Abstract
Air pollution contributes to acute exacerbations of asthma and the development of asthma in children and adults. Airway epithelial cells interface innate and adaptive immune responses and have been proposed to regulate much of the response to pollutants. Thymic stromal lymphopoietin (TSLP) is a pivotal cytokine linking innate and Th2 adaptive immune disorders and is upregulated by environmental pollutants, including ambient particulate matter (PM) and diesel exhaust particles (DEP). We now show that DEP and ambient fine PM upregulate TSLP mRNA and hsa-miR-375 in primary human bronchial epithelial cells (pHBEC). Moreover, transfection of pHBEC with anti-hsa-miR-375 reduced TSLP mRNA in DEP but not TNF-α treated cells. In silico pathway evaluation suggested the aryl hydrocarbon receptor (AhR) as one possible target of miR-375. DEP and ambient fine PM (3 μg/cm2), down regulated AhR mRNA. Transfection of mimic-hsa-miR-375 resulted in a small downregulation of AhR mRNA compared to resting AhR mRNA. AhR mRNA was increased in pHBEC treated with DEP after transfection with anti-hsa-miR-375. Our data show that two pollutants, DEP and ambient PM, upregulate TSLP in human bronchial epithelial cells by a mechanism that includes hsa-miR-375 with complex regulatory effects on AhR mRNA. The absence of this pathway in TNF-α-treated cells suggests multiple regulatory pathways for TSLP expression in these cells.
Keywords: TSLP, diesel exhaust particles, particulate matter, bronchial epithelial cells, miR-375, miRNA, Aryl hydrocarbon receptor, lung
Introduction
Epidemiologic and biologic evidence suggest that air pollution, including that derived from vehicular traffic, increases the global burden of respiratory and allergic diseases such as asthma (1–4). Air pollution contributes to acute exacerbations of asthma, as well as to the development of asthma in children and adults (5–8). Mechanisms for both these effects remain incompletely described, but airway epithelial cells, a primary pollutant target that interfaces innate and adaptive immune responses, have been proposed to regulate many of the responses to airborne pollutants (9–11).
Thymic stromal lymphopoietin (TSLP), an IL-7-like cytokine, is considered a pivotal cytokine linking innate and adaptive immune disorders (12–14). TSLP conditions the development of Th2 airway inflammation to innocuous antigen (15–17) and a TSLP soluble antagonist reduces airway inflammation (18). Airway epithelial cell-derived TSLP, including that induced by ambient particulate matter (PM) or diesel exhaust particles (DEP) promotes myeloid dendritic cells (mDC) that support Th2 polarization via upregulation of OX40 ligand and selective notch pathways (19–21). Bronchial epithelial cells are a major source of TSLP (12, 22–25) and in humans, TSLP is increased in asthmatic epithelium (22, 26–28). Variants of TSLP are associated with asthma (29–33) and we have shown association of a gene-environment interaction with a TSLP variant (34). Thus TSLP can be considered a candidate cytokine that bridges environmental exposures with innate and adaptive immune responses in the lung that can lead to allergic diseases such as asthma.
Little is known about the regulation of TLSP in airway epithelial cells. Microbes, viruses including double stranded RNA, and cytokines such as IL-13 and tumor necrosis factor (TNF)-α, upregulate TSLP in a variety of cell types (35, 36). Environmental pollutants, including ambient particulate matter (PM), diesel exhaust particles (DEP) and tobacco smoke, also upregulate TSLP in airway epithelial cells via pathways involving oxidative stress (19, 20, 37). Stimuli converge on nuclear factor (NF)-κB signaling since TSLP is upregulated by activation of nuclear factor (NF)-κB signaling in epithelial cells exposed to poly I:C and Toll-like receptor 3 activation (38, 39) and in double-stranded RNA stimulated keratinocytes (40, 41). Thymic stromal lymphopoietin is also upregulated by the caspase 1 activation of NF-κB in a human mast cell line (42).
MicroRNAs (miRNA) are evolutionarily conserved small non-coding RNA molecules that regulate gene expression. Epithelial cell-expressed miR-375 regulates epithelium-immune cross-talk in the gut by propagating a Th2 response (interleukins 13, 4, 5) via TSLP (43). We therefore hypothesized that TSLP upregulation in human bronchial epithelial cells in response to ambient pollutants would be mediated in part via the human miRNA, hsa-miR-375. We now demonstrate that DEP and ambient fine PM, but not TNF-α upregulated hsa-miR-375 and TSLP in primary culture human bronchial epithelial cells (pHBEC) and suggest that the aryl hydrocarbon receptor (AhR) is an intermediary in this pathway.
Material and Methods
Reagents
DMEM, MEM, penicillin-streptomycin, FBS, trypsin-EDTA solution, and PBS were purchased from GIBCO Life Technologies (Grand Island, NY). Bronchial epithelial cell growth medium (BEGM) and bronchial epithelial cell basal medium (BEBM) were purchased from Lonza (Walkersville, MD). Recombinant TNF-α was from Peprotech (Rocky Hill, NJ).
DEP were derived from a 1.6 l Volkswagen Diesel Engine (40 kW) according to U.S. test protocol FTP 72 (EPA, 1992) and were a kind gift of D.L. Costa (U.S. EPA). Ambient PM samples were collected using a high-volume three-stage impactor (ChemVol model 2400; Rupprecht & Patashnick, Albany, NY) to simultaneously collect PM10–2.5 (coarse PM), PM2.5–0.15 (fine PM), and PM0.15 (ultrafine) at 900 l/min. The 50% cut-off diameter for the well characterized Harvard ChemVol impactor’s fine PM stage is 2.5 μm (44). The coarse and fine PM fractions were collected on different collection substrates (McMaster-Carr, Robbinsville, NJ) and thus further fractionation of particles is not needed. All sampling substrates were pre-cleaned using sterile solutions prior to exposure and extracted after exposure in sterile and pyrogen-free water using sonication. Samples were obtained from Midtown Manhattan (Hunter College, New York, NY). Ambient fine PM and DEP were diluted in cell culture medium, vortexed (5 times, 10 sec), sonicated (1 min) and added to cells in the defined concentrations. Since particles sediment to the bottom of cell culture dishes, DEP and fine PM concentrations were based on the available surface area (μg/cm2). Only fine PM was used for these experiments as DEP are predominantly fine or ultrafine, and fine particles are more likely to bypass filtration mechanisms in the nose. Carbon particles were used as control particles when indicated. Endotoxin content of DEP and PM preparations were measured (PyroGene™ Recombinant Factor C Assay; Lonza; Walkersville, MD). Endotoxin activity in DEP and fine PM (100 μg/ml), a concentration 10 times higher than that used in experiments, was below the lower limit of detection (0.01 EU/ml).
Cells
Primary human bronchial epithelial cells (pHBEC) were obtained from Lonza (Walkersville, MD). All airway epithelial cells were cultured in bronchial epithelial cell basal medium supplemented with human epidermal growth factor (0.5 ng/ml), hydrocortisone (0.5 μg/ml), insulin (5 μg/ml), transferrin (10 μg/ml), epinephrine (0.5 μg/ml), tri-iodothyronine (6.5 ng/ml), gentamicin (50 μg/ml), amphotericin B (50 ng/ml), bovine pituitary extract (52 μg/ml), and retinoic acid (0.1 ng/ml) and plated on 12 well tissue culture plates (50,000 cells/cm2) coated with human collagen (Vitrogen-100; Cohesion Technologies). All studies were performed with cells in passage 2. After reaching confluence, cultures were deprived of hydrocortisone, epinephrine, and retinoic acid for 24 h before exposure studies.
RNA and protein quantification
RNA (including small microRNA) was isolated from HBEC using the miRNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA preparations contained 0.5–1.2 μg/μl RNA and we routinely used 0.5 μg RNA for RT reaction. For quantification of mRNA by real-time PCR, RNA was transcribed using the RT2 First Strand Kit (SABiosciences, Frederick, MD) and quantitative PCR (qPCR) was performed using a SYBR Green/ROX qPCR Master Mix and RT2 qPCR Primer Assay using specific primer (SA Biosciences, Frederick, MD; GAPDH: PPH00150E; AhR: PPH00457E; TSLP: PPH18939A) following the manufacturer’s instructions. Levels of respective transcripts were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript level as an internal control ΔCt(target)=Ct(target)−Ct(GAP-DH). Change in respective transcripts after treatment were assessed by ΔΔCt(target)=ΔCt(target, stimulated)−Ct(target, resting) and presented as fold increase (2−ΔΔCt). Data are expressed after normalizing to GAPDH rather than cell counts.
The expression of microRNAs was assayed using specific RT2 miRNA qPCR Primer Assays (SA Biosciences, Frederick, MD; hsa-miR-148a: MPH01182A; hsa-miR-375: MPH00191A; Human RNU6-2: MPH01653A) and a SYBR Green/ROX qPCR master mix according to the manufacturer’s instructions using reverse transcribed RNA after RT2 miRNA First Strand kit (SABiosciences). The ΔΔCt method for relative quantification using internal control RNU6-2 was used to determine relative expression of hsa-miR-375 and hsa-miR-148a.
Human TSLP protein in cell culture supernatant was measured by commercial ELISA. At confluence during passage 2, pHBEC were deprived of hydrocortisone, epinephrine, and retinoic acid (24 h) before treatment with defined doses of DEP (18h, 37°C). Supernatant was removed and TSLP measured by ELISA (R&D, Minneapolis, MI) according to the manufacturer’s instructions. The lower limit of detection of the ELISA is 3.5 pg/ml.
Transfection of microRNA mimic or inhibitor
For transfection studies, pHBEC were seeded (70% confluence) in vitrogen-coated 24-well plates. After 24 h, cells were transfected with a synthetic miRNA hsa-375 mimic oligonucleotide (mimic-hsa-miR-375; 10 nM; MSY0000728) or a negative control consisting of a scrambled oligonucleotide (syn-miR-control; AllStars Negative Control siRNA; Qiagen, Valencia, CA). Alternatively, for select experiments an miRNA inhibitor (anti-hsa-miR-375; 75 nM; miSCRIPT, MIN0000728 Qiagen, Valencia, CA) or mock inhibitor (anti-miR-control; miSCRIPT Inhibitor Negative Control; Qiagen, Valencia, CA) was used for transfection using HiPerFect transfection Reagent (SA Biosciences, Frederick, MD) according to the manufacturer’s manual. To assess transfection efficiency, control cells were routinely transfected with Silencer FAM-labeled negative control siRNA#1 (Life Technologies, Grand Island, NY; 10 nM and 75 nM for miRNA mimic and miRNA inhibitor experiments, respectively) and fluorescent cells were enumerated by FACS. Transfection efficiencies were 50–65% (data not shown).
Data Analysis
Data are presented as mean ± standard error (SE) of three independent experiments with different donors. Significance was determined by Student’s t test for comparisons of two variables or one-way ANOVA for multiple comparisons, with a p<0.05 considered significant. Page’s L test for trend testing with repeated measures was used for dose response studies.
Results
Expression of hsa-miR-375 and TSLP in HBEC
As expected from our previous studies, DEP and ambient fine PM (fine PM) upregulated TSLP mRNA in a dose-dependent manner (Fig. 1A, Fig. 1B). To assess miRNA intermediates, we focused our studies on hsa-miR-375 based on studies of the murine intestine and TSLP (43). Increasing doses of DEP and fine PM also resulted in a dose-dependent increase in hsa-miR-375 mRNA in pHBEC (Fig. 1A, 1B). In contrast, although hsa-miR-375 was upregulated in pHBEC treated with DEP (3 μg/cm2) or fine PM (3 μg/cm2) neither TNF-α (5 ng/ml), nor carbon particles (3 μg/cm2) upregulated hsa-miR-375 in these cells (Fig. 2).
Figure 1. DEP and ambient fine PM upregulate TSLP and hsa-miR-375 in a dose-dependent manner.
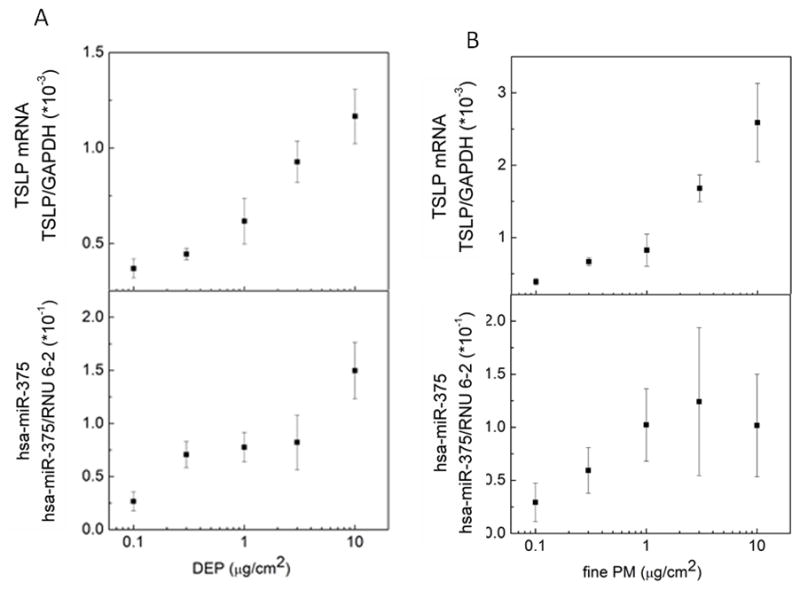
Confluent pHBEC were cultured (6h) with increasing concentrations of DEP or ambient fine PM. RNA was isolated and levels of TSLP mRNA and hsa-miR-375 measured (qPCR). Data are expressed as fold increase compared to GAPDH (TSLP) or RNU 6-2 (hsa-miR-375) (2−ΔΔCt; mean ± SE; n = 3 independent experiments).
Figure 2. DEP and ambient fine PM, but not TNF-α upregulate hsa-miR-375.
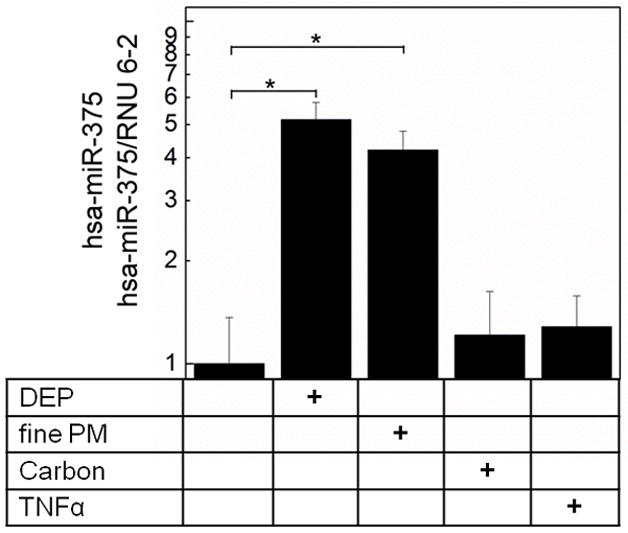
Confluent pHBEC were cultured (6h, 37°C) with DEP (3 μg/cm2), ambient fine PM (3 μg/cm2), carbon (3μg/cm2) or TNF-α (5 ng/ml). RNA was isolated and levels of hsa-miR-375 measured (qPCR). Data are expressed as fold increase compared to resting (2−ΔΔCt; mean ± SE; n = 3 independent experiments; * = p < 0.05).
Hsa-miR-375 regulation of TSLP in pHBEC
To further examine whether hsa-miR-375 upregulation was associated with TSLP mRNA, we transfected pHBEC with a mimic-hsa-miR-375 (syn-hsa-miR-375) or control oligonucleotide (syn-miR-control) (Fig. 3). As previously shown, DEP (3 μg/cm2), fine PM (3 μg/cm2), and TNF-α (5 ng/ml) upregulated TSLP mRNA in pHBEC (Fig. 3). TSLP mRNA was also upregulated in pHBEC transfected with mimic-hsa-miR-375 in the absence of additional stimuli. In contrast, no effect on TSLP mRNA was seen after transfection of pHBEC with syn-miR-control.
Figure 3. Synthetic mimic hsa-miR-375 induced TSLP expression.
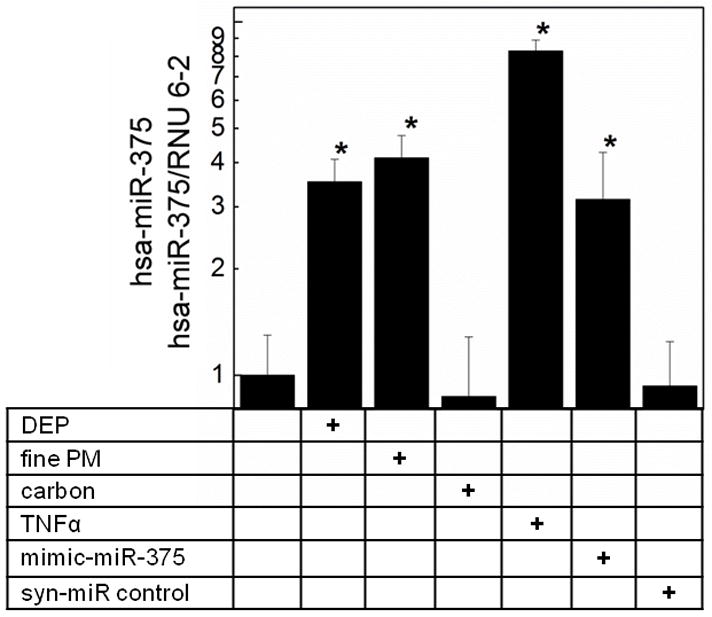
pHBEC were transfected with vehicle alone or with mimic-hsa-miR-375 or syn-miR control oligonucleotides (HiPerFect, 18h). Cells were stimulated as depicted (6h, 37°C), RNA was isolated and TSLP mRNA measured (qPCR, GAPDH internal control). Data are expressed as fold increase compared to resting vehicle control (2−ΔΔCt; mean ± SE; n = 3 independent experiments; * = p <0.05).
To determine whether hsa-miR-375 was necessary for upregulation of TSLP in pHBEC, we transfected pHBEC with anti-hsa-miR-375 before stimulation of pHBEC with defined agents. As shown in Fig. 4, TSLP mRNA was reduced in DEP-treated pHBEC transfected with anti-hsa-miR-375 compared to pHBEC treated with DEP alone or DEP-treated pHBEC transfected with anti-miR control. In contrast, transfection of pHBEC with anti-hsa-miR-375 did not inhibit TNF-α upregulation of TSLP mRNA. These data suggest that hsa-miR-375 was necessary for TSLP upregulated by DEP but not by TNF- α and suggest the potential for multiple pathways for TSLP regulation.
Figure 4. Upregulation of TSLP by DEP is dependent on hsa-miR-375.
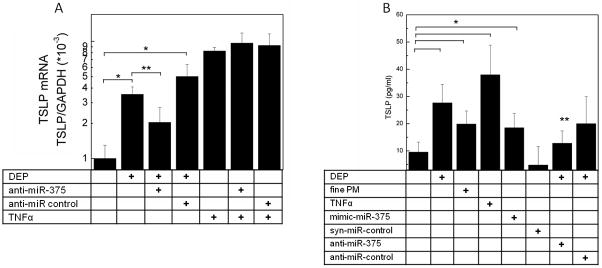
(4A)TSLP mRNA expression in pHBEC transfected with vehicle, anti-hsa-miR-375, or anti-miR control oligonucleotides (HiPerFect, 18h, 37°C). Cells were stimulated with defined agents (6h, 37°C), RNA isolated and TSLP mRNA measured (qPCR, GAPDH internal control). Data are expressed as fold increase compared to resting vehicle control (2−ΔΔCt; mean ± SE; n = 3 independent experiments; * = p < 0.05 vs. resting, ** = p < 0.05 vs. DEP). (4B) TSLP protein was measured (ELISA) in pHBEC supernates after treatment of pHBEC with defined stimuli (18h, 37°C) in cells transfected with mimic-hsa-miR-375, syn-miR control, anti-hsa-miR-375 or anti-miR control. Data are mean ± SEM (n = 3 independent experiments, * = p <0.05 vs. resting, ** = p < 0.05 vs. DEP.
To confirm whether hsa-miR-375 regulated TSLP protein released by pHBEC as well as TSLP mRNA, cells were cultured as described, supernatants removed after 18 h and TSLP protein measured by ELISA. As shown in Fig. 4B, TSLP protein was upregulated by DEP and fine PM, as well as by TNF-α. Consistent with mRNA measurements, TSLP protein was significantly increased in pHBEC transfected with mimic hsa-miR-375 but not control oligonucleotide. Conversely, the upregulation of TSLP protein by DEP was diminished in pHBEC transfected with anti-hsa-miR-375.
Aryl hydrocarbon and hsa-miR-375
The treatment of pHBEC with DEP or fine PM increased hsa-miR-375 as well as TSLP, suggesting the presence of an intermediary signal that might be downregulated by hsa-miR-375. In silico pathway evaluation (TargetScan 5.1 (April 2009) suggested the aryl hydrocarbon receptor (AhR) as one possible target of miR-375 thus predicting that upregulation of miR-375 would result in decreased AhR transcription. We therefore examined AhR mRNA and function in pHBEC exposed to defined stimuli. Increasing DEP and fine PM at doses shown to upregulate TSLP, suggested downregulation of AhR mRNA in a dose dependent manner (data not shown). In separate studies, DEP and fine PM, at doses used for TSLP mRNA studies (3 μg/cm2,6h), reduced AhR mRNA (n = 3, p < 0.05; Fig. 5), whereas carbon particles had no effect. TNF-α increased AhR mRNA (Fig. 5).
Figure 5. DEP and ambient fine PM down-regulate AhR expression.
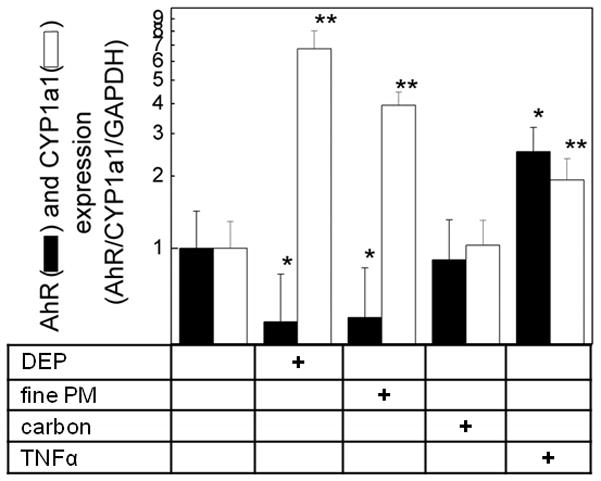
pHBEC were cultured (6h, 37°C) with DEP (3 μg/cm2), ambient fine PM (3 μg/cm2), carbon (3 μg/cm2) or TNF-α (5 ng/ml). RNA was isolated and levels of AhR and CYP1a1 measured (qPCR, GAPDH internal control). Data are expressed as fold change compared to resting (2−ΔΔCt; mean ± SE; n = 3 independent experiments; * = p<0.05 compared to resting AhR; ** = p<0.05 compared to resting CYP1a1).
To confirm functionality of the AhR and its response to DEP and fine PM, we measured the regulation of the aryl hydrocarbon hydroxylase cytochrome P450 CYP1a1, a downstream target gene. In data consistent with published literature (45), treatment of pHBEC with DEP or fine PM in the defined doses, upregulated CYP1a1 mRNA (6 h, n = 3, p < 0.05; Fig. 5). Treatment of pHBEC with carbon particles failed to change CYP1A1 mRNA levels. These data were consistent with the well-published effect of DEP and ambient particles on AhR function as measured by downstream AhR products.
To confirm an effect of hsa-miR-375 on AhR, we examined the effect of the mimic hsa-miR-375 on AhR mRNA (Fig. 6). Transfection of mimic hsa-miR-375 resulted in a small but significant downregulation of AhR mRNA compared to resting levels in pHBEC. Whereas AhR mRNA was decreased in pHBEC treated with DEP, AhR mRNA was increased in pHBEC that were treated with DEP after transfection with anti-hsa-miR-375. No change in AhR mRNA was noted between DEP treated cells and those treated with DEP and transfected with anti-miR control. These data supported the link between hsa-miR-375 and AhR mRNA expression in cells treated with an ambient pollutant. Furthermore, they suggested an indirect relationship between AhR mRNA and its effects on xenobiotic metabolizing enzymes.
Figure 6. AhR expression is regulated by hsa-miR-375.
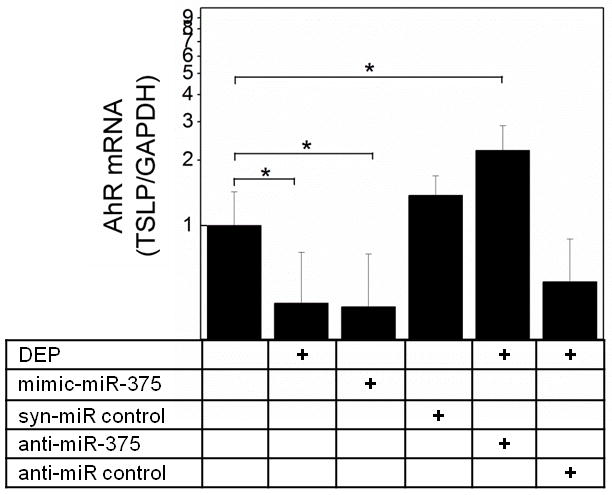
pHBEC were transfected with vehicle or with synthetic oligonucleotides (mimic hsa-miR-375, syn-hsa-miR control, anti- hsa-miR-375, anti- hsa-miR control). After 18h, cells were stimulated with DEP as depicted (6h, 37°C), RNA isolated and AhR mRNA measured (qPCR, GAPDH internal control). Data are expressed as fold change compared to resting vehicle control (2−ΔΔCt; mean ± SE; n=3 independent experiments; * = p < 0.05).
The regulatory effects of miRNA species are complex, and miRNA targets the 3′ UTR or less frequently the 5′ UTR of target mRNA (46). Target mRNA levels are subsequently reduced via several processes including transcript degradation, inhibition of translation, or mRNA decay (47). To determine whether hsa-miR-375 resulted in increased TSLP or AhR mRNA degradation, pHBEC were treated with actinomycin D (10 μg/ml) and mRNA levels measured by qPCR. As shown (Fig. 7A) no difference in TSLP mRNA degradation was noted between resting pHBEC, pHBEC transfected with mimic hsa-miR-375, or DEP-treated pHBEC in the absence or presence of anti-hsa-miR-375. In contrast, AhR mRNA degradation was increased in pHBEC transfected with mimic hsa-miR-375 compared to resting pHBEC, or those treated with DEP in the absence or presence of anti-hsa-miR-375. These data support the regulatory role of hsa-miR-375 on AhR mRNA levels.
Figure 7. TSLP and AhR mRNA degradation.
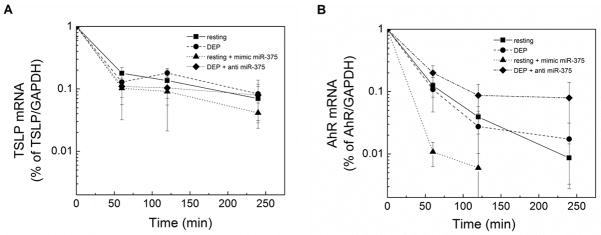
pHBEC were resting or transfected with mimic-hsa-miR-375 or anti- hsa-miR control and were treated with actinomycin D (10 μg/ml) in either the presence or absence of DEP (3 μg/cm2). Total RNA was isolated after 0, 1, 2, and 4 hours, and TSLP, AhR and GAPDH mRNA determined by qPCR. Data are presented as the percentage of mRNA relative to 0 time point after normalization to GAPDH (mean ± SE, n = 3 independent experiments).
Discussion
Thymic stromal lymphopoietin is a critical link between environmental pollutants, innate immunity and the adaptive Th2 response. Thus understanding TSLP regulation has potential to provide insight into manipulation of the Th2 response and to further our understanding mechanisms by which pollutants modify this response. Although activation of NF-kB upregulates TSLP in response to numerous stimuli, there is a large knowledge gap about additional pathways that contribute to the regulation of TSLP, particularly those induced by ambient pollutants. We now suggest that DEP and ambient fine PM regulate TSLP expression in part via an effect on hsa-miR-375, and propose that this regulation is mediated via an intermediate signal involving the AhR. These findings provide a novel mechanism for TSLP regulation in human bronchial epithelial cells.
DEP regulate numerous miRNA species (48). We focused on hsa-miR-375 because of its role in intestinal immunity (43). We now show upregulation of hsa-miR-375 by two ambient pollutants: DEP and ambient fine PM, and have previously shown that both these pollutants stimulate TSLP mRNA and protein and promote a Th2 response (19, 20). We also demonstrated that synthetic miR-375 upregulated TSLP mRNA and protein, whereas an anti-hsa-miR-375 oligonucleotide reduced TSLP. Inhibition of TSLP by anti-hsa-miR-375 although significant, was incomplete, most likely secondary to incomplete transfection efficiency of the oligonucleotides. In contrast to the increase in both TSLP and hsa-miR-375 in pHBEC after DEP or fine PM-treatment, TNF-α upregulated TSLP mRNA but not hsa-miR-375. Transfection of anti-hsa-miR-375 also failed to downregulate TSLP mRNA after TNF-α. These data are consistent with a role for hsa-miR-375 in the regulation of TSLP by ambient pollutants, but suggest the presence of additional regulatory pathways for other stimuli.
MicroRNA-375 has been extensively studied in malignant pathways leading to neoplastic transformation and miR-375 has been considered a putative tumor suppressor for numerous cancers (49). However, expression of miR-375 is also involved in metabolic pathways including glucose homeostasis (50) as well as in the regulation of murine mucosal immunity via TSLP expression (43) and in peripheral blood lymphocytes of the IL-10−/− mouse (51). Our studies now support a role for hsa-miR-375 in Th2 immune regulation in the lung, and its regulation by exogenous pollutants.
MicroRNA are short ribonucleic acid molecules whose binding results in gene silencing, yet we detected a concomitant increase in both TSLP mRNA and hsa-miR-375 in our studies. We therefore reasoned that the effects of miR-375 were mediated via an intermediary, and based on our in silico analysis, suggested the AhR as a target molecule. The AhR is a Per-Arnt-Sim domain containing conserved transcription factor that controls adaptation to environmental challenges. The AhR controls transcriptional responses to xenobiotic compounds including dioxins and polycyclic aromatic hydrocarbons via transcription of xenobiotic-metabolizing enzymes such as cytochrome P450 family members (CYP1A1, CYP1B1) (52). Recent studies suggest that the AhR is also an endogenous receptor that mediates additional physiologic functions. These include organ development and mucin production in epithelial cells (53) as well as immune functions (54). Aryl hydrocarbon receptor null mice overproduce transforming growth factor receptors in the liver (55) and overproduce IL-12 and interferon γ in ovalbumin-sensitized and challenged mice (56). Immune regulation and maintenance and expansion of innate lymphoid cells in the murine intestine requires expression of the AhR (57) and activation of the AhR also regulates ligand-specific and cell specific Treg and Th17 differentiation in murine models (58–60). Our study now suggests an additional role in immune regulation in the lung, mediated through TSLP expression in human airway epithelial cells.
Treatment of pHBEC with fine PM or DEP increased AhR target gene expression (CYP1A1). However, as predicted by our in silico analysis, we showed hsa-miR-375-dependent downregulation of AhR mRNA in response to fine PM and DEP. We confirmed a role for miR-375 in AhR mRNA regulation in HBEC with the use of transfected mimic hsa-miR-375 and anti-hsa-miR-375 and studies of AhR mRNA degradation. The disassociation of AhR activity and mRNA expression suggests a complex interplay of hsa-miR-375, AhR mRNA transcription and AhR targets. Disassociation of AhR expression and AhR target genes has been shown in other cells (45, 61). In the cytoplasm, the AhR is complexed to chaperonins (52) and ligand binding elicits nuclear localization where the Ahr –Arnt (aryl hydrocarbon receptor nuclear translocator) binds to an enhancer sequence (dioxin response element; DRE), and elicits transcription of DRE containing target genes including CYP1A1 and CYP2A2 (52). Our finding of downregulation of AhR mRNA by ambient pollutants and mimic hsa-miR-375, but increased AhR activity with xenobiotic target gene expression reinforce the complexity of AhR signaling.
Recent studies suggest that in addition to xenobiotic metabolizing enzymes, the AhR regulates inflammation and immune functions via NF-κB (62). Mice deficient in the AhR (AhR knockout;KO mice) have elevated pro-inflammatory cytokines in their lungs (63). Cigarette smoke exposed AhR null mice have increased NF-kB binding activity compared to wild-type mice, and have rapid loss of RelB consistent with premature degradation (63). The suggestion has been made that RelB inhibits inflammation via complex regulation of NF-κB pathway (64–67) and that AhR promotes loss of RelB protein, and thus activation of NF-κB by a non canonical pathway (63, 68, 69). These studies suggest an intriguing and potential mechanism by which hsa-miR-375 downregulation of the AhR might be one of multiple pathways leading to activation of NF-κB and thus upregulation of TSLP.
There are some potential limitations to these studies. We used human bronchial epithelial cells from multiple donors in primary culture. However, we used these cells in in vitro systems and the possibility exists that in vivo effects may differ. Future animal studies may help confirm these responses. We used primary cells for transfection and could not accomplish complete transfection of our cells. Despite incomplete transfection, we were able to identify effects of both our mimic and anti-miR-375, but not off-target effects of control oligonucleotides. We used two types of pollution particles for these studies as representative particles. These included diesel exhaust and ambient fine PM as respirable real-world particles (70). The possibility exists that other particles might induce different responses in the epithelial cells and we have not characterized the chemical components such as the organic or metal constituents that might contribute to the defined effects (71).
In summary, these studies suggest that two pollutants, DEP and ambient PM, upregulate TSLP in human bronchial epithelial cells by a mechanism that includes hsa-miR-375 with complex regulatory effects on AhR mRNA. The absence of this pathway in TNF-α-treated cells suggests multiple regulatory pathways for TSLP expression in these cells. The possibility exists that all these pathways converge on NF-κB. Identifying specific pathways regulating TSLP expression in the airway that lie upstream of NF-κB signaling would open up highly interesting possibilities for intervention strategies targeting TSLP regulation in human disease. These studies provide novel information that furthers our understanding of the complex regulation of innate immune responses.
Footnotes
This work was supported by National Institutes of Environmental Health Sciences grants Grants RO1 ES010187 (JR), T32 ES007267 (JR); EPA RD-83374201 (TG), 1R21HL092370-01 (GG), 1R01 HL095764-01 (GG), ES-000260 (ML)
References
- 1.Laumbach RJ, Kipen HM. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol. 2012;129:3–11. doi: 10.1016/j.jaci.2011.11.021. quiz 12–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riedl MA. The effect of air pollution on asthma and allergy. Curr Allergy Asthma Rep. 2008;8:139–146. doi: 10.1007/s11882-008-0024-8. [DOI] [PubMed] [Google Scholar]
- 3.Saxon A, Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat Immunol. 2005;6:223–226. doi: 10.1038/ni0305-223. [DOI] [PubMed] [Google Scholar]
- 4.Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 5.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 6.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J, Avol E, Kunzli N, Yao L, Peters J, Berhane K. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118:1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, Peters J. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunzli N, Bridevaux PO, Liu LJ, Garcia-Esteban R, Schindler C, Gerbase MW, Sunyer J, Keidel D, Rochat T. Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. 2009;64:664–670. doi: 10.1136/thx.2008.110031. [DOI] [PubMed] [Google Scholar]
- 9.Hogg JC, van Eeden S. Pulmonary and systemic response to atmospheric pollution. Respirology. 2009;14:336–346. doi: 10.1111/j.1440-1843.2009.01497.x. [DOI] [PubMed] [Google Scholar]
- 10.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 11.Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci (Lond) 2010;118:439–450. doi: 10.1042/CS20090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt W, de R, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 13.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol. 2009;101:1–25. doi: 10.1016/S0065-2776(08)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soumelis V. TSLP: from allergy to vaccine adjuvant. Eur J Immunol. 2012;42:293–295. doi: 10.1002/eji.201142337. [DOI] [PubMed] [Google Scholar]
- 15.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Huang G, Hu B, Song Y, Shi Y. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol. 2011;164:256–264. doi: 10.1111/j.1365-2249.2011.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol. 2008;28:147–156. doi: 10.1007/s10875-007-9149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185:6636–6645. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, Bullens SL, Seeber S, Fuentes ME, Labrijn AF, Graus YM, Miller LA, Schelegle ES, Hyde DM, Wu LC, Hymowitz SG, Martin F. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, Shelley M, Abbas AR, Austin CD, Jackman J, Wu LC, Heaney LG, Arron JR, Bradding P. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–111. e101–109. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 24.Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, Miyatake A, Enomoto T, Noguchi E, Yoshihara S, Ebisawa M, Saito H, Matsumoto K, Nakamura Y, Ziegler SF, Tamari M. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 25.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 27.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, Zamar D, Bosse Y, Kozyrskyj AL, James A, Laprise C, Daley D. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124:222–229. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias RA, Hancock DB, Baurley JW, Eng C, Stern DA, Celedon JC, Rafaels N, Capurso D, Conti DV, Roth LA, Soto-Quiros M, Togias A, Li X, Myers RA, Romieu I, Berg DJ, Hu D, Hansel NN, Hernandez RD, Israel E, Salam MT, Galanter J, Avila PC, Avila L, Rodriquez-Santana JR, Chapela R, Rodriguez-Cintron W, Diette GB, Adkinson NF, Abel RA, Ross KD, Shi M, Faruque MU, Dunston GM, Watson HR, Mantese VJ, Ezurum SC, Liang L, Ruczinski I, Ford JG, Huntsman S, Chung KF, Vora H, Calhoun WJ, Castro M, Sienra-Monge JJ, Del Rio-Navarro B, Deichmann KA, Heinzmann A, Wenzel SE, Busse WW, Gern JE, Lemanske RF, Jr, Beaty TH, Bleecker ER, Raby BA, Meyers DA, London SJ, Gilliland FD, Burchard EG, Martinez FD, Weiss ST, Williams LK, Barnes KC, Ober C, Nicolae DL. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;34:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, Avila L, Liang C, Lake SL, Hudson TJ, Spesny M, Fournier E, Sylvia JS, Freimer NB, Klanderman BJ, Raby BA, Celedon JC. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177:830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, Fujita K, Miyatake A, Enomoto T, Miyagawa T, Adachi M, Tanaka H, Niimi A, Matsumoto H, Ito I, Masuko H, Sakamoto T, Hizawa N, Taniguchi M, Lima JJ, Irvin CG, Peters SP, Himes BE, Litonjua AA, Tantisira KG, Weiss ST, Kamatani N, Nakamura Y, Tamari M. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43:893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffatt MF, I, Gut G, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Rogers L, Cheng Q, Shao Y, Fernandez-Beros ME, Hirschhorn JN, Lyon HN, Gajdos ZK, Vedantam S, Gregersen P, Seldin MF, Bleck B, Ramasamy A, Hartikainen AL, Jarvelin MR, Kuokkanen M, Laitinen T, Eriksson J, Lehtimaki T, Raitakari OT, Reibman J. Genetic variants of TSLP and asthma in an admixed urban population. PLoS One. 2011;6:e25099. doi: 10.1371/journal.pone.0025099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H, Ziegler SF. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91:877–886. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H, Nakao A. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122:1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3-and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidl B, Kalali B, Gerhard M, Ring J, Ollert M, Mempel M. Thymic stromal lymphopoietin induction by polyinosinic:polycytidylic acid in human keratinocytes is preferentially mediated through protein kinase R and retinoid-inducible gene I and not Toll-like receptor 3. J Allergy Clin Immunol. 2009;124:862–864. doi: 10.1016/j.jaci.2009.07.028. author reply 864–865. [DOI] [PubMed] [Google Scholar]
- 41.Vu AT, Chen X, Xie Y, Kamijo S, Ushio H, Kawasaki J, Hara M, Ikeda S, Okumura K, Ogawa H, Takai T. Extracellular double-stranded RNA induces TSLP via an endosomal acidification-and NF-kappaB-dependent pathway in human keratinocytes. J Invest Dermatol. 2011;131:2205–2212. doi: 10.1038/jid.2011.185. [DOI] [PubMed] [Google Scholar]
- 42.Moon PD, Kim HM. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-kappaB pathway in mast cells. Cytokine. 2011;54:239–243. doi: 10.1016/j.cyto.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H, Kredo-Russo S, Avnit-Sagi T, Cojocaru G, Zreik F, Bentwich Z, Poy MN, Artis D, Walker MD, Hornstein E, Pikarsky E, Ben-Neriah Y. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat Immunol. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 44.Demokritou P, I, Kavouras G, Harrison D, Koutrakis P. Development and evaluation of an impactor for a PM2.5 speciation sampler. J Air Waste Manag Assoc. 2001;51:514–523. doi: 10.1080/10473289.2001.10464296. [DOI] [PubMed] [Google Scholar]
- 45.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 46.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci U S A. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enfield KS, Pikor LA, Martinez VD, Lam WL. Mechanistic Roles of Noncoding RNAs in Lung Cancer Biology and Their Clinical Implications. Genet Res Int. 2012;2012:737416. doi: 10.1155/2012/737416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jardim MJ, Fry RC, Jaspers I, Dailey L, Diaz-Sanchez D. Disruption of microRNA expression in human airway cells by diesel exhaust particles is linked to tumorigenesis-associated pathways. Environ Health Perspect. 2009;117:1745–1751. doi: 10.1289/ehp.0900756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basu A, Alder H, Khiyami A, Leahy P, Croce CM, Haldar S. MicroRNA-375 and MicroRNA-221: Potential Noncoding RNAs Associated with Antiproliferative Activity of Benzyl Isothiocyanate in Pancreatic Cancer. Genes Cancer. 2011;2:108–119. doi: 10.1177/1947601911409212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha-and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaefer JS, Montufar-Solis D, Vigneswaran N, Klein JR. Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10−/− mice precedes expression in the colon. J Immunol. 2011;187:5834–5841. doi: 10.4049/jimmunol.1100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–645. doi: 10.1146/annurev-physiol-021909-135922. [DOI] [PubMed] [Google Scholar]
- 53.Chiba T, Uchi H, Tsuji G, Gondo H, Moroi Y, Furue M. Arylhydrocarbon receptor (AhR) activation in airway epithelial cells induces MUC5AC via reactive oxygen species (ROS) production. Pulm Pharmacol Ther. 2011;24:133–140. doi: 10.1016/j.pupt.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andreola F, Calvisi DF, Elizondo G, Jakowlew SB, Mariano J, Gonzalez FJ, De Luca LM. Reversal of liver fibrosis in aryl hydrocarbon receptor null mice by dietary vitamin A depletion. Hepatology. 2004;39:157–166. doi: 10.1002/hep.20004. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Sosa M, Elizondo G, Lopez-Duran RM, Rivera I, Gonzalez FJ, Vega L. Over-production of IFN-gamma and IL-12 in AhR-null mice. FEBS Lett. 2005;579:6403–6410. doi: 10.1016/j.febslet.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 57.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 58.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 59.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dohr O, Sinning R, Vogel C, Munzel P, Abel J. Effect of transforming growth factor-beta1 on expression of aryl hydrocarbon receptor and genes of Ah gene battery: clues for independent down-regulation in A549 cells. Mol Pharmacol. 1997;51:703–710. doi: 10.1124/mol.51.5.703. [DOI] [PubMed] [Google Scholar]
- 62.Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77:734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- 65.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Yoza BK, El Gazzar M, Hu JY, Cousart SL, McCall CE. RelB sustains IkappaBalpha expression during endotoxin tolerance. Clin Vaccine Immunol. 2009;16:104–110. doi: 10.1128/CVI.00320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011;90:439–446. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J Biol Chem. 2008;283:28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP. Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke-induced inflammation. Am J Pathol. 2011;179:125–133. doi: 10.1016/j.ajpath.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanek LW, Brown JS, Stanek J, Gift J, Costa DL. Air pollution toxicology--a brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol Sci. 2011;120(Suppl 1):S8–27. doi: 10.1093/toxsci/kfq367. [DOI] [PubMed] [Google Scholar]
- 71.Schwarze PE, Ovrevik J, Hetland RB, Becher R, Cassee FR, Lag M, Lovik M, Dybing E, Refsnes M. Importance of size and composition of particles for effects on cells in vitro. Inhal Toxicol. 2007;19(Suppl 1):17–22. doi: 10.1080/08958370701490445. [DOI] [PubMed] [Google Scholar]


