Abstract
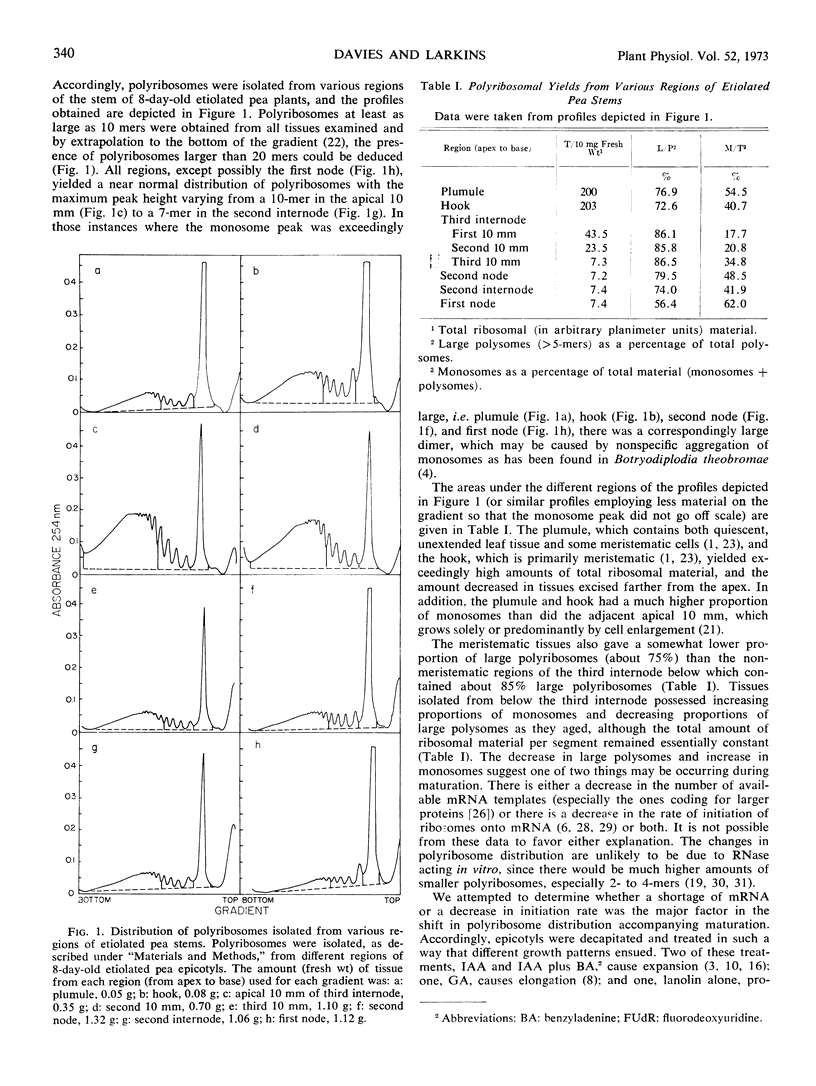
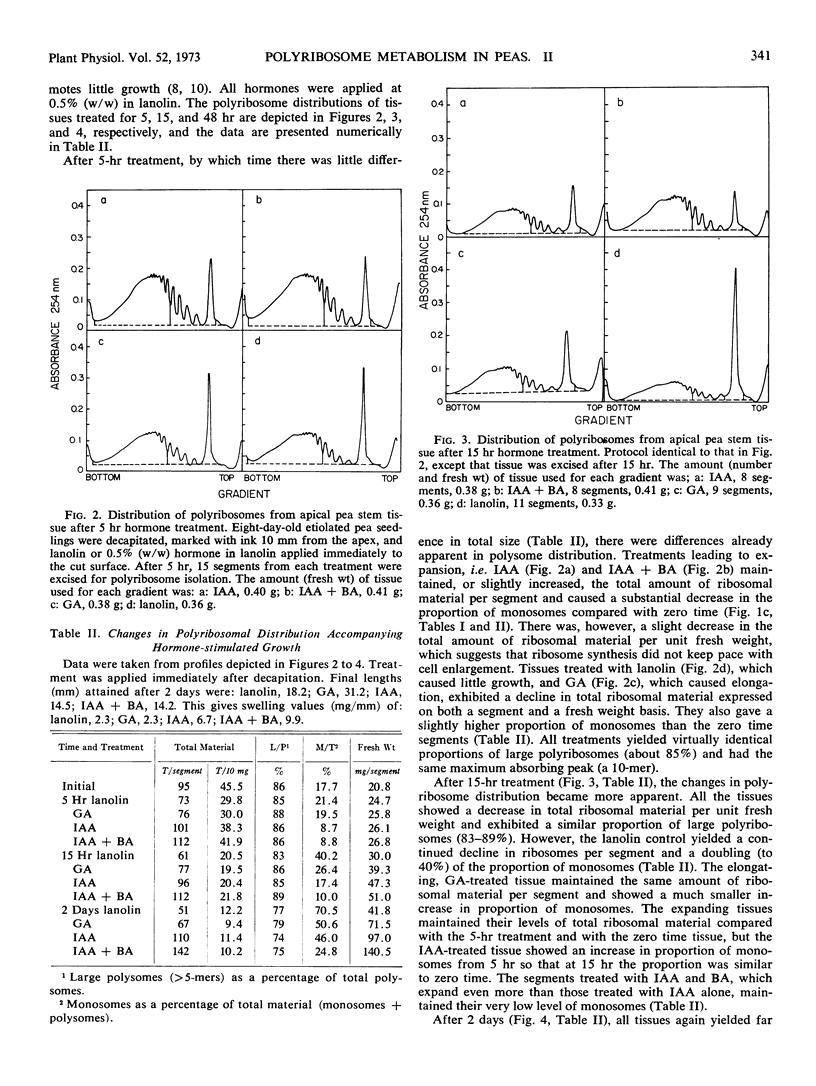
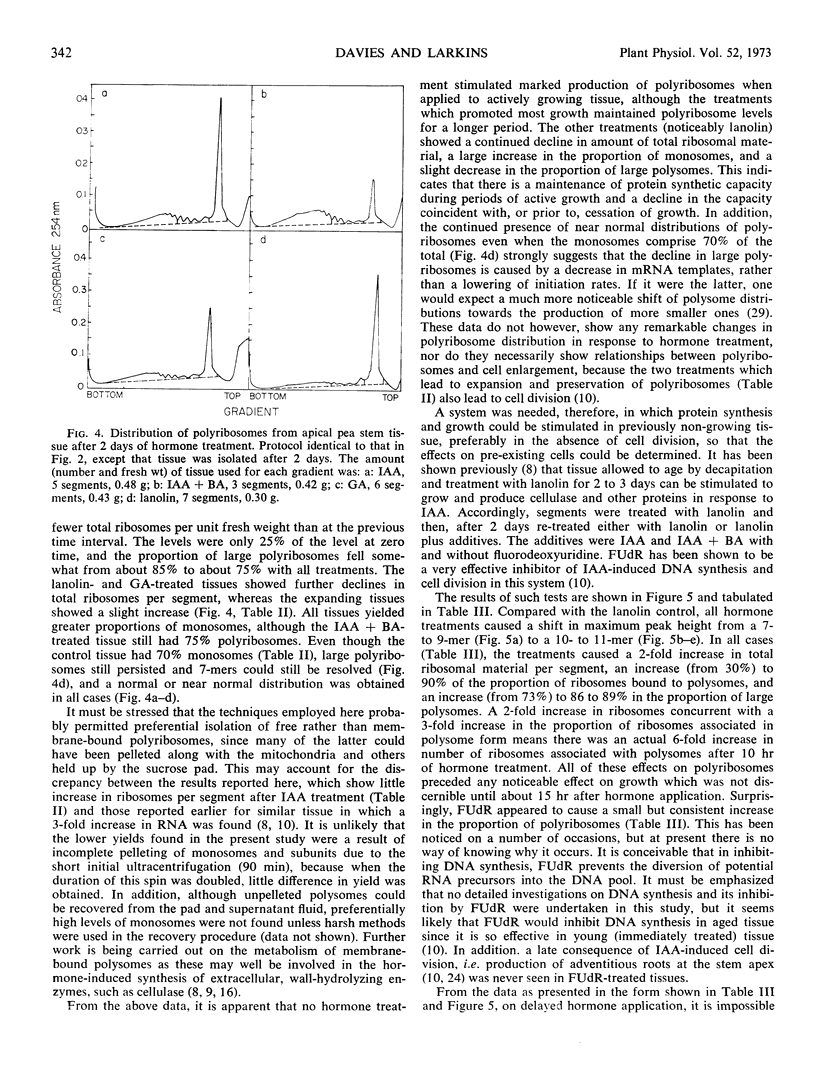
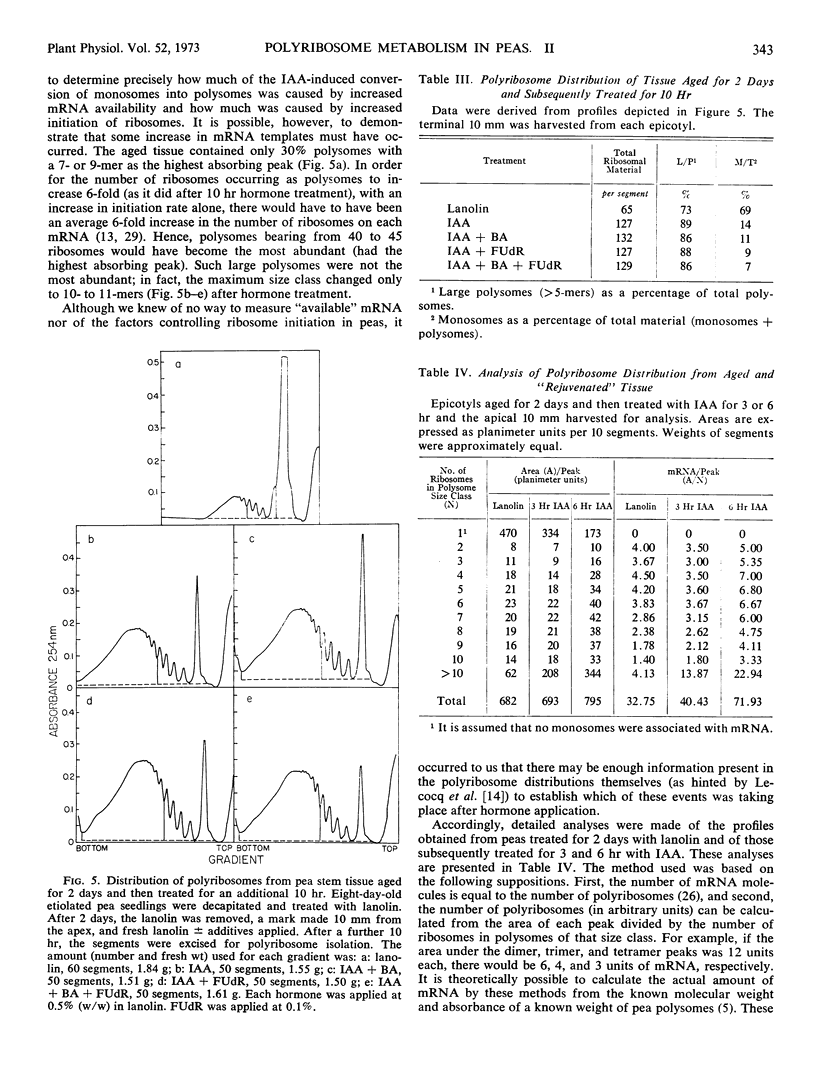
Polyribosomes as large as 10-mers (strands of messenger RNA bearing 10 ribosomes) were isolated from etiolated pea (Pisum sativum L. var. Alaska) stem tissue during all stages of development when methods were used which essentially eliminated ribonuclease activity during extraction. Actively growing tissue, harvested from the apical 10 mm, yielded many large polyribosomes and a low (<20%) proportion of monosomes. Similar tissue, allowed to age by applying lanolin to decapitated apices, showed a progressive decrease in number of larger polyribosomes and an increase in the proportion of monosomes. Hormone treatments, which prolonged growth and delayed aging, delayed the loss in large polyribosomes and the increase in proportion of monosomes. Growth-stimulating hormones, added to previously aged tissue, stimulated the production of many large polyribosomes in pre-existing cells.
It is suggested that (a) large polyribosomes occur in all regions of the pea stem, (b) changes in polyribosome distribution appear to precede changes in growth rate, (c) loss of larger polyribosomes is closely related to a decrease in mRNA templates followed more gradually by loss of ribosomes, (d) hormone-stimulated continuation of growth is accomplished through maintenance of available mRNA.
Methods are described, involving detailed analysis of polysome distribution, which, although they cannot be used to measure changes in initiation of ribosomes on to mRNA, do permit measurement of the amount of polysomal-associated mRNA present in tissues at different stages of growth. These analyses lead to the further suggestion that hormone stimulation of growth of previously nongrowing tissue is accomplished primarily through an increase in available mRNA prior to synthesis of ribosomes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birmingham B. C., Maclachlan G. A. Generation and suppression of microsomal ribonuclease activity after treatments with auxin and cytokinin. Plant Physiol. 1972 Mar;49(3):371–375. doi: 10.1104/pp.49.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambl R. M., Van Etten J. L. Protein synthesis during fungal spore germination. V. Evidence that the ungerminated conidiospores of Botryodiplodia theobromae contain messenger ribonucleic acid. Arch Biochem Biophys. 1970 Apr;137(2):442–452. doi: 10.1016/0003-9861(70)90461-3. [DOI] [PubMed] [Google Scholar]
- Cammarano P., Pons S., Romeo A., Galdieri M., Gualerzi C. Characterization of unfolded and compact ribosomal subunits from plants and their relationship to those of lower and higher animals: evidence for physicochemical heterogeneity among eucaryotic ribosomes. Biochim Biophys Acta. 1972 Nov 9;281(4):571–596. doi: 10.1016/0005-2787(72)90158-x. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Effects of indoleacetic acid on intracellular distribution of beta-glucanase activities in the pea epicotyl. Arch Biochem Biophys. 1968 Dec;128(3):595–600. doi: 10.1016/0003-9861(68)90068-4. [DOI] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Generation of cellulase activity during protein synthesis by pea microsomes in vitro. Arch Biochem Biophys. 1969 Feb;129(2):581–587. doi: 10.1016/0003-9861(69)90217-3. [DOI] [PubMed] [Google Scholar]
- Fan D. F., Maclachlan G. A. Massive synthesis of ribonucleic Acid and cellulase in the pea epicotyl in response to indoleacetic Acid, with and without concurrent cell division. Plant Physiol. 1967 Aug;42(8):1114–1122. doi: 10.1104/pp.42.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L. The density of ribosomes bearing messenger RNA in phage-infected and normal bacteria. J Cell Sci. 1971 May;8(3):649–658. doi: 10.1242/jcs.8.3.649. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Velez R., Mucha J. Resistance of messenger RNA-bound ribosomes to proteolytic dissociation. Exp Cell Res. 1972 Jun;72(2):431–435. doi: 10.1016/0014-4827(72)90011-0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Roberts N. E. In vivo labeling patterns of free polyribosomes: relationship to tape theory of messenger ribonucleic acid function. J Mol Biol. 1967 Jun 14;26(2):211–225. doi: 10.1016/0022-2836(67)90292-6. [DOI] [PubMed] [Google Scholar]
- Lecocq R. E., Cantraine F., Keyhani E., Claude A., Delcroix C., Dumont J. E. Quantitative evaluation of polysomes and ribosomes by density gradient centrifugation and electron microscopy. Anal Biochem. 1971 Sep;43(1):71–79. doi: 10.1016/0003-2697(71)90109-6. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H., Askey C., Aylmer C. Determination of sedimentation coefficients and polyribosomal size in nonisokinetic density gradient centrifugation. Anal Biochem. 1972 Apr;46(2):365–373. doi: 10.1016/0003-2697(72)90310-7. [DOI] [PubMed] [Google Scholar]
- STAEHELIN T., WETTSTEIN F. O., OURA H., NOLL H. DETERMINATION OF THE CODING RATIO BASED ON MOLECULAR WEIGHT OF MESSENGER RIBONUCLEIC ACID ASSOCIATED WITH ERGOSOMES OF DIFFERENT AGGREGATE SIZE. Nature. 1964 Jan 18;201:264–270. doi: 10.1038/201264a0. [DOI] [PubMed] [Google Scholar]
- Vassart G. M., Dumont J. E., Cantraine F. R. Simulation of polyribosome disaggregation. Biochim Biophys Acta. 1970 Nov 12;224(1):155–164. doi: 10.1016/0005-2787(70)90629-5. [DOI] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E., Cantraine F. R. Translational control of protein synthesis: a simulation study. Biochim Biophys Acta. 1971 Oct;247(3):471–485. doi: 10.1016/0005-2787(71)90034-7. [DOI] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Poort C. Rate of protein synthesis and polyribosome formation in the frog pancreas after fasting and feeding. Biochim Biophys Acta. 1971 Oct;247(3):468–470. doi: 10.1016/0005-2787(71)90033-5. [DOI] [PubMed] [Google Scholar]


