 1970 Jan 10;245(1):188–197. [PubMed] [Google Scholar]
- Kaufman P. B., Ghosheh N., Ikuma H. Promotion of growth and invertase activity by gibberellic Acid in developing Avena internodes. Plant Physiol. 1968 Jan;43(1):29–34. doi: 10.1104/pp.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Palmer J. M. The influence of growth regulating substances on the development of enhanced metabolic rates in thin slices of beetroot storage tissue. Plant Physiol. 1966 Sep;41(7):1173–1178. doi: 10.1104/pp.41.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in Xanthium leaf disks. Photosynthetic requirement and effect of daylength. Plant Physiol. 1969 Jun;44(6):912–922. doi: 10.1104/pp.44.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]


