Abstract
Background
We recently reported that Japanese had higher liver fat at a lower level of BMI compared with non-Hispanic whites (NHW).
Objective
We hypothesize that ethnic difference in fat storage capacity contributes to this ethnic difference in liver fat.
Design
To examine this, we assessed liver fat among 244 Japanese-American aged 40-49, using regional computed-tomography images, along with metabolic variables.
Results
Despite the similar BMI between Japanese-Americans and NHW men, Japanese-Americans had more liver fat (liver to spleen attenuation ratio: 1.03 ± 0.22 for Japanese-Americans, and 1.07 ± 0.15 for NHW men; p<0.05) and tended to have a greater disposition for fatty liver with an increase in BMI than NHW, indicating a clear difference between the two groups. In addition, liver fat is less in Japanese-Americans compared with Japanese men (1.03 ± 0.22 vs. 1.01 ± 0.16; p<0.05), despite of a much higher BMI. These ethnic differences support the hypothesis that higher fat storage capacity indeed seems to be associated with less liver fat. In all the groups, liver fat content strongly correlated with triglycerides, homeostasis model assessment-insulin resistance, and C-reactive protein (CRP). Nevertheless, these metabolic variables were worse in Japanese-Americans, despite of less liver fat, compared with Japanese. Moreover, CRP levels were least among Japanese with highest liver fat, and highest among NHW men with least liver fat, despite of a strong positive association between CRP and fatty liver within each population.
Conclusions
Fat content in the liver is intermediate for Japanese-Americans compared with Japanese and NHW men, which supports the hypothesis of less fat storage capacity among Japanese, closely linked to ethnic difference in predisposition to fatty liver.
Keywords: Ethnicity, Fatty liver, Genetic, Environmental, CRP
INTRODUCTION
Liver steatosis or fatty liver, an accumulation of excess fat in the liver, has been increasingly recognized to be closely associated with dyslipidemia and insulin resistance (1-4). A recent report showed that fatty liver rather than visceral adiposity, another well-established body composition related to metabolic risks (5-8), is closely linked with metabolic complication of obesity (9). In addition, its causal relation to excess energy influx into the body is possibly explained by selective insulin sensitivity in the liver (10).
Previously, we have reported that the Japanese in Japan had much higher prevalence of fatty liver as well as visceral adiposity compared with non-Hispanic whites in the Unites States (NHW), despite of a much lower BMI in the Japanese in Japan(11, 12). This could be a reason for similar metabolic profiles such as hypertension and dyslipidemia between the two populations.
Recently, Browning et al. reported that a very distinct ethnic difference exists in liver fat among African-Americans, Hispanic, and Caucasian, irrespective of metabolic variables such as insulin sensitivity (13). They also reported that the association between liver fat and visceral adiposity are strong and similar across different ethnic populations, whereas the association of liver fat with total adiposity was relatively weak and different by ethnicity. Namely, African-Americans, who have highest amount of subcutaneous adiposity have least liver fat as well as least visceral adiposity, and liver fat was least increased with an increasing total adiposity among the three ethnic populations. This finding together with ours strongly supports the idea that ethnic difference in liver fat is closely linked with that in fat storage capacity. In other words, a lower capacity of body fat stores within subcutaneous adiposity in response to an increased energy influx causes accumulation in non-adipose tissue such as liver, which is known as the concept of ectopic fat storage (14).
To examine this hypothesis, we newly examined the metabolic variables and body composition in Japanese Americans in Hawaii, who are a third or fourth generation of Japanese Americans without ethnic admixture, and compared the data with those in the Japanese in Japan and NHW. We would assume that liver fat content in Japanese-Americans is less than that in Japanese, despite of higher BMI and an apparent positive association between obesity and fatty liver, if our hypothesis is true.
METHODS
Subjects
The research design and methods have been described previously in detail (15). Briefly, we examined population-based samples of 867 men aged 40-49 without clinical cardiovascular disease, type 1 diabetes or other severe conditions from 2002 to 2006: 303 Japanese-Americans in Honolulu, HI, U.S., 313 Japanese in Kusatsu City, Shiga, Japan and 310 NHW living in Allegheny County, PA, U.S. CT images were available for all 313 Japanese, but only for 244 Japanese-Americans and 288 NHW subjects. However, the technical inability to acquire CT images such as extreme obesity or morbidity may not be the reason, since the comparisons for the image-available group vs. the entire population, among either Japanese-Americans or NHW men, shows no difference in BMI, age, or metabolic variables(16). The study was approved by the Institutional Review Boards of Hawaii University, Honolulu, U.S., Shiga University Medical Science, Otsu, Japan and the University of Pittsburgh, Pittsburgh, U.S.
Metabolic Risk Factors
Weight and height were measured on calibrated scales. Total adiposity was estimated using BMI, which is calculated as weight (kg) / height (m)2. Waist girth was measured at the level of the umbilicus while the participant was standing erect.
Venipuncture was performed early in the clinic visit after a 12-hour fast. Serum samples were analyzed at the Heinz Laboratory, Department of Epidemiology, University of Pittsburgh as described previously (15). Briefly, lipid levels were determined with the standardized methods of the Centers for Disease Control and Prevention, including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Fasting serum glucose was determined by an enzymatic procedure, and fasting insulin by radioimmunoassay (Linco Research Inc., St. Charles, MO). Homeostasis model assessment (HOMA-IR) was used to estimate insulin sensitivity: HOMA-IR = [Glu] (mg/dl) * [Insulin] (μU/ml) / 405 (17). High-sensitivity C-reactive protein (CRP) was determined by a colorimetric competitive ELISA at the Laboratory for Clinical Biochemistry Research, University of Vermont.
A self-administered questionnaire was used to obtain current medication lists, habitual alcohol drinking (Yes=1, No=0), and demographic information such as dietary habits. Alcohol drinking was assessed as whether the participant drank beer, wine, liquor, sake (Japanese rice wine), or other alcoholic beverages with quantity and frequency recorded. Ethanol consumption per day was estimated, assuming that concentrations of alcohol were five percent for beer, 12 percent for wine, 40 percent for liquor, and 16 percent for sake. Habitual alcohol drinking was defined as drinking 2 times per week or more.
Body Composition by CT imaging
CT images were taken to measure liver CT density. Scanning was performed using a GE-Imatron C150 Electron Beam Tomography scanner (GE Medical Systems, South San Francisco, U.S.) at all the three study sites. Images centered on the T12-L1 disc space and the L4-L5 disc space were used for assessing hepatic fat content and abdominal AT distribution, respectively. CT imaging provides information on spatial arrangement of tissues, with the contrast between tissues based upon differences in attenuation of energy from X-rays. Attenuation values for CT are expressed using water as a reference value of 0 Hounsfield unit (HU). Adipose tissue displays attenuation values that are negative to those of water, and in a range from −160 to 0 HU, whereas skeletal muscle in a range from 0 to 100 HU (18).
In the current study, to assess hepatic fat content, CT attenuation in HU was determined in three regions of interest (ROI) for the liver and the spleen, each ROI of ~120 mm2. ROI for the liver was placed manually to avoid major vessels. Liver to spleen ratio (L/S ratio) is used as an index of liver fat content (19-21), using spleen as an internal control (22, 23). On the CT images of the abdomen, the area for AT and the psoas muscles were measured electronically by defining for each tissue as a range of CT attenuation values: 0 to −160 HU for AT; and 0 to 100 HU for muscle, as previously described (2). To determine the respective areas of VAT and SAT, a separation line was drawn manually using a cursor along abdominal wall musculature in continuity with fascia of the para-spinal muscles. Again, these measurements were performed by a cross-sectional image at L4-5, and were estimates for AT volume (distribution) (24, 25). All CT images were analyzed at the University of Pittsburgh using image analysis software by one trained reader (SliceOmatic, Tomovision, Montreal, Canada for hepatic fat content and AccuImage; AccuImage Diagnostic Corporation, San Francisco for VAT and SAT).
Statistical Analysis
General linear models were used to examine the ethnic difference in body composition and metabolic variables and to assess the association of liver CT density with selected variables. Two-way ANOVA was used to assess BMI-ethnicity interaction for liver fat, and BMI was used as a continuous variable, though the data was presented as categorized for ease of visualization. L/S ratio was also presented as categorized despite of using as a continuous variable, and ANCOVA was used to assess the ethnic difference. A P value <0.05 was considered statistically significant. All tests were based on a two-sided level of significance. Statistical analyses were performed using SPSS 17.0 program (SPSS Inc., Chicago, IL).
Results
Basic clinical charactereristics (Table 1, unadjusted values)
Table 1.
Basic Clinical Characteristics and Body Composition Assessed by CT in Japanese-Americans in Honolulu, HI, Japanese in Kusatsu City, Shiga, Japan and non-Hispanic white men in Allegheny County, PA, aged 40-49.
| Japanese men (n=314) |
Japanese-America n men (n=244) |
Non-Hispanic white men (n=288) |
|
|---|---|---|---|
| Age (years) | 45.1 ± 2.8** | 45.9 ± 2.8 | 45.0 ± 2.8** |
| Weight (kg) | 68.6 ± 9.6** | 79.9 ± 14.1 | 90.3 ± 14.9** |
| BMI (kg/m2) | 23.6 ± 2.9** | 27.8 ± 4.3 | 27.8 ± 4.2 |
| Alcohol intake (g/day) | 27 ± 29** | 18 ± 34 | 10 ± 14** |
| Metabolic parameters | |||
| Triglyceride (mg/dl) | 155 ± 81* | 184 ± 136 | 152 ± 100 |
| HOMA-IR | 2.77 ± 1.51** | 4.29 ± 3.11 | 3.89 ± 2.76 |
| Diabetes prevalence (%) | 6.1%* | 13.1% | 3.1%** |
| CRP (mg/dl) | 0.74 ± 1.79** | 1.36 ± 2.41 | 1.62 ± 2.36** |
|
CT imaging of the Abdomen |
|||
| VAT (cm2) | 133 ± 50 | 175 ± 76 | 172 ± 73 |
| SAT (cm2) | 136 ± 58** | 225 ± 100 | 252 ± 112 |
| V/S ratio | 1.05 ± 0.33** | 0.83 ± 0.33 | 0.74 ± 0.28* |
| Psoas muscle (cm2) | 25 ± 5** | 27 ± 5 | 29 ± 5** |
| Psoas muscle (HU) | 54 ± 5** | 59 ± 8 | 54 ± 6** |
|
CT imaging of the Liver
and the Spleen |
|||
| Liver (HU) | 59 ± 9 | 58 ± 13 | 60 ± 8 |
| Spleen (HU) | 58 ± 4** | 57 ± 6 | 56 ± 5 |
| Liver / Spleen ratio | 1.01 ± 0.16* | 1.03 ± 0.22 | 1.07 ± 0.15* |
p<0.05
p<0.01 compared with J-Americans, values are unadjusted means± SD.
Clinical characteristics and body composition parameters for Japanese-American, along with previous data for Japanese and NHW men (11) are presented in Table 1.
By design, age was similar among Japanese, Japanese-Americans, and NHW men, though Japanese-Americans (45.9 ± 2.8) were slightly (0.9 year) older than Japanese (45.1 ± 2.8) and NHW men (45.0 ± 2.8). Japanese-Americans were much heavier compared with Japanese (79.9 ± 14.1 vs. 68.6 ± 9.6 kg; p<0.01) and lighter compared with NHW men (79.9 ± 14.1 vs. 90.3 ± 14.9kg; p<0.01). Since Japanese-Americans were shorter compared with NHW men, BMI was almost the same between Japanese-Americans and NHW men (27.8 ± 4.3 vs. 27.8 ± 4.2; ns).
Ethanol intake in Japanese Americans (18 ± 34g/day) was similar to that in African-Americans (13 ± 22g/day) and was somewhere in the middle for that in Japanese (27 ± 29g/day) and NHW men (10 ± 14g/day).
Despite of very similar BMI between Japanese Americans and NHW men, diabetes prevalence was much higher among Japanese Americans compared with NHW men.
Ethnic difference in liver fat
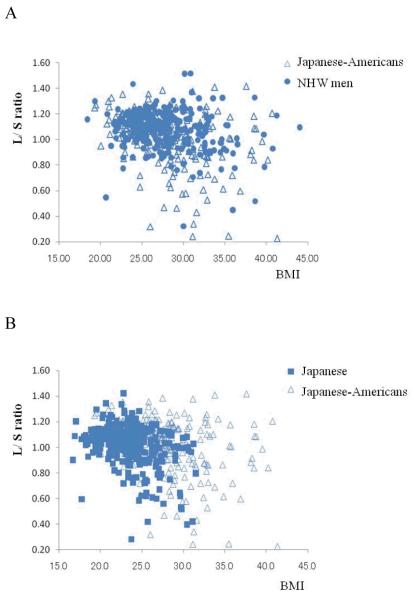
As shown in Figure 1A, despite of a very similar BMI, compared with NHW men, Japanese-Americans had higher liver fat content (liver / spleen ratio; 1.07 ± 0.15 for NHW vs. 1.03 ± 0.22 for Japanese-Americans, p<0.05) which tended to become more significant with increasing BMI (ethnicity by BMI interaction, p=0.09). These ethnic differences in liver fat content were still significant even after adjusted for age, BMI and ethanol intake (g/day). On the other hand, compared with Japanese, Japanese-Americans had a lower liver fat content (liver / spleen ration: 1.01 ± 0.16 for Japanese vs. 1.03 ± 0.22; p<0.05), regardless of BMI (Figure 1B, 1C).
Figure 1.
The raw data were plotted against BMI and liver to spleen ratio (L/S ratio) in Figure 1A and 1B. The data for Japanese-Americans (open triangle) and non-Hispanic white (NHW) men (solid circle), and those for Japanese-Americans and Japanese (solid rectangle), were superimposed each other in Figure 1A and 1B, respectively. In Figure 1C, subjects were stratified by BMI in Japanese-Americans (cross), Japanese men (solid rectangle), and NHW men (solid circle), separately and mean values (±SEM) of BMI for each quartile are plotted against mean values (±SEM) of L/S ratio for respective quartiles. As shown in Figure 1A and 1B, raw data plots for Japanese-Americans were partly overlapped with both those for NHW men (1A) and those for Japanese (1B).
Association with visceral adiposity (VAT)
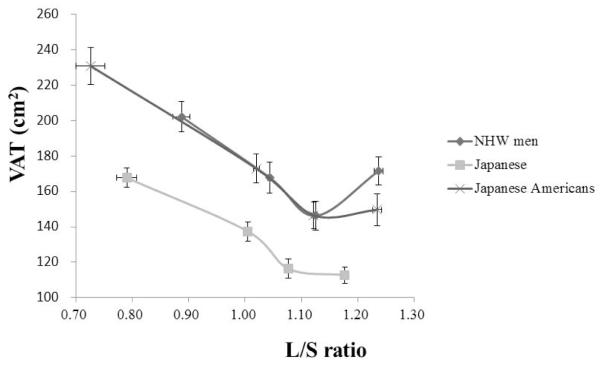
As shown in Figure 2, a strong positive association exists between liver fat content and VAT in Japanese-Americans. We previously observed similar association in Japanese and NHW men (11). Regression lines of liver fat and VAT had similar slopes across the three groups, unlike for those between liver fat and BMI (Figure 1C).
Figure 2.
Subjects were stratified by liver fat content (L/S ratio) in Japanese-Americans (cross), Japanese men (solid rectangle), and NHW men (solid circle), separately and mean values (±SEM) of L/S ratio for each quartile are plotted against mean values (±SEM) of visceral adiposity (VAT) for respective quartiles. The association between VAT and L/S ratio were similar among the four different ethnic groups.
Association with metabolic variables
CRP was lower in Japanese-Americans compared with NHW men (1.36 ± 2.41 vs. 1.62 ± 2.36 mg/dl, respectively; p<0.01), despite of a similar level of BMI and VAT and a higher degree of liver fat content, as well as similar level of triglyceride (184 ± 136 vs. 152 ± 100 mg/dl, respectively; ns), HOMA-IR (4.29 ± 3.11 vs. 3.89 ± 2.76, respectively; ns) and higher prevalence of diabetes in Japanese-Americans than in NWH men (13.1% vs. 3.1%, respectively; p<0.01), which are all well-known correlates of higher CRP. In multiple regression analaysis among age, BMI, VAT, L/Sratio, and ethnicity, higher BMI, lower L/Sratio, and ethnicity explained 52% of variance of logCRP (p<0.01).
Discussion
In the current study, Japanese-Americans living in Hawaii, who have a similar ethnic background to Japanese, yet a similar amount of adiposity to NHW men, were newly assessed for liver fat content. As expected, liver fat content was less in Japanese-Americans compared with Japanese, despite of a much higher BMI in Japanese-Americans and an apparent positive association between fatty liver and obesity in each population group. This supports our hypothesis that there is indeed a major population difference in fat storage capacity, which explains for a propensity for fatty liver in Japanese.
On the other hand, Japanese-Americans had higher liver fat content compared with NHW men, despite of the same degree of obesity (BMI). Moreover, the regression line of liver fat and BMI for Japanese-Americans was similar to that for Japanese, rather than that for NHW men (Figure 1C). Since Japanese-Americans have similar ethnic background with Japanese, this finding has reemphasized the previously observed ethnic difference in body composition between Japanese and NHW men.
Browning et al. recently reported the ethnic difference in liver fat in NHW, African-American, and Hispanics with large number (n=2170) using MR spectroscopy (13). They have found that there was a clear ethnic difference in liver fat and African-Americans had least liver fat with greater capacity of body fat stores within subcutaneous adiposity, especially in lower extremity. Moreover, they also reported the strong positive association between visceral fat accumulation and fatty liver along with serum triglyceride levels, which was also observed in the current study (Figure 2 and previous figure(11)).
Vague et al. have first recognized the association of body fat distribution with metabolic risk, independent of total adiposity, i.e. upper-body obesity is metabolically worse than lower body obesity (26). Since then, the development of body composition analyses by CT and MRI, revealed that upper body obesity is characterized by accumulation of visceral adiposity, which has consistently been demonstrated to correlate with insulin resistance (6, 8). More recently, increased content of fat within liver and skeletal muscle, has been also reported to be closely associated with insulin resistance (1, 2, 27). In contrast, lower body adiposity, namely gluteal-femoral subcutaneous adiposity has a weak association with insulin resistance and may instead counterbalance the influence of abdominal adiposity (28). These observations can be well explained by the concept of ectopic fat storage proposed by Ravussin et al., i.e. when energy influx due to excess energy intake and less energy expenditure, exceeds one’s capacity of body fat stores within subcutaneous adiposity, fat accumulation in non-adipose tissue such as liver and muscle occurs, which compromises insulin signaling within the tissue, a phenomenon known as insulin resistance (14). Indeed, in each population, we have observed that liver fat content as well as VAT were unfavorably associated with HOMA-IR and CRP, as also reported recently (29).
However, in the current study, despite of less liver fat, Japanese-Americans had higher prevalence of diabetes mellitus, higher insulin resistance (HOMA-IR) and higher level of inflammatory marker, CRP, which are all well-known strong cardiovascular risk markers. Moreover, despite of unfavorable body fat distributions, CRP was much lower among Japanese-Americans as well as Japanese compared with NHW men. This contradictory finding across different populations clearly shows that the associations between body composition and metabolic variables should be considered by ethnicity.
These ethnic differences in the association between body composition and metabolic variables, can be partly explained by differences in dietary lifestyle, especially dietary fat intake.
Firstly, with regard to body composition, it has been reported that dietary saturated fat induced expansion of adipose tissue mass more effectively than polyunsaturated fat (30). Among obese adolescents, higher plasma level of saturated fatty acid and lower level of omega-3 fatty acid was observed compared with non-obese counterparts (31). Secondly, with regard to the ethnic difference in the association between body composition and metabolic variables, higher saturated fat intake and lower polysaturated fat intake, especially n-3 fatty acid intake, has been reported to be associated not only with higher prevalence of obesity but also with higher CRP (31, 32).
Therefore, higher intake of saturated fatty acid and lower intake of n-3 fatty acid may link higher capacity to store fat and higher CRP, which can lead to a contradictory combination of less liver fat and higher CRP across different populations with different dietary fat intake.
Although we have not collected detailed dietary data, the INTERMAP Study, which is designed to assess the association of nutrition with blood pressure with one of the most comprehensive dietary assessment internationally, includes the detail information about food intake in Japanese and NHW men, who are well matched for age and BMI with our study. According to INTERMAP data (33), despite of increasing westernized dietary lifestyle in Japanese in Japan, there was a distinct difference in dietary lifestyle such as the amount of saturated fat and fish intake. Also, in an ancillary study of the INTERMAP(33, 34), we have observed Japanese men in Japan had higher intake of polyunsaturated/saturated fatty acid ratio and omega-3 fatty acid, compared with Japanese-Americans living in Hawaii. Therefore, there is a possibility that lower intake of saturated fatty acid among Japanese partly explains the observed lower amount of subcutaneous adiposity with higher liver fat content and lower CRP, which may contribute to lower cardiovascular event risk in Japanese despite of unfavorable fat distribution for metabolic risks (16, 32).
In conclusion, we have observed that liver fat content was indeed higher among Japanese-Americans compared with NHW men, who have had similar BMI, and Japanese-Americans are also more prone to hepatic steatosis with increasing BMI. This suggests that a reduced capacity to store fat in response to positive net energy balance seen in Japanese was inherited to this immigrants and ethnic difference in body composition truly exists. Also, the association of liver fat with CRP can be ethnicity-specific and might explain for less cardiovascular events seen in Japanese.
ACKNOWLEDGEMENTS
This research was supported by grants R01 HL68200 and HL071561 from the US National Institutes of Health, and Grant-in-aid for Scientific Research (A) 17209023 and Grant-in aid for Young Scientists (B) 18790396 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
ABBREVIATIONS
- NHW
non-Hispanic whites
- BMI
body mass index
- CRP
C-reactive protein
- CT
computed tomography
- TG
triglyceride
- HOMA
homeostasis model assessment
- IR
insulin resistance
- AT
adipose tissue
- VAT
visceral adipose tissue
- SAT
subcutaneous adipose tissue
- L/S ratio
Liver to spleen ratio
- MRI
magnetic resonance imaging
Footnotes
We also declare that we do not have any conflict of interest for this manuscript.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346(16):1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E906–16. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 3.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care. 2006 Aug;29(8):1845–50. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann Med. 2005;37(5):347–56. doi: 10.1080/07853890510037383. [DOI] [PubMed] [Google Scholar]
- 5.Albu JB, Kovera AJ, Johnson JA. Fat distribution and health in obesity. Ann N Y Acad Sci. 2000 May;904:491–501. doi: 10.1111/j.1749-6632.2000.tb06505.x. [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990 Jul-Aug;10(4):497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 7.Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003 May;19(5):457–66. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002 Mar;282(3):E657–63. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009 Feb;119(2):315–22. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azuma K, Kadowaki T, Cetinel C, Kadota A, El-Saed A, Kadowaki S, et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism. 2009 Aug;58(8):1200–7. doi: 10.1016/j.metabol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond) 2006 Jul;30(7):1163–5. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009 Mar;49(3):791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002 Jun;967:363–78. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 15.Sekikawa A, Ueshima H, Kadowaki T, El-Saed A, Okamura T, Takamiya T, et al. Less Subclinical Atherosclerosis in Japanese Men in Japan than in White Men in the United States in the Post-World War II Birth Cohort. Am J Epidemiol. 2007 Jan 22; doi: 10.1093/aje/kwk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008 Aug 5;52(6):417–24. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986 Jun;250(6 Pt 1):E736–45. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 19.Longo R, Ricci C, Masutti F, Vidimari R, Croce LS, Bercich L, et al. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993 Apr;28(4):297–302. [PubMed] [Google Scholar]
- 20.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980 Dec;137(3):727–9. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 21.Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997 Jul;27(1):108–13. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JC, Kronmal RA, Carr JJ, McNitt-Gray MF, Wong ND, Loria CM, et al. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005 May;235(2):403–14. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- 23.Stanford W, Burns TL, Thompson BH, Witt JD, Lauer RM, Mahoney LT. Influence of body size and section level on calcium phantom measurements at coronary artery calcium CT scanning. Radiology. 2004 Jan;230(1):198–205. doi: 10.1148/radiol.2301020807. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol. 2004 Sep;97(3):948–54. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004 Aug;80(2):271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vague J. Sexual differentiation, a factor affecting the forms of obesity. Presse Med. 1947 May 24;55(30):339–40. [PubMed] [Google Scholar]
- 27.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000 Jul;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 28.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004 Feb;27(2):372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 29.Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009 Sep;32(9):1734–6. doi: 10.2337/dc09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillabeer G, Lau DC. Regulation of new fat cell formation in rats: the role of dietary fats. J Lipid Res. 1994 Apr;35(4):592–600. [PubMed] [Google Scholar]
- 31.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005 Dec;82(6):1178–84. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 32.Innis SM. Dietary lipids in early development: relevance to obesity, immune and inflammatory disorders. Curr Opin Endocrinol Diabetes Obes. 2007 Oct;14(5):359–64. doi: 10.1097/MED.0b013e3282be90b9. [DOI] [PubMed] [Google Scholar]
- 33.Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003 Sep;17(9):623–30. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueshima H, Okayama A, Saitoh S, Nakagawa H, Rodriguez B, Sakata K, et al. Differences in cardiovascular disease risk factors between Japanese in Japan and Japanese-Americans in Hawaii: the INTERLIPID study. J Hum Hypertens. 2003 Sep;17(9):631–9. doi: 10.1038/sj.jhh.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]