Abstract
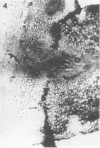
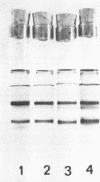
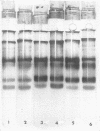
Peroxidase activity and localization in the abscission zone of bean leaves were studied histochemically and by gel electrophoresis. Deblading of bean leaves resulted in an increase in peroxidase activity in the abscission zone 2 to 4 days after deblading with highest activity just prior to separation. In debladed plants, the cell division in six to eight layers of cells preceded separation. An ethylene treatment (8 microliters per liter) induced separation of debladed petioles in approximately 24 hours and of intact plants in 36 to 48 hours. Ethylene treatment produced similar results in both debladed and intact plants. In ethylene-treated plants, whether debladed or not, enzyme localization was restricted to only two to three layers of cells with no cell division apparent prior to separation. Infrequent cell divisions were observed after treatment with 2-chloroethylphosphonic acid (1000 micrograms per liter) (Ethephon); however, other changes were similar to those observed with ethylene. Deblading and ethylene treatment resulted in changes in the six peroxidase isozymes observed in the abscission zone. Only four were observed in samples collected 2 centimeters below the abscission zone. Peroxidase bands IV and V increased significantly in debladed and ethylene-treated plants and peroxidase VI decreased only in debladed plants. The changes in peroxidase activity were invariably observed prior to separation in all treatments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Abscission: role of cellulase. Plant Physiol. 1969 Mar;44(3):447–452. doi: 10.1104/pp.44.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Holm R. E. Enhancement of RNA synthesis, protein synthesis, and abscission by ethylene. Plant Physiol. 1966 Oct;41(8):1337–1342. doi: 10.1104/pp.41.8.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971 Aug;48(2):208–212. doi: 10.1104/pp.48.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Jong D. W. An investigation of the role of plant peroxidase in cell wall development by the histochemical method. J Histochem Cytochem. 1967 Jun;15(6):335–346. doi: 10.1177/15.6.335. [DOI] [PubMed] [Google Scholar]
- Dela Fuente R. K., Leopold A. C. Senescence processes in leaf abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1496–1502. [PMC free article] [PubMed] [Google Scholar]
- Fowler J. L., Morgan P. W. The relationship of the peroxidative indoleacetic Acid oxidase system to in vivo ethylene synthesis in cotton. Plant Physiol. 1972 Apr;49(4):555–559. doi: 10.1104/pp.49.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. C., Lane H. C. COMPOSITIONAL AND PHYSIOLOGICAL CHANGES ASSOCIATED WITH THE CHEMICAL DEFOLIATION OF COTTON. Plant Physiol. 1952 Oct;27(4):754–768. doi: 10.1104/pp.27.4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H. Induction of peroxidase activity by ethylene in sweet potato. Plant Physiol. 1970 Jul;46(1):172–174. doi: 10.1104/pp.46.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leopold A. C. The mechanism of foliar abscission. Symp Soc Exp Biol. 1967;21:507–516. [PubMed] [Google Scholar]
- McCown B. H., Beck G. E., Hall T. C. Plant leaf and stem proteins. I. Extraction and electrophoretic separation of the basic, water-soluble fraction. Plant Physiol. 1968 Apr;43(4):578–582. doi: 10.1104/pp.43.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Leopold A. C. Analysis of the Auxin Control of Bean Leaf Abscission. Plant Physiol. 1963 May;38(3):262–267. doi: 10.1104/pp.38.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Uritani I., Imaseki H. De novo synthesis of peroxidase isozymes in sweet potato slices. Plant Physiol. 1971 Apr;47(4):493–498. doi: 10.1104/pp.47.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonier T., Rodriguez-Tormes F., Yoneda Y. Studies on Auxin Protectors. IV. The Effect of Manganese on Auxin Protector-I of the Japanese Morning Glory. Plant Physiol. 1968 Jan;43(1):69–72. doi: 10.1104/pp.43.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B. D. Anatomical aspects of abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1512–1544. [PMC free article] [PubMed] [Google Scholar]
- Yang S. F. Biosynthesis of ethylene. Ethylene formation from methional by horseradish peroxidase. Arch Biochem Biophys. 1967 Nov;122(2):481–487. doi: 10.1016/0003-9861(67)90222-6. [DOI] [PubMed] [Google Scholar]