Abstract
Degradation of the multifunctional amino acid proline is associated with mitochondrial oxidative respiration. The two-step oxidation of proline is catalyzed by proline oxidase and Δ1-pyrroline-5-carboxylate (P5C) dehydrogenase, which produce P5C and glutamate, respectively. In animal and plant cells, impairment of P5C dehydrogenase activity results in P5C-proline cycling when exogenous proline is supplied via the actions of proline oxidase and P5C reductase (the enzyme that converts P5C to proline). This proline is oxidized by the proline oxidase-FAD complex that delivers electrons to the electron transport chain and to O2, leading to mitochondrial reactive oxygen species (ROS) overproduction. Coupled activity of proline oxidase and P5C dehydrogenase is therefore important for maintaining ROS homeostasis. In the genome of the fungal pathogen Cryptococcus neoformans, there are two paralogs (PUT1 and PUT5) that encode proline oxidases and a single ortholog (PUT2) that encodes P5C dehydrogenase. Transcription of all three catabolic genes is inducible by the presence of proline. However, through the creation of deletion mutants, only Put5 and Put2 were found to be required for proline utilization. The put2Δ mutant also generates excessive mitochondrial superoxide when exposed to proline. Intracellular accumulation of ROS is a critical feature of cell death; consistent with this fact, the put2Δ mutant exhibits a slight, general growth defect. Furthermore, Put2 is required for optimal production of the major cryptococcal virulence factors. During murine infection, the put2Δ mutant was discovered to be avirulent; this is the first report highlighting the importance of P5C dehydrogenase in enabling pathogenesis of a microorganism.
Keywords: Cryptococcus neoformans, nitrogen, virulence, proline oxidase, P5C dehydrogenase
PROLINE is an important biological proteinogenic amino acid that is necessary for primary metabolism in organisms including animals, plants, fungi, and bacteria. This imino acid, as it is more precisely known, has multiple physiological functions. Apart from being an osmoprotectant, proline can serve as a transient storage of organic nitrogen, stabilize proteins and membranes during stressful conditions, prevent protein aggregation during its folding or refolding, and scavenge reactive oxygen species (ROS) such as superoxide anions (Rudolph and Crowe 1985; Carpenter and Crowe 1988; Ignatova and Gierasch 2006; Kaul et al. 2008). However, the exact mechanism by which proline protects against stresses remains poorly understood. Intracellular proline must be present at appropriate levels to confer stress-protective effects, and an excess of free proline has been shown to be detrimental to cell growth or protein functions in numerous organisms (Davis 2000; Morita et al. 2002; Deuschle et al. 2004; Maxwell and Nomura and Takagi 2004). The transport, anabolism, and catabolism of proline therefore need to be tightly regulated for proper cellular defense, adaptation, and growth.
Degradation of proline that takes place in eukaryotic mitochondria or prokaryotic plasma membrane results in high-energy output and may provide amino nitrogen and reducing power to post-stress recovering cells (Atkinson 1977; Peng et al. 1996; Verbruggen et al. 1996; Hare and Cress 1997). Hence, the process of proline catabolism is also beneficial, in addition to its intracellular accumulation. The four-electron oxidation steps that convert proline to glutamate, with Δ1-pyrroline-5-carboxylate (P5C) as an intermediate, occur in all living systems (Figure 1A). Oxidation of proline to P5C is first catalyzed by the O2-dependent proline oxidase (also known as proline dehydrogenase). The resulting P5C is nonenzymatically transformed into glutamate-γ-semialdehyde (GSA), which acts as a substrate for P5C dehydrogenase to generate glutamate. Glutamate is an important precursor molecule for all other cellular nitrogenous compounds.
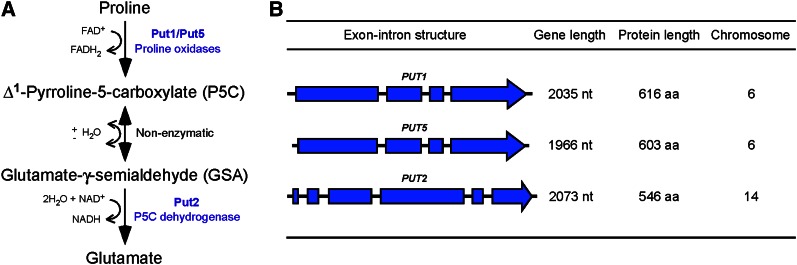
Figure 1.
The C. neoformans genome contains two paralogs that encode proline oxidases and a single ortholog that encodes P5C dehydrogenase. (A) Scheme representing the proline degradation pathway. (B) Exon–intron structures of PUT1, PUT5, and PUT2 and their chromosomal locations.
Conversely, uncoupled proline oxidase activity can also be detrimental depending on the behavior of other catabolic and biosynthesis enzymes of this metabolism. In animals and plants, when proline oxidase activation is not accompanied by P5C dehydrogenase activation, intracellular levels of the toxic intermediate P5C or GSA (thought to be more chemically reactive and causative) as well as ROS are increased and redox homeostasis is altered, ultimately leading to cell death. Excessive ROS is generated from the P5C-proline cycle that involves the constant interconversion between P5C and proline, catalyzed by activities of the cytosolic P5C reductase and the mitochondrial proline oxidase (Phang et al. 2008; Miller et al. 2009). This seemingly futile cycle controls the cellular accumulation of the proline-derived metabolite P5C/GSA and provides an excess of electron flow to the mitochondrial electron transport chain (mtETC) that is partially diverted to O2, resulting in superoxide anion formation. P5C/GSA has high reactivity with various cellular compounds and may act as a signal molecule to elicit deleterious effects in animal, plant, and yeast cells (Mezl and Knox 1976; Maxwell and Davis 2000; Deuschle et al. 2001, 2004; Donald et al. 2001; Nomura and Takagi 2004). Incomplete proline oxidation can also enhance the transference of reducing equivalents to the mitochondria altering the NADP+/NADPH ratio at the cytosol, potentially impacting redox-sensitive pathways such as the defense-associated oxidative pentose phosphate pathway (Hare and Cress 1997).
In the model ascomycete Saccharomyces cerevisiae, expression of PUT1-encoded proline oxidase and PUT2-encoded P5C dehydrogenase is activated by the pathway-specific transcription factor Put3 in the presence of proline and the absence of easily assimilated nitrogen sources such as ammonium or glutamine (Brandriss and Magasanik 1979; Axelrod et al. 1991; Huang and Brandriss 2000). Transcriptional regulation of PUT1 and PUT2 is therefore subjected to nitrogen catabolite/metabolite repression, a regulatory phenomenon that enables preferential utilization of metabolically favored nitrogen sources. Proline oxidase and P5C dehydrogenase use FAD and NAD+ as electron acceptors to generate FADH2 and NADH, respectively, delivering electrons for mitochondrial respiration. Transport of proline into yeast cells occurs mainly through the general amino acid permease Gap1 and high-affinity proline-specific permease Put4 (Vandenbol et al. 1989; Jauniaux and Grenson 1990). Activation of the nitrogen catabolite repression-sensitive GAP1 and PUT4 is induced by the globally acting GATA transcription factors Gln3 and/or Gat1 (Daugherty et al. 1993; Stanbrough and Magasanik 1996).
Knowledge gained from pioneering studies of gene regulation in model fungi has steadily laid the foundation for modern-day research into dissecting the mechanisms of virulence in pathogenic fungi. One of the leading fatal fungal infections is cryptococcal meningoencephalitis. Cryptococcus neoformans predominantly infects immunocompromised patients, while its sister species Cryptococcus gattii generally infects immunocompetent individuals (Heitman 2010). Historically, differential media were used to distinguish the different basidiomycete species, capitalizing on their unique ability to metabolize different substrates. For example, C. neoformans is unable to utilize D-proline while C. gattii is among the rare few microorganisms that can assimilate this dextrorotatory form of proline (Dufait et al. 1987; Ngamskulrungroj et al. 2012).
In the more clinically prevalent C. neoformans, regulation of proline (L-proline, unless specified otherwise) utilization is particularly interesting because a functional GATA factor (Gat1/Are1) is not required for assimilation of this traditionally nonpreferred nitrogen source, an observation that is different from the assimilation seen in the ascomycetes (Kmetzsch et al. 2011; Lee et al. 2011, 2012). Initiation of C. neoformans infection occurs via inhalation of airborne basidiospores or desiccated yeast cells, and one of the initial lines of host defense that this pathogen encounters is reactive oxygen, and nitrogen species burst from alveolar phagocytes. In addition, C. neoformans has to endure various stresses found in different body locations of the host such as nutrient limitation for successful proliferation, dissemination, and colonization. Thus, the observation of Gat1/Are1 independence in proline utilization, coupled with the supporting literature that proline catabolism is likely a key regulator of virulence, led us to investigate the role of the proline catabolic enzymes in C. neoformans pathobiology.
In this study, we examined how the C. neoformans proline catabolic genes are transcriptionally regulated and both genetically and phenotypically characterized their enzymatic functions in relation to nitrogen and carbon source utilization, balance of mitochondrial ROS formation, as well as stress-adaptation and virulence-associated mechanisms.
Materials and Methods
Strains and media
All fungal strains used in this study are listed in Supporting Information, Table S1 and were grown in YPD (1% yeast extract, 2% Bacto-peptone, 2% glucose) or YNB (0.45% yeast nitrogen base w/o amino acids and ammonium sulfate, 2% glucose, 10 mM nitrogen source) unless specified otherwise. C. neoformans biolistic transformants were selected on YPD medium supplemented with 200 µg/ml G418 (Sigma) or 100 µg/ml nourseothricin (Werner BioAgents). L-3,4-dihydroxyphenylalanine (L-DOPA) medium was prepared as described previously (Missall et al. 2005). Escherichia coli Mach-1 cells served as the host strain for transformation and propagation of all plasmids using lysogeny broth supplemented with either 100 µg/ml ampicillin (Sigma) or 50 µg/ml kanamycin (Sigma) (Sambrook et al. 1989).
Bioinformatic analyses
C. neoformans genes were identified using annotation from the H99 genome sequence from the Broad Institute (http://www.broadinstitute.org/annotation/genome/cryptococcus_neoformans/MultiHome.html). Gene annotations from the Broad Institute are designated by their nomenclature “CNAG_#####.#.” Sequence analyses were performed using BLAST and MacVector 9.5 (MacVector Inc., Cary NC) (Altschul et al. 1990). Sequence alignments were created using ClustalW v1.4 within MacVector (Thompson et al. 1994). Sequence traces generated at the Australian Genome Research Facility (Brisbane, QLD, Australia) were analyzed using Sequencher 4.7 (Gene Codes, Ann Arbor MI). Putative mitochondrial localization signals were examined using MitoProt (http://ihg.gsf.de/ihg/mitoprot.html).
Construction and complementation of C. neoformans mutant strains
All primers and plasmids used in this study are listed in Table S2 and Table S3, respectively. Gene deletion mutants were created using overlap PCR and biolistic transformation as described previously (Davidson et al. 2002). For example, to construct the put1Δ mutant strain in the H99 background, the 2035-bp PUT1 (CNAG_02049.2) coding sequence was replaced with the neomycin phosphotransferase II-encoding selectable marker NEO using a construct created by overlap PCR combining an ∼1-kb fragment upstream from the PUT1 start codon, the NEO marker, and an ∼1-kb fragment downstream from the PUT1 stop codon. Strain H99 genomic DNA and plasmid pJAF1 were used as PCR templates (Fraser et al. 2003). The construct was transformed into C. neoformans cells via particle bombardment and transformants selected on YPD plates supplemented with G418. Deletion of PUT1 was confirmed by diagnostic PCR and Southern blot (Southern 2006). To complement the put1Δ mutant, the C. neoformans PUT1 gene, including an ∼1-kb promoter and terminator, was amplified from genomic DNA using high-fidelity PCR, cloned into pCR2.1-TOPO (Invitrogen) to give pELYL1, and sequenced. The PUT1 fragment of pELYL1 was then subcloned into pCH233, creating the complementation construct pELYL5. pELYL5 was subsequently linearized and biolistically transformed into the put1Δ mutant. Stable transformants were selected on YPD supplemented with nourseothricin, and complemented strains containing a single copy of the wild-type PUT1 gene were identified by Southern blot.
Cross-species complementation
Full-length C. neoformans PUT1, PUT5, and PUT2 ORFs amplified from strain H99 complementary DNA (cDNA) were fused with ∼1-kb promoter and terminator sequences of S. cerevisiae BY4741 PUT1 or PUT2 via high-fidelity overlap PCR (Brachmann et al. 1998). These fusion genes were cloned into pCR2.1-TOPO, sequenced, and subcloned into the yeast shuttle vector YCplac111 (Gietz and Sugino 1988). Likewise, the control S. cerevisiae PUT1 and PUT2 genes, including their ∼1-kb promoter and terminator, were amplified from strain BY4741 genomic DNA, cloned into pCR2.1-TOPO, sequenced, and subcloned into YCplac111. Plasmids were transformed into S. cerevisiae put1Δ or put2Δ strains obtained from the BY4741 deletion library using the lithium acetate/heat-shock method (Gietz and Schiestl 2007). Prior to transformation, these previously auxotrophic strains had URA3, HIS3, and MET15 restored, but not LEU2, to remove background interference through supplementation of potential nitrogen sources (uracil, histidine, methionine). Transformants were first selected on YNB (2% glucose, 10 mM ammonium sulfate) lacking leucine; thereafter, LEU2 was restored in the strains. Likewise, URA3, HIS3, MET15, and LEU2 were restored in the wild-type and mutant control strains. Functional complementation of proline oxidase and P5C dehydrogenase activity was verified through growth on YNB (2% glucose, 10 mM proline) lacking leucine.
Construction and screening of library for C. neoformans proline catabolism defective mutants
An Agrobacterium-mediated mutagenesis library of C. neoformans H99 was created as described previously (Idnurm et al. 2004). The majority of transformants harbored a single copy of randomly integrated T-DNA and were mitotically stable. To identify genes involved in proline catabolism, replica spotting of the insertional library consisting of 12,000 clones was carried out on YNB supplemented with proline or ammonium (10 mM each nitrogen source). Mutants that grew on ammonium but failed to grow on proline-containing medium were selected for further analysis. The T-DNA insertion site was mapped using GenomeWalker according to the manufacturer’s instructions (Clontech). BLAST analysis of these sequences was performed to reveal which loci were affected.
Quantitative real-time PCR
C. neoformans strains were grown in YNB supplemented with 10 mM of the specified nitrogen source and shaken at 30° for 16 hr. Overnight cultures were harvested, cell pellets were frozen and lyophilized, total RNA was isolated using TRIzol reagent (Invitrogen), and cDNA was generated using the SuperscriptIII First-Strand Synthesis System (Invitrogen). Primers for PUT1 (CNAG_02049.2), PUT5 (CNAG_02048.2), and PUT2 (CNAG_05602.2) were designed to span exon–exon boundaries and tested to verify that they bind specifically to cDNA but not to genomic DNA. Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green Supermix (Applied Biosystems) and an Applied Biosystems 7900HT Fast Real Time PCR System with the following cycling conditions: denaturation at 95° for 10 min, followed by amplification and quantification in 45 cycles at 95° for 15 sec and 60° for 1 min, with melting-curve profiling at 95° for 2 min, 60° for 15 sec, and 95° for 15 sec. Dissociation analysis confirmed the amplification of a single PCR product for each primer pair and an absence of primer dimer formation. Relative gene expression was quantified using SDS software 1.3.1 (Applied Biosystems) based on the 2−ΔΔCT method (Livak and Schmittgen 2001). The housekeeping actin-encoding gene ACT1 was used as a control for normalization. One-way analysis of variance was performed using the unpaired, two-tailed t-test in GraphPad Prism Version 5.0c. P-values of <0.05 were considered statistically significant.
Oxidative and nitrosative stress assays
Starter C. neoformans cultures were prepared by growth in YPD at 30° overnight with shaking and diluted to a concentration of 1 × 107 cells/ml in water. A 50-µl drop containing 5 × 105 cells was then inoculated into YNB [proline and glutamate (10 mM each nitrogen source)] supplemented with the stressors menadione (0– 0.003 mM), tert-Butyl hydroperoxide (0–0.3 mM), sodium nitrite (0–3 mM), and S-nitrosoglutathione (GSNO) (0–9 mM) and shaken at 30° for an additional 16 hr. Growth density was measured by monitoring the turbidity with an optical density at a wavelength of 600 nm (OD600nm). Each experimental condition was repeated in triplicate. One-way analysis of variance was performed using the unpaired, two-tailed t-test in GraphPad Prism Version 5.0c. P-values of <0.05 were considered statistically significant.
Mitochondrial staining, confocal microscopy, and flow cytometry
C. neoformans strains were grown in YPD and shaken at 30° for 16 hr; thereafter, cells were washed and transferred to YNB supplemented with 10 mM proline for a further 4 hr. Cells were then stained with MitoSOX Red (Invitrogen) at a final concentration of 5 μM or with MitoTracker Red CMXRos (Invitrogen) at a final concentration of 25 nM for 15 min at 30°. After staining, cells were washed and resuspended in PBS at a density of 1 × 107 cells/ml. Twenty yeast cells of each strain were randomly chosen for fluorescence microscopy analysis. Images were collected on a Zeiss Axioplan 2 imaging confocal microscope using a 66× oil immersion Plan-Apochromat objective. Fluorochromes were excited using a 514-nm Argon/2 laser for MitoSOX and a 543-nm HeNe1 laser for MitoTracker. Differential interference contrast images were collected simultaneously with the fluorescence images using the transmitted light detector. Images were processed using AIM 4.2 LSM software. MitoSOX fluorescence was also analyzed using a BD Accuri C6 flow cytometry. The parameters for FACS were set at excitation of 488 nm, and for emission the data were collected at FSC, SSC, and 675LP (FL3) channels. Cell debris represented by distinct low forward and side scatter were gated out for analysis. All experiments were done in triplicate. One-way analysis of variance was performed using the unpaired, two-tailed t-test in GraphPad Prism Version 5.0c. P-values of <0.05 were considered statistically significant.
Growth and melanization assays
Starter C. neoformans cultures were prepared by growth in YPD at 30° overnight with shaking, diluted to OD600nm = 0.05 in water, and then further diluted 10-fold in series. Each diluted cell suspension was spotted onto YPD and YNB supplemented with the specified nitrogen source or L-DOPA medium. Results were imaged after 2–3 days incubation at 30° (nitrogen utilization assay), 30° and 37° (melanization assay), or 37° (high-temperature growth assay).
Capsule assays
C. neoformans strains were inoculated into RPMI supplemented with 10% fetal bovine serum (Gibco) and incubated at 37° for 2 days. To visualize the capsule, cells were stained with India ink (Becton Dickinson) and observed under a ZEISS Axioplan 2 epifluorescent/light microscope. Pictures were taken with an Axiocam grayscale digital camera using the AxioVision AC imaging software.
Murine inhalation model of cryptococcosis
For murine virulence assays, C. neoformans were used to infect 6-week-old female BALB/c mice by nasal inhalation (Cox et al. 2000). For every tested strain, 10 mice were each inoculated with a 50-µl drop containing 5 × 105 cells. Mice were weighed before infection and daily thereafter; animals were killed using CO2 inhalation once their body weight had decreased to 80% of the pre-infection weight or at 50 days post infection. In addition, brain, lungs, liver, and spleen were harvested from killed put2Δ-infected mice; organs were weighed, homogenized, and plated onto YPD supplemented with 50 µg/ml chloramphenicol (Sigma) for selective isolation of yeast colony forming units (CFUs). Survival was plotted against time, and P-values were calculated by plotting a Kaplan–Meier survival curve and performing a log-rank (Mantel–Cox) test using Graphpad Prism Version 5.0c. P-values of <0.05 were considered statistically significant.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes by the National Health and Medical Research Council. The protocol was approved by the Molecular Biosciences Animal Ethics Committee (AEC) of The University of Queensland (AEC approval no. SCMB/008/11/UQ/NHMRC). Infection was performed under methoxyflurane anesthesia, and all efforts were made to minimize suffering through adherence to the Guidelines to Promote the Wellbeing of Animals Used for Scientific Purposes as put forward by the National Health and Medical Research Council (Australia).
Results
Bioinformatic analyses reveal conserved and duplicated genes predicted to be involved in proline utilization in C. neoformans
While transcriptional studies relating to nitrogen metabolite repression-associated regulation of the C. neoformans proline oxidase and P5C dehydrogenase-encoding genes have been performed, these putative proline degradation pathway genes have not been properly characterized at the molecular level (Kmetzsch et al. 2011; Lee et al. 2011, 2012). Unlike the genome of the model hemiascomycete S. cerevisiae that contains a single proline oxidase-encoding gene, chromosome 6 of C. neoformans var. grubii strain H99 contains two tandemly arrayed genes that are both predicted to encode proline oxidases (Figure 1, A and B) (Lee et al. 2011). These two Cryptococcus paralogs were previously named PUT1 (CNAG_02049.2) and PUT5 (CNAG_02048.2), for proline utilization 1 and 5 (Kmetzsch et al. 2011; Lee et al. 2011). A similar scenario is observed in the model plant Arabidopsis thaliana where two proline dehydrogenase isoforms have been functionally characterized (Funck et al. 2010). In contrast to what was seen for proline oxidase, the C. neoformans H99 genome encodes only a single P5C dehydrogenase on chromosome 14 (Figure 1, A and B). This Cryptococcus ortholog was previously designated PUT2 (CNAG_05602.2) for proline utilization 2 (Lee et al. 2011). Thus, unlike the model filamentous ascomycete Aspergillus nidulans, where the proline catabolism-associated genes form a cluster within a single chromosome, the C. neoformans proline oxidase and P5C dehydrogenase genes are unlinked, like those of S. cerevisiae (Figure S1) (Arst and MacDonald 1978).
The duplication of the putative proline oxidase gene is an interesting occurrence into which we sought to gain further insights. Both genomic DNA and cDNA of these genes were PCR-amplified and sequenced, verifying the predicted exon–intron structure. This exercise demonstrated that the position of the three introns in PUT1 is precisely conserved in PUT5, providing evidence that these genes are indeed paralogs (Figure 1B). Using this sequence information, a comparison of Put1 and Put5 revealed 67% identity at the protein level, suggesting that the duplication event is not recent (Figure S2). In support of this hypothesis, analysis of the sequenced genomes of the C. neoformans var. neoformans strain JEC21 (estimated to have diverged from C. neoformans var. grubii ∼24.5 million years ago) and the C. gattii strain R265 (estimated to have diverged from C. neoformans var. grubii ∼49 million years ago) revealed that the duplication is also present in these isolates, consistent with the duplication event preceding the divergence of these species.
Transcription of all the predicted proline catabolic genes of C. neoformans is regulated by pathway-specific induction
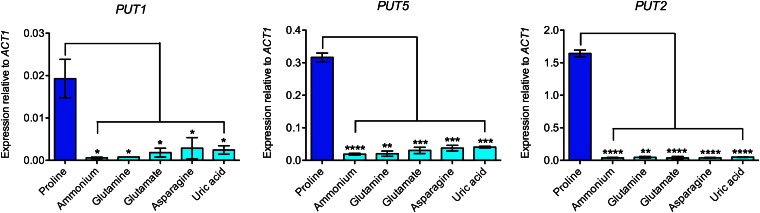
To provide support for our bioinformatics-based prediction, we analyzed the transcriptional regulation of the putative proline catabolic genes using qRT-PCR on RNA extracted from wild-type H99 grown in minimal YNB medium supplemented with a range of different sole nitrogen sources (Figure 2). While only basal levels of expression of PUT1, PUT5, and PUT2 were observed when cells were cultured in ammonium, glutamine, glutamate, asparagine, or uric acid, transcription of these genes was significantly upregulated (>10-fold increase) during growth in proline. This result indicates that the expression of PUT1, PUT5, and PUT2 is inducible only in the presence of proline, suggesting that these genes are indeed proline metabolism-related genes. Nonetheless, the result provides no indication as to whether PUT1 or PUT5 is likely to be the true functional proline oxidase ortholog required for proline assimilation or whether both PUT1 and PUT5 are enzymatically active.
Figure 2.
Transcription of all the proline catabolic enzyme-encoding genes is regulated by pathway-specific induction. cDNA from wild-type H99 grown in YNB supplemented with proline, ammonium, glutamine, glutamate, asparagine, or uric acid (10 mM each) was amplified via qRT-PCR using primers for the proline catabolic genes PUT1, PUT5, and PUT2, normalized against the control gene ACT1. Expression of PUT1, PUT5, and PUT2 was induced in the presence of proline but not in the presence of the other nitrogen sources (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Error bars represent standard errors across three biological replicates.
Cross-species complementation provides evidence that C. neoformans PUT2 is a true functional ortholog encoding P5C dehydrogenase, but gives no indication of the functionality of PUT1 and PUT5
We tested the enzymatic functions of C. neoformans PUT1, PUT5, and PUT2 by heterologous expression in S. cerevisiae strains lacking either the proline oxidase or P5C dehydrogenase-encoding gene (Figure S3). These well-characterized S. cerevisiae put1Δ and put2Δ mutant strains are unable to utilize proline as a source of nitrogen for growth (Brandriss 1983; Wang and Brandriss 1986). Transformation of the S. cerevisiae put2Δ strain with a plasmid harboring the full-length cDNA of C. neoformans PUT2 enabled the strain to catabolize proline, albeit to a poorer extent compared to a full-length S. cerevisiae PUT2. This result indicates that C. neoformans PUT2 encodes an enzyme with P5C dehydrogenase activity. However, transformation of the S. cerevisiae put1Δ strain with plasmids harboring the full-length cDNA of either C. neoformans PUT1 or PUT5 did not rescue the strains’ proline utilization defect, whereas a full-length S. cerevisiae PUT1 restored the proline oxidase function. Difficulties associated with complementing the S. cerevisiae put1Δ strain with proline oxidase cDNA of other organisms have previously been reported (Kiyosue et al. 1996). Hence, the enzymatic functions of C. neoformans PUT1 and PUT5 remain inconclusive.
Reverse genetics approach reveals that PUT5-encoded proline oxidase and PUT2-encoded P5C dehydrogenase are required for proline utilization in C. neoformans
We therefore adopted another approach to characterize the functions of the C. neoformans putative proline catabolic genes. Deletion mutants each lacking PUT1, PUT5, or PUT2 were created via homologous recombination in strain H99. In addition, a double mutant lacking both PUT1 and PUT5 was constructed as we reasoned that the single put1Δ or put5Δ mutant might still possess an active proline oxidase isoform. This is unlike the case for the put2Δ mutant where degradation of proline to glutamate, a prerequisite for recycling of nitrogen, is expected to be completely blocked. The generated proline oxidase deletion mutants (put1Δ, put5Δ, double put1Δ put5Δ) as well as the P5C dehydrogenase deletion mutant (put2Δ) were all viable; the proline oxidase deletion mutants’ growth appears indistinguishable from wild-type on rich undefined YPD medium, whereas the put2Δ mutant exhibited a mild growth defect (see Put2 is required for optimal production of the major virulence factors).
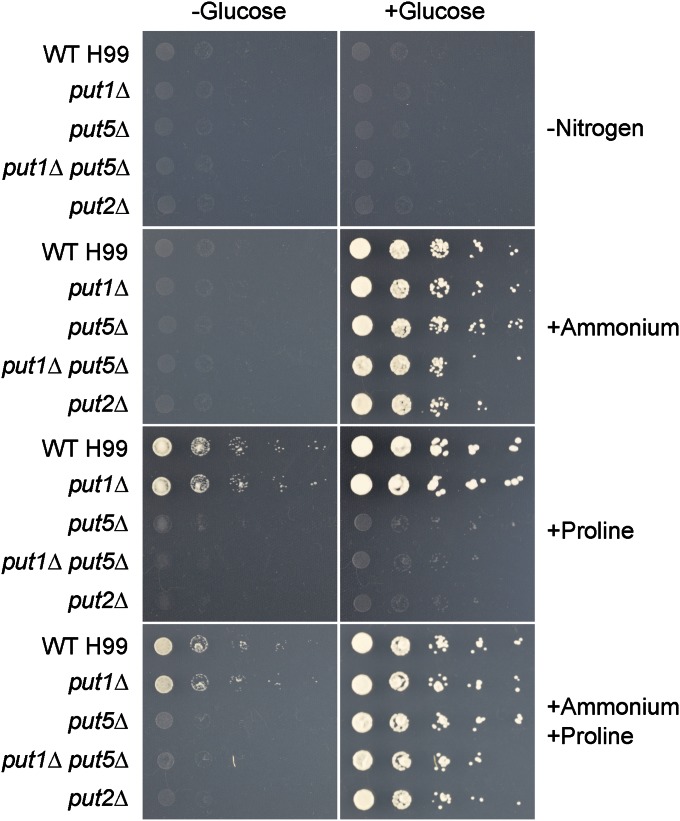
On YNB-defined medium supplemented with proline as the sole nitrogen source or sole nitrogen and carbon source (i.e., with or without the addition of glucose as a source of carbon), the put1Δ mutant displayed wild-type growth while the put5Δ, double put1Δ put5Δ, and put2Δ mutants were unable to proliferate (Figure 3). This result indicates that, unlike S. cerevisiae that can assimilate only proline as a nitrogen source, C. neoformans follows the ability of fungi such as A. nidulans in being able to utilize proline as both a nitrogen and a carbon source (Brandriss and Magasanik 1979; Sophianopoulou et al. 1993). Importantly, C. neoformans Put1 does not play a role in proline utilization under this specific condition; instead, it is Put5 that appears to be the major proline oxidase responsible for proline degradation. Nonetheless, the MitoProt computer-based prediction program clearly indicates a characteristic mitochondrial import signal at the N terminus of Put1 and Put5 with a probability of 92 and 98%, respectively, suggesting that both these proline oxidases are likely in the correct subcellular localization to support proline catabolism (Claros and Vincens 1996). Like Put5, Put2 is also essential for proline assimilation. Complementation of the put5Δ, double put1Δ put5Δ, and put2Δ mutants subsequently restored the wild-type phenotype, verifying the proline utilization functions of Put5 and Put2 (Figure S4).
Figure 3.
PUT5-encoded proline oxidase and PUT2-encoded P5C dehydrogenase are required for proline assimilation. Tenfold spot dilution assays for nitrogen or nitrogen and carbon source utilization showed that the put1Δ mutant exhibited wild-type growth on YNB supplemented with 10 mM proline (with or without 2% glucose as a carbon source). In contrast, the put5Δ, double put1Δ put5Δ, and put2Δ mutants were unable to proliferate on proline as the sole nitrogen or carbon source. The ammonium-supplemented YNB plates served as a control to demonstrate that, unlike proline, ammonium can be utilized by C. neoformans only as a nitrogen source but not as a carbon source.
Forward genetics approach involving Agrobacterium-mediated insertional mutagenesis did not reveal any additional proline catabolic enzymes in C. neoformans
P5C is thought to form a pH-dependent, nonenzymatic equilibrium with GSA. Recent findings in uric acid catabolism raise the question of whether this dogma should be accepted prima facie; in addition to urate oxidase, two new biological enzymes (HIU hydrolase and OHCU decarboxylase) were recently discovered to play a role in the conversion of uric acid to allantoin (Ramazzina et al. 2006). To provide unbiased support for the bioinformatics and phenotypic-based model of C. neoformans proline catabolism, a T-DNA insertional library of strain H99 was created using Agrobacterium tumefaciens-mediated transformation. Insertional mutagenesis is a powerful tool for identifying novel genes and their functions, and the frequency of random integration is very high using this transkingdom DNA delivery approach. In an attempt to locate as-yet-identified genetic loci involved in proline utilization, we replica-spotted 12,000 purified individual transformants onto YNB supplemented with proline or ammonium (control) as the sole nitrogen source. Only a single mutant, 65B8, failed to grow on proline but grew on ammonium. We then mapped the T-DNA insertion site of the 65B8 strain and found that its disrupted gene encodes P5C dehydrogenase. Hence, our sequence analysis did not reveal any additional proline catabolic genes beyond what has been bioinformatically predicted or phenotypically characterized.
C. neoformans put5Δ and double put1Δ put5Δ mutants display reduce tolerance against certain oxidative and nitrosative stressors
In S. cerevisiae, metabolically engineered proline-accumulated cells generated through enhancing the biosynthesis pathway and/or concomitant silencing of the catabolic pathway are more tolerant to a wide array of stresses (Takagi et al. 2000, 2005; Morita et al. 2002). For example, S. cerevisiae put1Δ mutants display increased resistance against oxidative damage (Chen et al. 2006). To promote intracellular proline accumulation in C. neoformans, the proline oxidase deletion mutants were grown in YNB supplemented with proline and glutamate as the sole nitrogen sources. Glutamate is the end product of proline catabolism and the precursor for proline biosynthesis; in S. cerevisiae, PRO3-encoded P5C reductase that converts P5C to proline is constitutively expressed (Brandriss and Falvey 1992). Concurrently, these cultures were subjected to various oxidative (menadione, tert-Butyl hydroperoxide) and nitrosative (sodium nitrite, GSNO) stresses; numerous reactive oxygen/nitrogen species (ROS/RNS)-generating chemicals were assayed as each compound acts on a different cellular target.
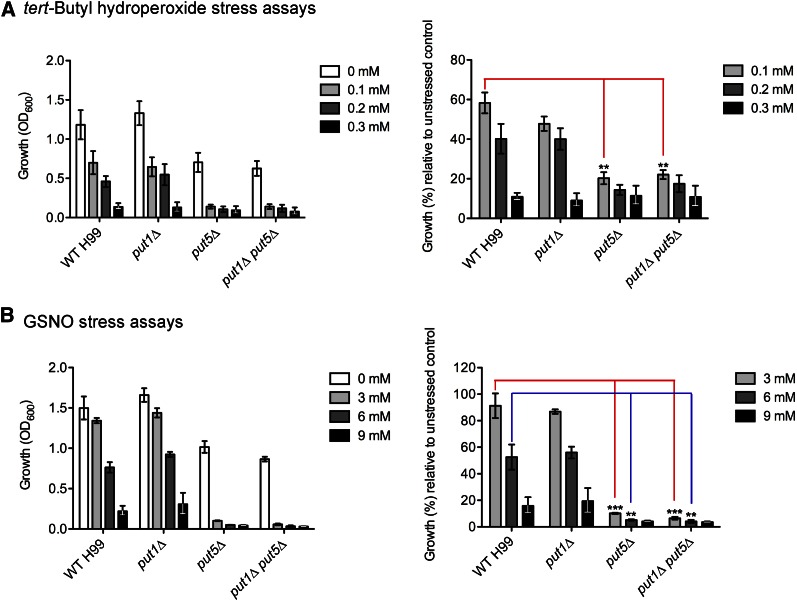
Spectrophotometric OD600nm readings revealed that tolerance against menadione and sodium nitrite is equivalent between the wild-type strain and proline oxidase deletion mutants (Figure S5). Interestingly, the put5Δ and double put1Δ put5Δ mutants exhibit decreased resistance against tert-Butyl hydroperoxide and GSNO as compared to the wild-type and put1Δ strains (Figure 4). This suggests that lack of proline oxidase activity may potentially impair the C. neoformans cellular antioxidant system in handling the stresses generated by ROS/RNS-producing reagents. Therefore, unlike the scenario in S. cerevisiae, proline accumulation in C. neoformans cells may not necessarily result in enhanced resistance against oxidative or nitrosative stresses. Instead, the predicted occurrence of unregulated proline accumulation is likely to be slightly inhibitory to general cell growth, as observed by the put5Δ and double put1Δ put5Δ mutants’ slower growth rate relative to the wild-type and put1Δ strains in control cultures (YNB plus proline and glutamate) devoid of any stressors.
Figure 4.
The put5Δ and double put1Δ put5Δ mutants are less tolerant against the oxidative stressor tert-Butyl hydroperoxide and nitrosative stressor GSNO. Wild-type H99 and proline oxidase deletion mutant strains were grown in YNB [proline and glutamate (10 mM each)] supplemented with (A) 0–0.3 mM tert-Butyl hydroperoxide or (B) 0–9 mM GSNO. The put5Δ and double put1Δ put5Δ mutants exhibited a significantly lower growth percentage relative to the unstressed control when subjected to these individual stressors as compared to the wild-type or put1Δ strains (**P < 0.01, ***P < 0.001). Growth percentage relative to unstressed control is defined by dividing each stressed strain’s growth in OD600 with the same strain’s growth in OD600 when unstressed. Error bars represent standard errors across three biological replicates.
C. neoformans put2Δ mutant accumulates excessive mitochondrial ROS in the presence of proline
In S. cerevisiae put2Δ mutants, exposure to proline has been shown to result in growth inhibition and an increase in intracellular ROS levels (Deuschle et al. 2001; Nomura and Takagi 2004). Through external supplementation of proline, we hypothesized that the predicted P5C-proline cycling would provide a constant substrate for proline oxidase-linked FAD reduction in the C. neoformans put2Δ mutant, resulting in an increased rate of electron transfer to the mtETC and to O2 and leading to elevated superoxide production. Generation and accumulation of ROS is a critical feature of programmed cell death in animals, plants, and fungi, and this ROS-induced oxidative stress may be a factor causing the general growth retardation phenotype in the C. neoformans put2Δ mutant (Jabs 1999).
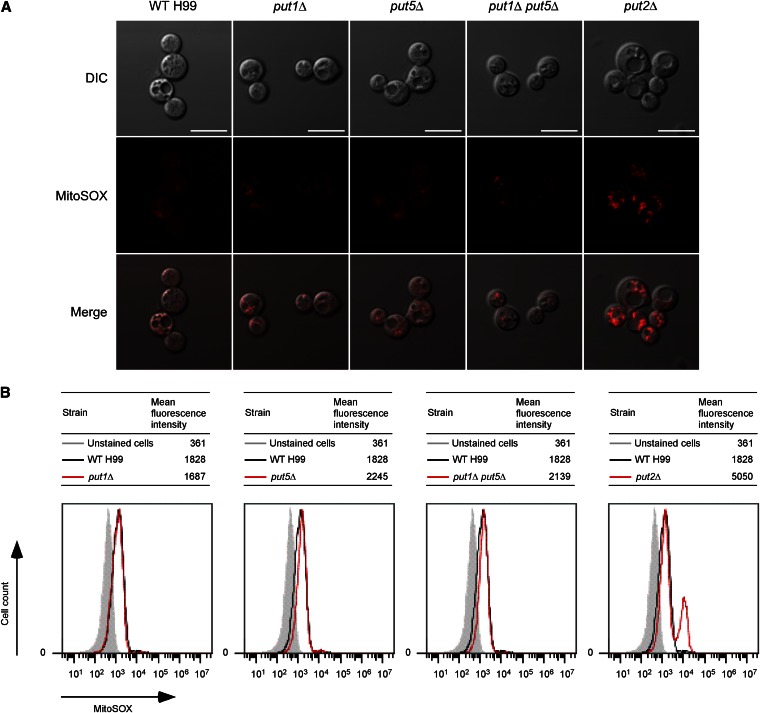
To determine whether the put2Δ mutant has abnormal intrinsic ROS levels, C. neoformans strains were first grown overnight in rich YPD medium after which they were transferred to YNB supplemented with proline for an additional 4 hr. Strains were then stained with the fluorogenic MitoSOX Red probe used to selectively detect mitochondrial superoxide ions (the predominant ROS in mitochondria) of live cells. In a subset (∼1/3 of the entire population) of put2Δ cells, the extent of ROS formation was by far higher than that generated in the wild-type or proline oxidase deletion mutant strains as seen by a more intense red fluorescence caused by oxidation of the MitoSOX reagent by superoxide (Figure 5A). Because confocal microscopic imaging only allows semiquantitative detection of superoxide generation in relatively few live cells, we also simultaneously employed flow cytometry that enables a quantitative measure of a larger population of live cells. The flow cytometry analysis also revealed two major distinct populations of put2Δ cells, with a combined 3.9 ± 0.3-fold increase of mean MitoSOX fluorescence intensity relative to the wild-type strain (WT vs. put2Δ, P = 0.0002) (Figure 5B and Figure S6). It remains to be determined why a subpopulation of put2Δ cells displayed wild-type fluorescence intensity while another subpopulation exhibited significantly enhanced fluorescence. A single population generating wild-type levels of superoxide production was restored in the complemented put2Δ + PUT2 strain, verifying the role of P5C dehydrogenase in controlling mitochondrial ROS levels in C. neoformans (Figure S6). Uncoupled coordination of proline oxidase and P5C dehydrogenase activities therefore leads to an imbalance in ROS homeostasis.
Figure 5.
The put2Δ mutant generates excessive superoxide ions in the mitochondria when exposed to proline. Wild-type H99 and proline catabolic deletion mutant strains were briefly subjected to culture in YNB supplemented with 10 mM proline and stained with MitoSOX, and the accumulation of ROS in the mitochondria was assessed by fluorescence microscopy and flow cytometry. (A) Representative confocal images showing a subpopulation of put2Δ cells generate enhanced MitoSOX fluorescence compared to the wild-type or proline oxidase deletion mutant strains. Bar, 10 μm. (B) Representative histograms of flow cytometry showing two major distinct populations of put2Δ cells with one subpopulation exhibiting wild-type MitoSOX fluorescence and another subpopulation producing enhanced fluorescence. Overall, the put2Δ mutant generated a significant increase in mean fluorescence intensity of oxidized MitoSOX relative to the wild-type or complemented put2Δ + PUT2 strains (Figure S6). Unstained C. neoformans cells were used as a negative control.
Given that mitochondria are organelles known to play an important role in apoptosis, we questioned whether this anomaly in ROS levels of the put2Δ mutant impairs mitochondrial function. Various aspects of mitochondrial dysfunction can be detected by examining for perturbation in membrane potential using MitoTracker dyes such as CMXRos. Confocal microscopy showed that all the proline catabolic deletion mutants, including the put2Δ strain, were able to retain this fluorescence dye, suggesting that mitochondrial membrane potential is unperturbed and that the respiratory system is still intact in all these mutants (Figure S7).
Put2 is required for optimal production of the major virulence factors
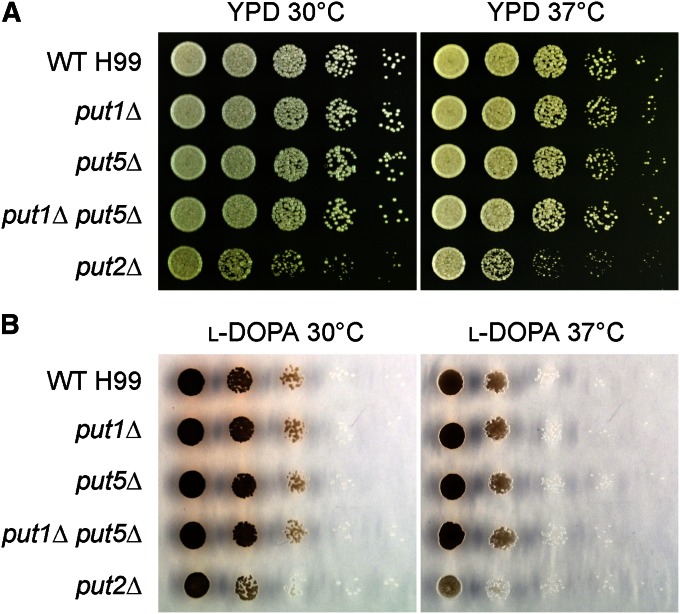
The ability of C. neoformans to successfully infect a host is largely dependent on several well-established virulence factors, namely, ability to grow at mammalian body temperature as well as synthesis of the antioxidant pigment melanin and antiphagocytic polysaccharide capsule. Therefore, we tested whether the proline catabolic enzymes influence the expression of these classical virulence traits (Figure 6 and not shown). No changes in the ability to grow at high temperature or in the production of melanin and capsule were observed in the put1Δ, put5Δ, and double put1Δ put5Δ mutants, indicating that proline oxidase is dispensable for virulence factor expression. In contrast, the mild growth defect of the put2Δ mutant that may be caused by general growth retardation or death of a subset of cells was slightly exacerbated at 37° on YPD medium. The put2Δ mutant also exhibited a subtle but reproducible decrease in melanin production at 30° on L-DOPA medium, with this melanization defect slightly more pronounced at 37°. No change in capsule size, however, was observed in the put2Δ cells when induced in serum. Virulence factor expression was restored to wild-type levels in the complemented put2Δ + PUT2 strain (Figure S8). Collectively, P5C dehydrogenase is crucial for optimal production of at least two of the well-known virulence components of C. neoformans.
Figure 6.
P5C dehydrogenase is needed for optimal growth at high temperature and for optimal production of melanin. (A) Tenfold spot dilution assays on YPD medium demonstrated that the put2Δ mutant exhibited a slightly slower growth rate compared to wild type at 30°, and this growth defect was exacerbated at 37°. (B) Tenfold spot dilution assays on L-DOPA medium showed that the put2Δ mutant produced slightly less melanin than wild type at 30° and that this melanization defect was exacerbated at 37°.
The put2Δ mutant is severely compromised for virulence in a murine inhalation model of cryptococcosis
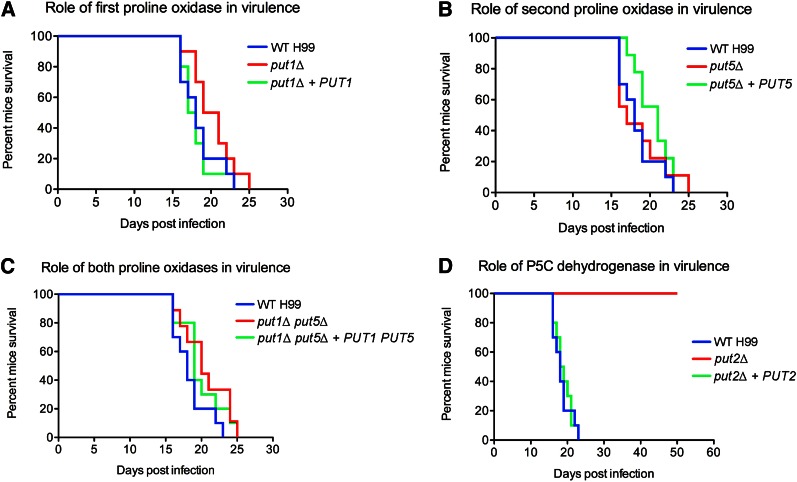
A recent study in S. cerevisiae has demonstrated that P5C directly inhibits the mitochondrial respiration, reiterating our hypothesis that proline catabolism is likely to be important for C. neoformans virulence (Nishimura et al. 2012). Given that our overarching interest in C. neoformans lies in better understanding its pathogenicity in a mammalian host, we investigated the role of the proline catabolic enzymes during infection in vivo (Figure 7). Mice intranasally infected with the put1Δ, put5Δ, and double put1Δ put5Δ mutants succumbed to infection at approximately the same rate as mice infected with the wild-type strain (killing occurred between 16 and 23 days post infection; median survival of mice = 18.0 days), indicating that proline oxidase is not required for pathogenesis. In stark contrast, the put2Δ mutant was unable to kill any mice even after 50 days, indicating that P5C dehydrogenase is critical for pathogenesis (WT vs. put2Δ, P < 0.0001). Infection of mice with the complemented put2Δ + PUT2 strain restored wild-type virulence (killing occurred between 16 and 23 days post infection; median survival of mice = 18.5 days), verifying the role of P5C dehydrogenase in pathogenesis.
Figure 7.
The put2Δ mutant is avirulent during infection of a murine host. Ten mice were each intranasally infected with either 5 × 105 cells of (A) wild-type H99, put1Δ, put1Δ +PUT1, (B) put5Δ, put5Δ +PUT5, (C) double put1Δ put5Δ, put1Δ put5Δ + PUT1 PUT5, and (D) put2Δ or put2Δ +PUT2, and survival was monitored daily. Mice infected with the put1Δ, put1Δ +PUT1, put5Δ, put5Δ +PUT5, double put1Δ put5Δ, put1Δ put5Δ + PUT1 PUT5, and put2Δ +PUT2 strains progressed to morbidity as quickly as mice infected with the wild-type strain. In contrast, mice infected with the put2Δ strain remained healthy throughout the experiment.
To more precisely characterize the effect of the loss of P5C dehydrogenase on C. neoformans survival and dissemination inside the host body, we examined the fungal burden of various organs at the endpoint of the experiment. Despite showing no sign of disease at 50 days post infection, the put2Δ-infected mice had a low number of CFUs in 9 of 10 tested lung samples and an even lower number of CFUs in 3 of 10 tested brain samples when selected on YPD supplemented with chloramphenicol. These CFUs were confirmed to express resistance to G418 and were unable to utilize proline as a nitrogen source, consistent with C. neoformans put2::NEO cells being isolated from the mice. No cells were detected in the liver or spleen of each of these 10 mice. Thus, the majority of the killed put2Δ-inoculated mice did not clear the cryptococcal infection; dormant-lying put2Δ cells were primarily located in the lungs, the initial site of infection. Nevertheless, compared to the robust proliferation and widespread dissemination characteristic of a typical wild-type H99 infection (Ormerod et al. 2013), the put2Δ mutant is attenuated in these virulence capacities.
Discussion
C. neoformans has emerged as one of the most medically important pathogens of the immunocompromised population (Heitman 2010). To establish infection in a host, C. neoformans needs to sense and adapt to low-nitrogen and carbon concentrations in the human lungs, brain, and other anatomical sites. The multifunctional amino acid, proline, can act as a nitrogen or carbon source for C. neoformans and is a potential source of nutrients during infection. For this to occur, proline needs to be degraded into the more usable glutamate that serves as a nitrogen donor for all other cellular nitrogen-containing compounds (for example, amino acid biosynthesis), or it can be converted to α-ketoglutarate by NAD-dependent glutamate dehydrogenase that feeds into the Krebs/TCA cycle for energy generation (Cooper 1982; Magasanik 1992).
The proline oxidase gene duplication that occurred millions of years ago has been retained in both C. neoformans and C. gattii. From an evolutionary perspective, this implies that the selective pressure that Cryptococcus faces in the environment or during infection may have resulted in the retention of both paralogs. Such an advantage usually increases the overall fitness of an organism and has previously been described in the model plant A. thaliana where two functional proline dehydrogenases with nonredundant but partially overlapping functions exist; ProDH1 is the main proline utilization enzyme while ProDH2 is believed to play a role in proline homeostasis under salt stress (Funck et al. 2010). In C. neoformans, Put5 is the major proline oxidase responsible for proline assimilation as well as for protection against certain oxidative and nitrosative stresses. Sensitivity of microorganisms to the toxic activity of oxidants is of particular importance, as one of the primary mechanisms of host defense against microbial infection is oxidative burst. Regardless of the abated nutrient acquisition and stress-sensitive phenotypes of the put5Δ mutant in vitro, Put5 is dispensable for virulence in vivo. This suggests that, in the absence of proline catabolism, other nitrogen and carbon sources can still be readily acquired by C. neoformans within the host and that the activity of other existing stress response pathways of C. neoformans is sufficient to confer protection against oxidative and nitrosative insults by host immune cells.
The role of the other proline oxidase paralog, PUT1, remains ambiguous, and our attempted cross-species complementation experiment failed to provide any insights into its enzymatic functionality. The failure of both the C. neoformans PUT1 and PUT5 cDNA to complement the S. cerevisiae put1Δ strain could be due to multiple factors, including codon usage bias or divergence of the C. neoformans proline oxidases’ mitochondrial targeting signal peptides, resulting in incorrect subcellular localization of the expressed proteins. While we created fusion genes to engineer the S. cerevisiae PUT1 promotor and terminator sequences to flank the C. neoformans PUT1 and PUT5 ORFs, the predicted mitochondrial transit peptide of S. cerevisiae Put1 was not incorporated into the N terminus of C. neoformans Put1 or Put5 as the exchange of sequences within an ORF may potentially interfere with the functionality test. Therefore, it remains open to speculation why the native C. neoformans proline oxidase genes do not enable the expression of sufficient amounts of active and/or correctly targeted protein in S. cerevisiae, whereas the C. neoformans P5C dehydrogenase gene produced a functional protein with its native mitochondrial transit peptide.
C. neoformans PUT2-encoded P5C dehydrogenase is the second enzyme of the proline degradation pathway that is required for proline utilization as well as for control of mitochondrial ROS production. All aerobic organisms generate ROS as metabolic by-products primarily through aerobic respiration; ROS can damage DNA, proteins, and lipids, causing cytotoxicity. Multiple lines of evidence suggest that ROS can also regulate apoptosis, a morphologically distinct form of programmed cell death. In fact, a growing list of fungi have been demonstrated to exhibit stress-induced cell death such as in the model ascomycetes S. cerevisiae and A. nidulans, as well as in the fungal pathogens Candida albicans, Colletotrichum trifolii, and C. neoformans (Madeo et al. 1997; Cheng et al. 2003; Phillips et al. 2003; Chen and Dickman 2005; Ikeda and Sawamura 2008; Semighini et al. 2011).
In animals, plants, and probably in the fungus C. neoformans (as we have suggestive evidence here), impairment of P5C dehydrogenase activity that causes intense P5C-proline cycling or proline oxidase overexpression leads to ROS accumulation and induction of apoptosis in cells (Maxwell and Davis 2000; Donald et al. 2001; Yoon et al. 2004; Phang et al. 2008; Miller et al. 2009). In fact, the proline oxidase gene is a target of the pro-apoptotic and tumor suppressor protein p53 in humans (Polyak et al. 1997). While definitive proof underlying the mechanism of ROS accumulation is lacking for most proline oxidases, mounting evidence points to the ability of the enzyme to load electrons into the mtETC or directly into O2, causing elevated production of superoxide ions. P5C dehydrogenase therefore plays a vital role in eukaryotes by irreversibly removing P5C from potential P5C-proline cycling, resulting in an overall reduction of ROS production from electron runoff.
Interestingly, certain prokaryotes have evolved strategies to specifically avoid P5C/GSA accumulation by coupling proline oxidase and P5C dehydrogenase activities. For example, in bacterial species such as Bradyrhizobium japonicum and Helicobacter pylori, the proline catabolic enzymatic activities are fused into a unique bifunctional enzyme to channel the reaction intermediate and thus prevent the release of P5C/GSA (Krishnan and Becker 2006). In plants lacking P5C dehydrogenase, the inability to oxidize P5C to glutamate and, in turn, to α-ketoglutarate may also cause a reduction in mitochondrial NADPH production, which can affect the regeneration of reduced glutathione by NADPH-dependent glutathione reductase and slow down the ascorbate/glutathione cycle, leading to enhanced mitochondrial ROS levels (Moller 2001). In C. neoformans, P5C dehydrogenase is additionally required for proper expression of the major virulence factors. Accordingly, yeast mutants lacking P5C dehydrogenase are profoundly attenuated in virulence during a murine inhalation model of cryptococcosis. To our knowledge, this is the first report recognizing P5C dehydrogenase as an important mediator that enables pathogenesis of a microorganism.
In conclusion, proline oxidases have been shown to potentiate ROS-mediated cell death in various kingdoms of life, and this paradigm likely extends to the basidiomycete pathogen C. neoformans. The role of P5C dehydrogenase in suppressing proline oxidase–FAD complex activity that, in turn, controls apoptosis might therefore be an underappreciated ancient function of the enzyme that has been conserved throughout evolution.
Supplementary Material
Acknowledgments
We acknowledge a University of Queensland International Research Tuition Award and a University of Queensland Research Scholarship awarded to I.R.L. and the National Health and Medical Research Council CDA 569673 Grant awarded to J.A.F.
Footnotes
Communicating editor: J. Heitman
Literature Cited
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, MacDonald D. W., 1978. Reduced expression of a distal gene of the prn gene cluster in deletion mutants of Aspergillus nidulans: genetic evidence for a dicistronic messenger in an eukaryote. Mol. Gen. Genet. 163: 17–22. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E., 1977. Cellular Energy Metabolism and Its Regulation. Academic Press, New York. [Google Scholar]
- Axelrod J. D., Majors J., Brandriss M. C., 1991. Proline-independent binding of PUT3 transcriptional activator protein detected by footprinting in vivo. Mol. Cell. Biol. 11: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., et al. , 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Brandriss M. C., 1983. Proline utilization in Saccharomyces cerevisiae: analysis of the cloned PUT2 gene. Mol. Cell. Biol. 3: 1846–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. C., Falvey D. A., 1992. Proline biosynthesis in Saccharomyces cerevisiae: analysis of the PRO3 gene, which encodes delta 1-pyrroline-5-carboxylate reductase. J. Bacteriol. 174: 3782–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. C., Magasanik B., 1979. Genetics and physiology of proline utilization in Saccharomyces cerevisiae: mutation causing constitutive enzyme expression. J. Bacteriol. 140: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter J. F., Crowe J. H., 1988. Modes of stabilization of a protein by organic solutes during desiccation. Cryobiology 25: 459–470. [Google Scholar]
- Chen C., Dickman M. B., 2005. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 102: 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Wanduragala S., Becker D. F., Dickman M. B., 2006. Tomato QM-like protein protects Saccharomyces cerevisiae cells against oxidative stress by regulating intracellular proline levels. Appl. Environ. Microbiol. 72: 4001–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Park T. S., Chio L. C., Fischl A. S., Ye X. S., 2003. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol. Cell. Biol. 23: 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros M. G., Vincens P., 1996. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 241: 779–786. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., 1982. Nitrogen metabolism in Saccharomyces cerevisiae, pp. 39–99 in The Molecular Biology of the Yeast Saccharomyces, edited by Strathern J. N., Jones E. W., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Cox G. M., Mukherjee J., Cole G. T., Casadevall A., Perfect J. R., 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty J. R., Rai R., el Berry H. M., Cooper T. G., 1993. Regulatory circuit for responses of nitrogen catabolic gene expression to the GLN3 and DAL80 proteins and nitrogen catabolite repression in Saccharomyces cerevisiae. J. Bacteriol. 175: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. C., Blankenship J. R., Kraus P. R., de Jesus Berrios M., Hull C. M., et al. , 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148: 2607–2615. [DOI] [PubMed] [Google Scholar]
- Deuschle K., Funck D., Hellmann H., Daschner K., Binder S., et al. , 2001. A nuclear gene encoding mitochondrial Delta-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 27: 345–356. [DOI] [PubMed] [Google Scholar]
- Deuschle K., Funck D., Forlani G., Stransky H., Biehl A., et al. , 2004. The role of [Delta]1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 16: 3413–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald S. P., Sun X. Y., Hu C. A., Yu J., Mei J. M., et al. , 2001. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 61: 1810–1815. [PubMed] [Google Scholar]
- Dufait R., Velho R., De Vroey C., 1987. Rapid identification of the two varieties of Cryptococcus neoformans by D-proline assimilation. Mykosen 10: 483. [PubMed] [Google Scholar]
- Fraser J. A., Subaran R. L., Nichols C. B., Heitman J., 2003. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck D., Eckard S., Muller G., 2010. Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis. BMC Plant Biol. 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H., 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2: 31–34. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A., 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534. [DOI] [PubMed] [Google Scholar]
- Hare P. D., Cress W. A., 1997. Metabolic implications of stess-induced proline accumulation in plants. Plant Growth Regul. 21: 79–102. [Google Scholar]
- Heitman J., 2010. Cryptococcus: From Human Pathogen to Model Yeast. ASM Press, Washington, DC. [Google Scholar]
- Huang H. L., Brandriss M. C., 2000. The regulator of the yeast proline utilization pathway is differentially phosphorylated in response to the quality of the nitrogen source. Mol. Cell. Biol. 20: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A., Reedy J. L., Nussbaum J. C., Heitman J., 2004. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryot. Cell 3: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova Z., Gierasch L. M., 2006. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. USA 103: 13357–13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R., Sawamura K., 2008. Bacterial and H2O2 stress-induced apoptosis-like events in Cryptococcus neoformans. Res. Microbiol. 159: 628–634. [DOI] [PubMed] [Google Scholar]
- Jabs T., 1999. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 57: 231–245. [DOI] [PubMed] [Google Scholar]
- Jauniaux J. C., Grenson M., 1990. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur. J. Biochem. 190: 39–44. [DOI] [PubMed] [Google Scholar]
- Kaul S., Sharma S. S., Mehta I. K., 2008. Free radical scavenging potential of L-proline: evidence from in vitro assays. Amino Acids 34: 315–320. [DOI] [PubMed] [Google Scholar]
- Kiyosue T., Yoshiba Y., Yamaguchi-Shinozaki K., Shinozaki K., 1996. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8: 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L., Staats C. C., Simon E., Fonseca F. L., Oliveira D. L., et al. , 2011. The GATA-type transcriptional activator Gat1 regulates nitrogen uptake and metabolism in the human pathogen Cryptococcus neoformans. Fungal Genet. Biol. 48: 192–199. [DOI] [PubMed] [Google Scholar]
- Krishnan N., Becker D. F., 2006. Oxygen reactivity of PutA from Helicobacter species and proline-linked oxidative stress. J. Bacteriol. 188: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. R., Chow E. W., Morrow C. A., Djordjevic J. T., Fraser J. A., 2011. Nitrogen metabolite repression of metabolism and virulence in the human fungal pathogen Cryptococcus neoformans. Genetics 188: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. R., Lim J. W., Ormerod K. L., Morrow C. A., Fraser J. A., 2012. Characterization of an Nmr homolog that modulates GATA factor-mediated nitrogen metabolite repression in Cryptococcus neoformans. PLoS ONE 7: e32585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Madeo F., Frohlich E., Frohlich K. U., 1997. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139: 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., 1992. Regulation of nitrogen utilization, pp. 283–317 in The Molecular and Cellular Biology of the Yeast Saccharomyces: Gene Expression, edited by Jones E. W., Pringle J. R., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Maxwell S. A., Davis G. E., 2000. Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proc. Natl. Acad. Sci. USA 97: 13009–13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezl V. A., Knox W. E., 1976. Properties and analysis of a stable derivative of pyrroline-5-carboxylic acid for use in metabolic studies. Anal. Biochem. 74: 430–440. [DOI] [PubMed] [Google Scholar]
- Miller G., Honig A., Stein H., Suzuki N., Mittler R., et al. , 2009. Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J. Biol. Chem. 284: 26482–26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missall T. A., Moran J. M., Corbett J. A., Lodge J. K., 2005. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot. Cell 4: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller I. M., 2001. PLANT MITOCHONDRIA AND OXIDATIVE STRESS: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 561–591. [DOI] [PubMed] [Google Scholar]
- Morita Y., Nakamori S., Takagi H., 2002. Effect of proline and arginine metabolism on freezing stress of Saccharomyces cerevisiae. J. Biosci. Bioeng. 94: 390–394. [DOI] [PubMed] [Google Scholar]
- Ngamskulrungroj P., Chang Y., Roh J., Kwon-Chung K. J., 2012. Differences in nitrogen metabolism between Cryptococcus neoformans and C. gattii, the two etiologic agents of Cryptococcosis. PLoS ONE 7: e34258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A., Nasuno R., Takagi H., 2012. The proline metabolism intermediate Delta1-pyrroline-5-carboxylate directly inhibits the mitochondrial respiration in budding yeast. FEBS Lett. 586: 2411–2416. [DOI] [PubMed] [Google Scholar]
- Nomura M., Takagi H., 2004. Role of the yeast acetyltransferase Mpr1 in oxidative stress: regulation of oxygen reactive species caused by a toxic proline catabolism intermediate. Proc. Natl. Acad. Sci. USA 101: 12616–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod K. L., Morrow C. A, Chow E. W., Lee I. R., Arras S. D., et al. , 2013. Comparative genomics of serial isolates of Cryptococcus neoformans reveals gene associated with carbon utilization and virulence. G3:Genes, Genomes, Genetics 3: 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Lu Q., Verma D. P., 1996. Reciprocal regulation of delta 1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol. Gen. Genet. 253: 334–341. [DOI] [PubMed] [Google Scholar]
- Phang J. M., Donald S. P., Pandhare J., Liu Y., 2008. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 35: 681–690. [DOI] [PubMed] [Google Scholar]
- Phillips A. J., Sudbery I., Ramsdale M., 2003. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. USA 100: 14327–14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Xia Y., Zweier J. L., Kinzler K. W., Vogelstein B., 1997. A model for p53-induced apoptosis. Nature 389: 300–305. [DOI] [PubMed] [Google Scholar]
- Ramazzina I., Folli C., Secchi A., Berni R., Percudani R., 2006. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat. Chem. Biol. 2: 144–148. [DOI] [PubMed] [Google Scholar]
- Rudolph A. S., Crowe J. H., 1985. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22: 367–377. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T., 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Semighini C. P., Averette A. F., Perfect J. R., Heitman J., 2011. Deletion of Cryptococcus neoformans AIF ortholog promotes chromosome aneuploidy and fluconazole-resistance in a metacaspase-independent manner. PLoS Pathog. 7: e1002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophianopoulou V., Suarez T., Diallinas G., Scazzocchio C., 1993. Operator derepressed mutations in the proline utilisation gene cluster of Aspergillus nidulans. Mol. Gen. Genet. 236: 209–213. [DOI] [PubMed] [Google Scholar]
- Southern E., 2006. Southern blotting. Nat. Protoc. 1: 518–525. [DOI] [PubMed] [Google Scholar]
- Stanbrough M., Magasanik B., 1996. Two transcription factors, Gln3p and Nil1p, use the same GATAAG sites to activate the expression of GAP1 of Saccharomyces cerevisiae. J. Bacteriol. 178: 2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H., Sakai K., Morida K., Nakamori S., 2000. Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 184: 103–108. [DOI] [PubMed] [Google Scholar]
- Takagi H., Takaoka M., Kawaguchi A., Kubo Y., 2005. Effect of L-proline on sake brewing and ethanol stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 71: 8656–8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J., 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbol M., Jauniaux J. C., Grenson M., 1989. Nucleotide sequence of the Saccharomyces cerevisiae PUT4 proline-permease-encoding gene: similarities between CAN1, HIP1 and PUT4 permeases. Gene 83: 153–159. [DOI] [PubMed] [Google Scholar]
- Verbruggen N., Hua X. J., May M., Van Montagu M., 1996. Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proc. Natl. Acad. Sci. USA 93: 8787–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Brandriss M. C., 1986. Proline utilization in Saccharomyces cerevisiae: analysis of the cloned PUT1 gene. Mol. Cell. Biol. 6: 2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. A., Nakamura Y., Arakawa H., 2004. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J. Hum. Genet. 49: 134–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.