Abstract
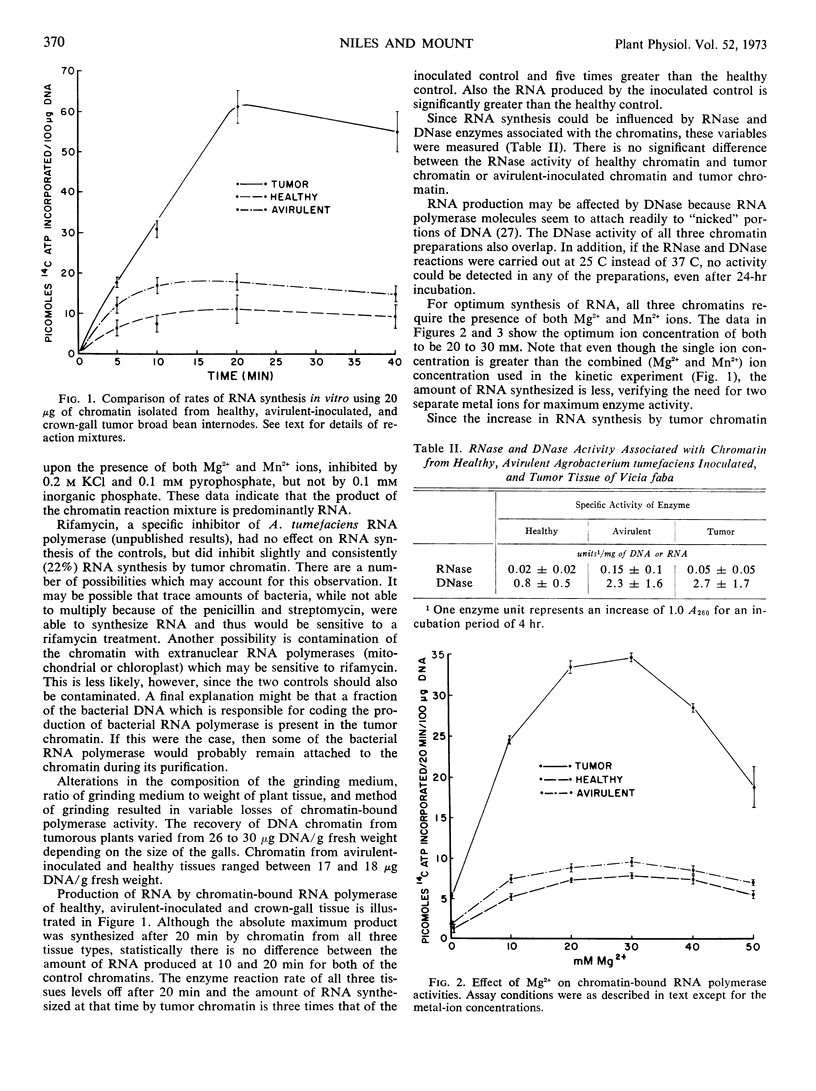
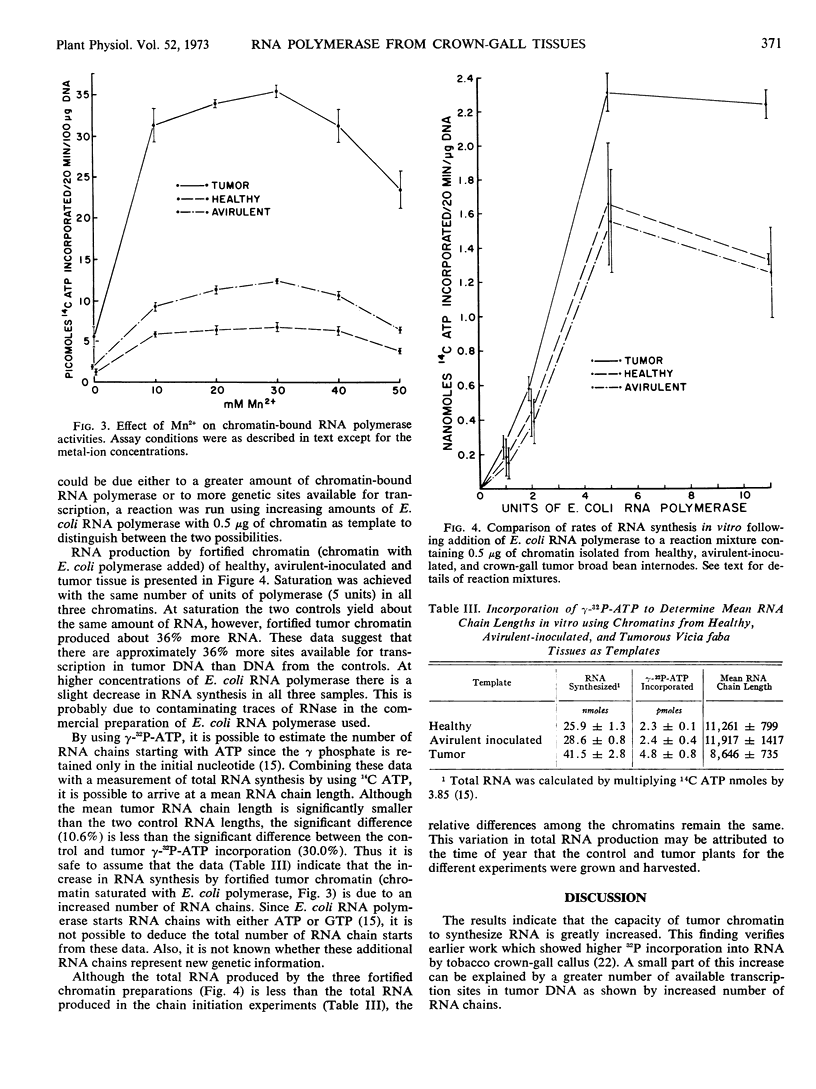
Chromatin was extracted from healthy, avirulent Agrobacterium tumefaciens inoculated, and crown-gall tumor Vicia faba internodes of the same age. Chromatin from crown-gall tissue produced 5 times more RNA per 100 micrograms of DNA than chromatin from the healthy tissue. When template availability was compared using chromatin with saturating amounts of Escherichia coli RNA polymerase, chromatin from crown-gall tissue had 36% more available template than the controls. In addition, when γ-32P-ATP was incorporated into the RNA synthesizing reaction mixture, with saturating amounts of E. coli RNA polymerase, there were twice as many RNA chain starts in tumor as in control tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. C. On the origin of the cancer cells. Am Sci. 1970 May-Jun;58(3):307–320. [PubMed] [Google Scholar]
- Dahmus M. E., Bonner J. Nucleoproteins in regulation of gene function. Fed Proc. 1970 May-Jun;29(3):1255–1260. [PubMed] [Google Scholar]
- Gahan P. B., Sheikh K., Maggi V., Stroun M., Smith A. R.W. Electrophoretic analysis of hydrolases from grown gall tissues. FEBS Lett. 1971 Feb 12;13(1):53–55. doi: 10.1016/0014-5793(71)80662-2. [DOI] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Purves W. K. Ribonucleic Acid Synthesis by Cucumber Chromatin: Developmental and Hormone-induced Changes. Plant Physiol. 1970 Oct;46(4):581–585. doi: 10.1104/pp.46.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupila-Ahvenniemi S. Morphogenesis of crown gall. Adv Morphog. 1968;7:45–78. doi: 10.1016/b978-1-4831-9954-2.50006-9. [DOI] [PubMed] [Google Scholar]
- Maitra U., Hurwitz H. The role of DNA in RNA synthesis, IX. Nucleoside triphosphate termini in RNA polymerase products. Proc Natl Acad Sci U S A. 1965 Sep;54(3):815–822. doi: 10.1073/pnas.54.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb A. J., McComb J. A., Duda C. T. Increased ribonucleic Acid polymerase activity associated with chromatin from internodes of dwarf pea plants treated with gibberellic Acid. Plant Physiol. 1970 Aug;46(2):221–223. doi: 10.1104/pp.46.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Parsons C. L., Beardsley R. E. Bacteriphage activity in homogenates of crown gall tissue. J Virol. 1968 Jun;2(6):651–651. doi: 10.1128/jvi.2.6.651-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi K. K. Ribonuclease induction in cells transformed by Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1207–1214. doi: 10.1073/pnas.56.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D., Mascarenhas J. P. Auxin and cyclic 3',5'-adenosine monophosphate during the isolation of chromatin from Avena coleoptiles: effects on cell free RNA synthesis. Biochem Biophys Res Commun. 1972 Apr 14;47(1):131–141. doi: 10.1016/s0006-291x(72)80020-2. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Veldstra H., Warnaar S. O., Mulder G., Cohen J. A. Formation of complexes between DNA isolated from tobacco crown gall tumours and RNA complementary to Agrobacterium tumefaciens DNA. Biochim Biophys Acta. 1967 Sep 26;145(2):523–525. doi: 10.1016/0005-2787(67)90075-5. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Chadha K. C. Liberation of Agrobacterium tumefaciens DNA from the crown gall tumor cell DNA by shearing. Biochem Biophys Res Commun. 1970 Aug 24;40(4):968–972. doi: 10.1016/0006-291x(70)90998-8. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. DNA-DNA hybridization studies between bacterial DNA, crown gall tumor cell DNA and the normal cell DNA. Life Sci II. 1970 Aug 8;9(15):889–892. doi: 10.1016/0024-3205(70)90058-5. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Patterns of nucleic acids synthesis in normal and crown gall tumor tissue cultures of tobacco. Arch Biochem Biophys. 1968 Jun;125(3):817–823. doi: 10.1016/0003-9861(68)90519-5. [DOI] [PubMed] [Google Scholar]
- Stroun M. The natural release of nucleic acids from bacteria into plant cells and the transcription of host cell DNA. FEBS Lett. 1970 Jul 3;8(6):349–352. doi: 10.1016/0014-5793(90)80011-7. [DOI] [PubMed] [Google Scholar]
- Vogt V. Breaks in DNA stimulate transcription by core RNA polymerase. Nature. 1969 Aug 23;223(5208):854–855. doi: 10.1038/223854a0. [DOI] [PubMed] [Google Scholar]
- WOOD H. N., BRAUN A. C. Studies on the regulation of certain essential biosynthetic systems in normal and crown-gall tumor cells. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1907–1913. doi: 10.1073/pnas.47.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]


