Abstract
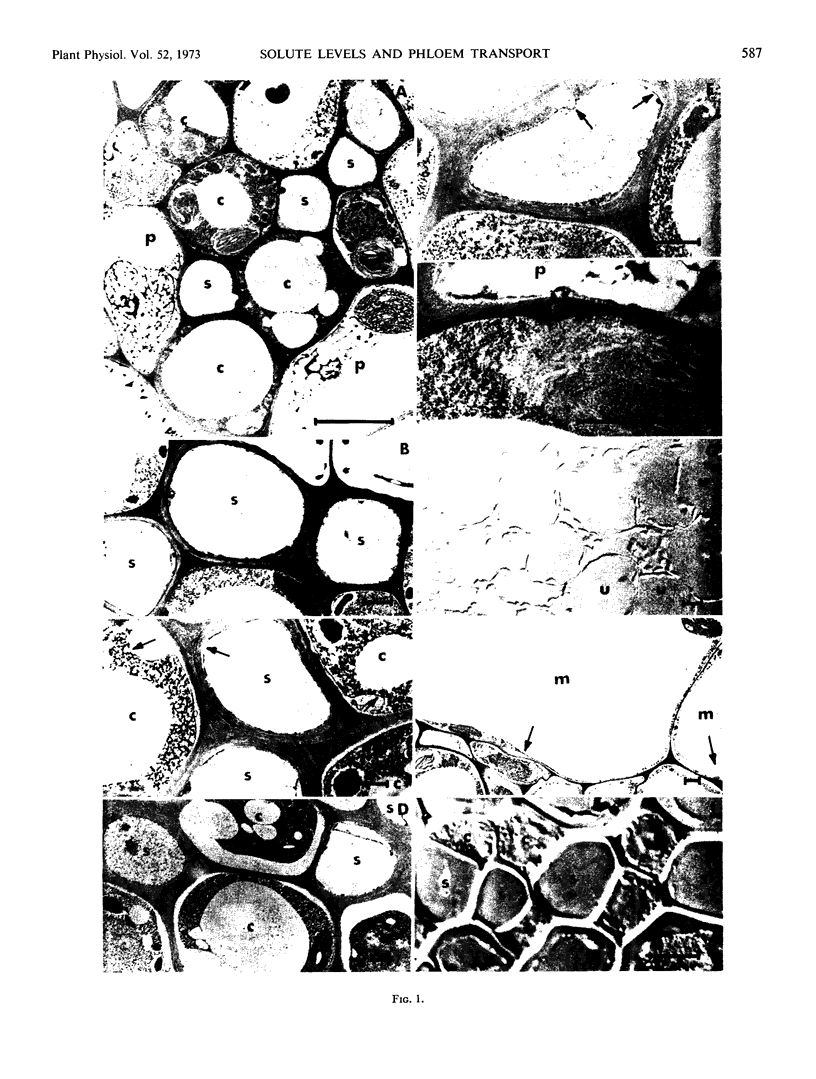
The distribution of solutes in the various cells of sugar beet (Beta vulgaris L.) source leaves, petioles, and sink leaves was studied in tissue prepared by freeze-substitution. The differences in degree of cryoprotection indicated that sieve elements and companion cells of the source leaf, petiole, and sink leaf contain a high concentration of solute. The osmotic pressure of various types of cells was measured by observing incipient plasmolysis in freeze-substituted tissues equilibrated with a series of mannitol solutions prior to rapid freezing. Analysis of source leaf tissue revealed osmotic pressure values of 13 bars for the mesophyll and 30 bars for the sieve elements and companion cells. The osmotic pressure of the mesophyll of sink leaves was somewhat higher.
The sharp concentration increase at the membrane of the sieve element-companion cell complex of the source leaf indicates active phloem loading from the free space at this site. Active loading of the phloem is presumably needed to move the sugar from the chloroplasts of the mesophyll to the sieve tubes against the concentration gradient. The osmotic pressure of the mature sieve element-companion cell complex appears to be approximately the same in source leaf, path, and sink leaf tissue. There is a distinct difference in concentration between the mature sieve element-companion cell complex in the sink and the surrounding mesophyll. The solute distribution suggests that sugar is actively accumulated from the free space by the developing sink leaf tissue.
The osmotic values observed in the various cells are consistent with the operation of a mass flow mechanism of translocation driven by active phloem loading and by active accumulation of sugar by sink tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrier G. E., Loomis W. E. Absorption and Translocation of 2,4-Dichlorophenoxyacetic Acid and P by Leaves. Plant Physiol. 1957 May;32(3):225–231. doi: 10.1104/pp.32.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebbler G. F. Cryoprotective compounds. Review and discussion of structure and function. Cryobiology. 1966 Jul-Aug;3(1):2–11. doi: 10.1016/s0011-2240(66)80144-x. [DOI] [PubMed] [Google Scholar]
- Fisher D. B. Artifacts in the Embedment of Water-soluble Compounds for Light Microscopy. Plant Physiol. 1972 Feb;49(2):161–165. doi: 10.1104/pp.49.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Cataldo D. A. Leaf structure and translocation in sugar beet. Plant Physiol. 1969 Jan;44(1):45–54. doi: 10.1104/pp.44.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Evaluation of Selected Parameters in a Sugar Beet Translocation System. Plant Physiol. 1965 Sep;40(5):942–947. doi: 10.1104/pp.40.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Sucrose Translocation in the Sugar Beet. Plant Physiol. 1965 Jul;40(4):685–690. doi: 10.1104/pp.40.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Geiger D. R. Mechanism of inhibition of translocation by localized chilling. Plant Physiol. 1973 Feb;51(2):372–377. doi: 10.1104/pp.51.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning B. E., Pate J. S., Briarty L. G. Specialized "transfer cells" in minor veins of leaves and their possible significance in phloem translocation. J Cell Biol. 1968 Jun;37(3):C7–12. doi: 10.1083/jcb.37.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel H. T. Measurement of turgor pressure and its gradient in the Phloem of oak. Plant Physiol. 1968 Jul;43(7):1042–1048. doi: 10.1104/pp.43.7.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köcher H., Leonard O. A. Translocation and Metabolic Conversion of C-Labeled Assimilates in Detached and Attached Leaves of Phaseolus vulgaris L. in Different Phases of Leaf Expansion. Plant Physiol. 1971 Feb;47(2):212–216. doi: 10.1104/pp.47.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E. Solute potentials of sucrose solutions. Plant Physiol. 1972 Jul;50(1):196–198. doi: 10.1104/pp.50.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trip P. Sugar transport in conducting elements of sugar beet leaves. Plant Physiol. 1969 May;44(5):717–725. doi: 10.1104/pp.44.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T. The symplast concept. A general theory of symplastic transport according to the thermodynamics of irreversible processes. J Theor Biol. 1970 Feb;26(2):181–214. doi: 10.1016/s0022-5193(70)80012-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. H. Translocation of Organic Substances in Trees. III. The Removal of Sugars from the Sieve Tubes in the White Ash (Fraxinus Americana L.). Plant Physiol. 1958 May;33(3):213–217. doi: 10.1104/pp.33.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]



