Abstract
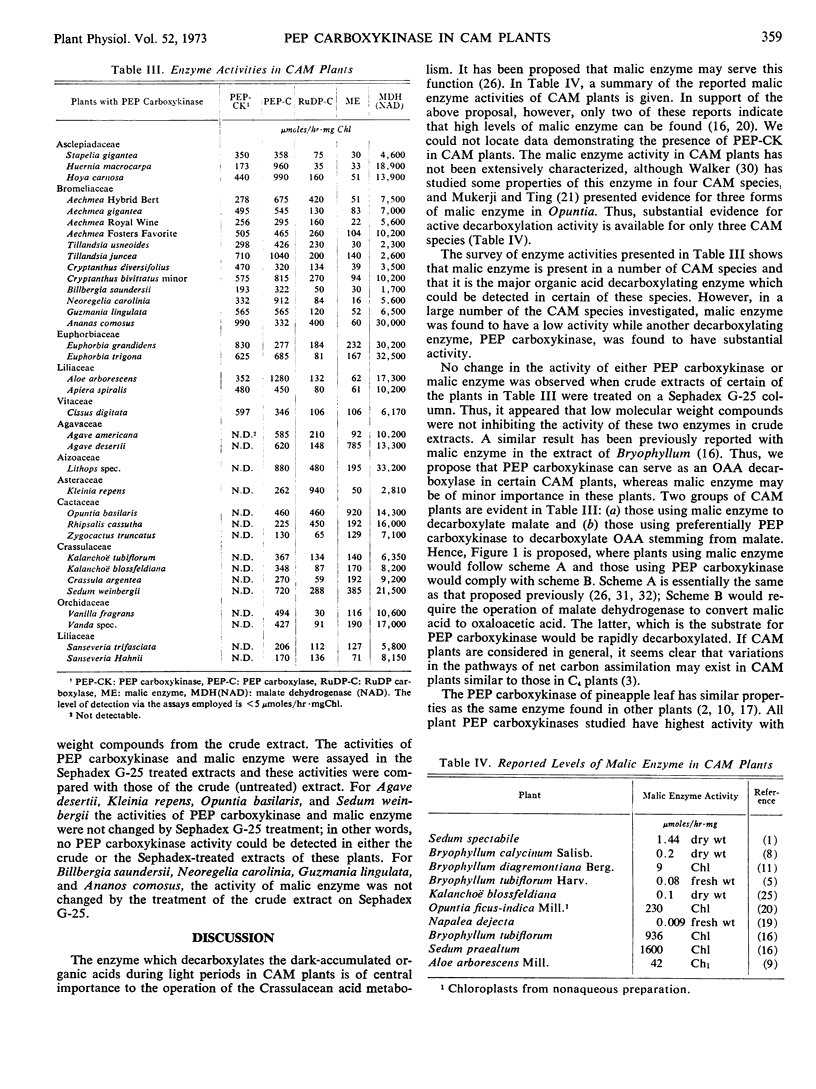
Phosphoenolpyruvate carboxykinase has been found in significant activities in a number of plants exhibiting Crassulacean acid metabolism. Thirty-five species were surveyed for phosphoenolpyruvate carboxykinase, phosphoenolpyruvate carboxylase, ribulose diphosphate carboxylase, malic enzyme, and malate dehydrogenase (NAD). Plants which showed high activities of malic enzyme contained no detectable phosphoenolpyruvate carboxykinase, while plants with high activities of the latter enzyme contained little malic enzyme. It is proposed that phosphoenolpyruvate carboxykinase acts as a decarboxylase during the light period, furnishing CO2 for the pentose cycle and phosphoenolpyruvate for gluconeogenesis.
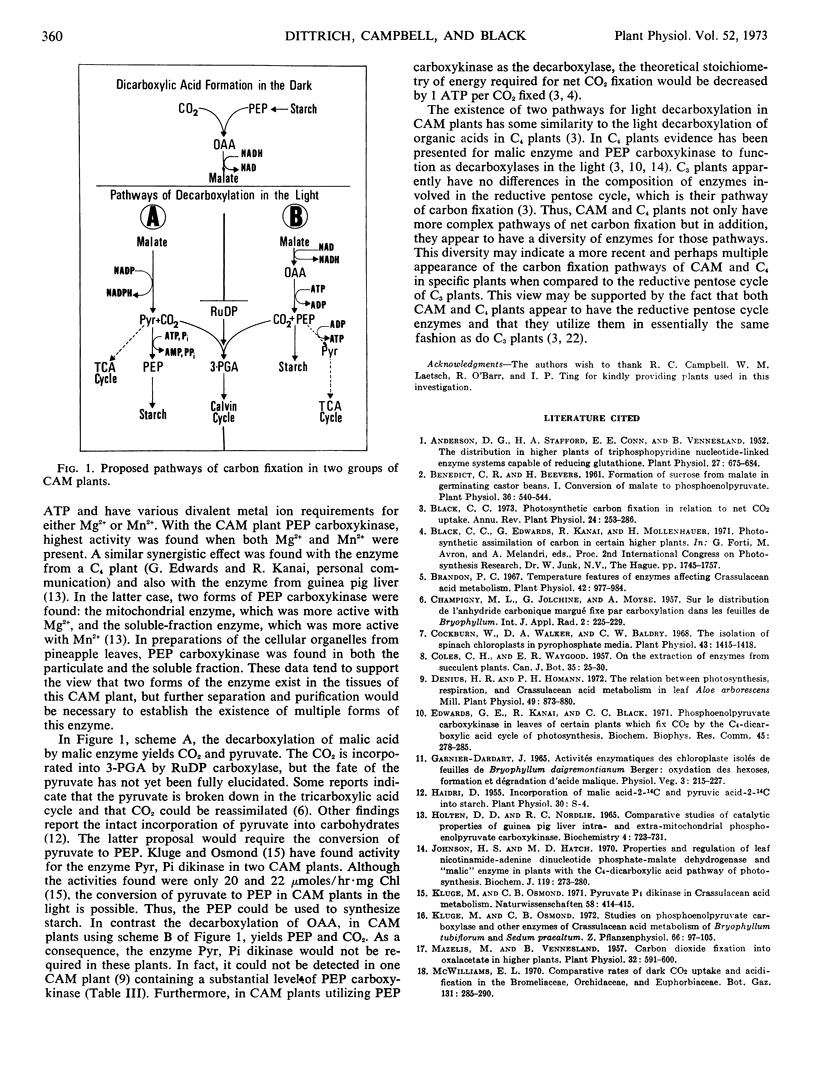
Some properties of phosphoenolpyruvate carboxykinase in crude extracts of pineapple leaves were investigated. The enzyme required Mn2+, Mg2+, and ATP for maximum activity. About 60% of the activity could be pelleted, along with chloroplasts and mitochondria, in extracts from leaves kept in the dark overnight.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., Stafford H. A., Conn E. E., Vennesland B. THE DISTRIBUTION IN HIGHER PLANTS OF TRIPHOSPHOPYRIDINE NUCLEOTIDE-LINKED ENZYME SYSTEMS CAPABLE OF REDUCING GLUTATHIONE. Plant Physiol. 1952 Oct;27(4):675–684. doi: 10.1104/pp.27.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C. R., Beevers H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961 Sep;36(5):540–544. doi: 10.1104/pp.36.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon P. C. Temperature features of enzymes affecting crassulacean Acid metabolism. Plant Physiol. 1967 Jul;42(7):977–984. doi: 10.1104/pp.42.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denius H. R., Homann P. H. The Relation between Photosynthesis, Respiration, and Crassulacean Acid Metabolism in Leaf Slices of Aloe arborescens Mill. Plant Physiol. 1972 Jun;49(6):873–880. doi: 10.1104/pp.49.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Kanai R., Black C. C. Phosphoenolpyruvate carboxykinase in leaves of certain plants whick fix CO 2 by the C 4 -dicarboxylic acid cycle of photosynthesis. Biochem Biophys Res Commun. 1971 Oct 15;45(2):278–285. doi: 10.1016/0006-291x(71)90814-x. [DOI] [PubMed] [Google Scholar]
- HOLTEN D. D., NORDLIE R. C. COMPARATIVE STUDIES OF CATALYTIC PROPERTIES OF GUINEA PIG LIVER INTRA- AND EXTRAMITOCHONDRIAL PHOSPHOENOLPYRUVATE CARBOXYKINASES. Biochemistry. 1965 Apr;4:723–731. doi: 10.1021/bi00880a018. [DOI] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelis M., Vennesland B. Carbon Dioxide Fixation into Oxalacetate in Higher Plants. Plant Physiol. 1957 Nov;32(6):591–600. doi: 10.1104/pp.32.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S. K., Ting I. P. Malate dehydrogenase (decarboxylating) (NADP) isoenzymes of Opuntia stem tissue. Mitochondrial, chloroplast, and soluble forms. Biochim Biophys Acta. 1968 Oct 8;167(2):239–249. doi: 10.1016/0005-2744(68)90202-7. [DOI] [PubMed] [Google Scholar]
- Neales T. F. Effect of ambient carbon dioxide concentration on the rate of transpiration of Agave americana in the dark. Nature. 1970 Nov 28;228(5274):880–882. doi: 10.1038/228880b0. [DOI] [PubMed] [Google Scholar]
- SIMON E. W. The effect of digitonin on the cytochrome c oxidase activity of plant mitochondria. Biochem J. 1958 May;69(1):67–74. doi: 10.1042/bj0690067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Reich R., Witt H. T. Electrochromism of chlorophylls and carotenoids in multilayers and in chloroplasts. Naturwissenschaften. 1971 Aug;58(8):414–414. doi: 10.1007/BF00591523. [DOI] [PubMed] [Google Scholar]
- WALKER D. A. Physiological studies on acid metabolism. 7. Malic enzyme from Kalanchoe crenata: effects of carbon dioxide concentration. Biochem J. 1960 Feb;74:216–223. doi: 10.1042/bj0740216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. A. Pyruvate carboxylation and plant metabolism. Biol Rev Camb Philos Soc. 1962 May;37:215–256. doi: 10.1111/j.1469-185x.1962.tb01611.x. [DOI] [PubMed] [Google Scholar]


