Abstract
The retina specific G protein-coupled receptor kinases, GRK1 and GRK7, have been implicated in the shutoff of the photoresponse and adaptation to changing light conditions via rod and cone opsin phosphorylation. Recently, we have defined sites of phosphorylation by cAMP-dependent protein kinase (PKA) in the amino termini of both GRK1 and GRK7 in vitro. To determine the conditions under which GRK7 is phosphorylated in vivo, we have generated an antibody that recognizes GRK7 phosphorylated on Ser-36, the PKA phosphorylation site. Using this phospho-specific antibody, we have shown that GRK7 is phosphorylated in vivo and is located in the cone inner and outer segments of mammalian, amphibian and fish retinas. Using Xenopus laevis as a model, GRK7 is phosphorylated under dark-adapted conditions, but becomes dephosphorylated when the animals are exposed to light. The conservation of phosphorylation at Ser-36 in GRK7 in these different species (which span a 400 million-year evolutionary period), and its light-dependent regulation, indicate that phosphorylation plays an important role in the function of GRK7. Our work demonstrates for the first time that cAMP can regulate proteins involved in the photoresponse in cones and introduces a novel mode of regulation for the retinal GRKs by PKA.
Keywords: PKA, cAMP, GRK7, retina, cone, photoreceptor
Rhodopsin kinase (GRK1) and GRK7 are retina-specific G protein-coupled receptor kinases that have been implicated in the termination of the photoresponse in cones (Cideciyan et al. 2003, Liu et al. 2005, Rinner et al. 2005, Tachibanaki et al. 2005, Wada et al. 2006). GRK7 is expressed in all vertebrate cones so far examined, with the exception of mice and rats, which do not have the gene for GRK7 (Hisatomi et al. 1998, Weiss et al. 1998, Chen et al. 2001, Weiss et al. 2001). In contrast, GRK1 is expressed in the rods of all vertebrates but is also expressed in some vertebrate cones. Examples of the heterogeneity of GRK expression in cones include mice and rats, which only express GRK1, pigs and dogs, which only express GRK7, and a number of vertebrates where GRK1 and GRK7 are coexpressed in cones, such as zebrafish, carp (Rinner et al. 2005, Shimauchi-Matsukawa et al. 2005, Wada et al. 2006), monkeys and humans (Chen et al. 2001, Weiss et al. 2001, Maeda et al. 2003). This variability in expression could result in differences in visual properties that may be attributed to evolutionary adaptation to different environmental lighting. Electroretinogram (ERG) analysis comparing patients with Oguchi disease, whose retinas lack GRK1, and patients with Enhanced S Cone syndrome, whose cones lack either GRK7 or both GRK1 and GRK7, indicate that both kinases may contribute to deactivation of the photoresponse in human cones (Cideciyan et al. 2003). This introduces the interesting question of whether these 2 kinases have similar or distinct modes of regulation that control their activities under different light conditions. Our laboratory has proposed a novel mechanism for regulating GRK1 and GRK7 activity via phosphorylation by cAMP-dependent protein kinase (PKA) (Horner et al. 2005). GRK1 and GRK7 are phosphorylated by PKA in vitro on Ser-21 and Ser-36, respectively, which results in reduced activity in vitro (Horner et al. 2005). Ser-36 is conserved in all GRK7 orthologs from fish to mammals, suggesting that it plays an important role in the function of this kinase. Since it is known that cAMP levels in vertebrate photoreceptor cells are high in the dark and decline in the light (Farber et al. 1981, Cohen & Blazynski 1990, Weiss et al. 1995, Traverso et al. 2002), phosphorylation by PKA may mediate the control of GRK7 activity by light.
In the present report, we use a phosphospecific antibody to define the presence of phosphorylated GRK7 in vivo in mammalian, amphibian and fish cones. In living animals, GRK7 is phosphorylated within approximately 60 min of dark-adaptation and dephosphorylated within 60 min of light-adaptation. Increased phosphorylation in the dark can be mimicked in retinas incubated ex vivo with reagents that increase cAMP, demonstrating that this cyclic nucleotide can regulate the phosphorylation of phototransduction-associated proteins in cones. These data reveal the existence of a previously unknown and potentially important mechanism for the regulation of GRK7.
Materials and Methods
Antibodies
A polyclonal antibody directed against Xenopus laevis GRK7, anti-XGRK7, was raised in rabbits against the synthetic peptide, CLNDPSREATGGGGNSG, which contains an amino-terminal cysteine for conjugation to keyhole limpet hemocyanin (KLH) followed by amino acids corresponding to 527–541 of X. laevis GRK7 (Bethyl Laboratories Inc., Montgomery, TX). A polyclonal antibody that recognizes GRK7 phosphorylated at Ser-36 (anti-pGRK7) was also generated in rabbits by Bethyl Laboratories using the sequence, CQRRRR(pS)LMLP, which contains an amino-terminal cysteine for conjugation to KLH, followed by a sequence corresponding to amino acids 31–40 of pig and bovine GRK7. The phosphopeptide and nonphosphopeptide corresponding to this sequence were also obtained from Bethyl Laboratories. The anti-Flag antibody was purchased from Sigma (St. Louis, MO). For experiments described in Fig. 6, the anti-XGRK7 and anti-pGRK7 antibodies were conjugated directly to the fluorophores Alexafluor-488 and Alexafluor-555, respectively (Invitrogen, Carlsbad, CA).
Fig. 6. Phosphorylated GRK7 in cones of dark- and light-adapted Xenopus frogs.
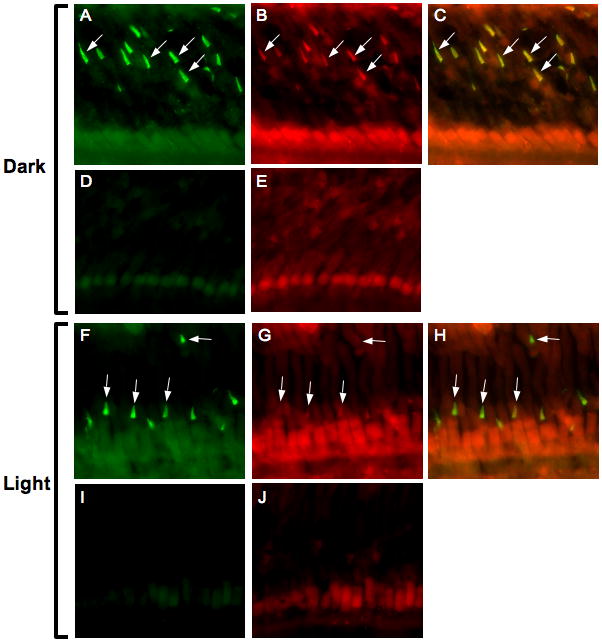
Frogs were dark-adapted overnight, then euthanized and the eyes removed under infrared illumination (A–E) or light-adapted for 3 hours before euthanasia and removal of the eyes (F–J). Retinas were stained with anti-XGRK7 (1:200; A, F), or anti-pGRK7 (1:250; B, G) directly conjugated to Alexa Fluor 488 or 555, respectively. Nonspecific antibodies were used at equivalent concentrations to visualize background staining (D, E and I, J). Figs. C and H represent the merging of images A and B or F and G, respectively. White arrows indicate a few examples of cone outer segments, where most of the GRK7 appears to be localized.
Cloning of Xenopus laevis GRK7
The primers, GCCATGTGTGACATGGGAGGACTAGA and CATCTAAAGAATAGAGCAAACCCCTGACTT, were synthesized based on the sequence of the 5′ and 3′ ends of the coding regions, respectively, of Xenopus tropicalis GRK7, derived from the Department of Energy Joint Genome Institute database (http://www.jgi.doe.gov/). These primers were used to amplify the 2 paralogs of the Xenopus laevis GRK7 cDNAs (XGRK7a and XGRK7b) from X. laevis retina RNA using the SMART RACE cDNA Amplification kit (Clontech, Mountain View, CA). The 5′ and 3′ noncoding regions were amplified using primers against the cloned fragment of the cDNA and the primers included with the RACE kit. The accession numbers for Xenopus laevis GRK7a and GRK7b are EU621673 and EU621674, respectively.
Expression and purification of human GRK7 from HEK-293 cells
Flag-tagged, human GRK7 was expressed in HEK-293 cells cultured in DME/F-12 with 10% fetal bovine serum (complete medium). One day after subculture, cells were cotransfected with 4 μg human GRK7 cDNA inserted into the pFLAG-CMV2 vector (Sigma-Aldrich, Inc., St. Louis, MO) and 2 μg pRSV-T antigen per 10-cm dish using diethylaminoethyl dextran as described previously (Weiss et al. 1994). After DMSO shock, the culture medium was replaced with 10 ml complete medium containing 1 mM mevalonolactone (Sigma-Aldrich, Inc) to ensure that the carboxyl terminus of GRK7 is isoprenylated. Approximately 72 h after transfection, cells were harvested, frozen once, then homogenized in Tris-buffered saline (TBS) containing 0.5% n-dodecyl maltoside and Protease Inhibitor Cocktail (Sigma-Aldrich, Inc.), followed by centrifugation at 40,000 × g for 30 min at 4 °C. Flag-tagged, human GRK7 was isolated using M2 anti-Flag monoclonal antibody resin (Anti-Flag Affinity Gel; Sigma-Aldrich, Inc) as described previously (Horner et al. 2005). The amount of purified GRK7 was determined by SDS-PAGE using BSA as a standard. The gel was stained with SYPRO Red (Molecular Probes, Eugene, OR) and visualized using the STORM imaging system (General Electric Healthcare Life Sciences, Piscataway, NJ).
Immunoblot analysis of in vitro-phosphorylated human GRK7
To evaluate the specificity of the anti-pGRK7 antibody, Flag-tagged human GRK7, isolated as described above, was phosphorylated in vitro and analyzed by immunoblot analysis. Reaction mixtures containing 750 ng GRK7 and 200 μM ATP were incubated with or without 7,500 units of PKA catalytic subunit (New England Biolabs, Ipswich, MA) for 1 h at 30 °C in buffer supplied by the manufacturer. The reaction was stopped by the addition of 5X Laemmli buffer (Laemmli 1970). Samples from the reaction mixtures containing 225 ng GRK7 were chromatographed by SDS-PAGE and transferred to nitrocellulose for immunoblot analysis. The anti-pGRK7 antibody was preincubated overnight with or without a 10-fold excess by weight of the phosphopeptide or nonphosphopeptide (Bethyl Laboratories Inc.), followed by incubation with the blots as described in the legend to Figure 2A.
Fig. 2. Elevated cAMP increases phosphorylated GRK7 in HEK-293 cells.
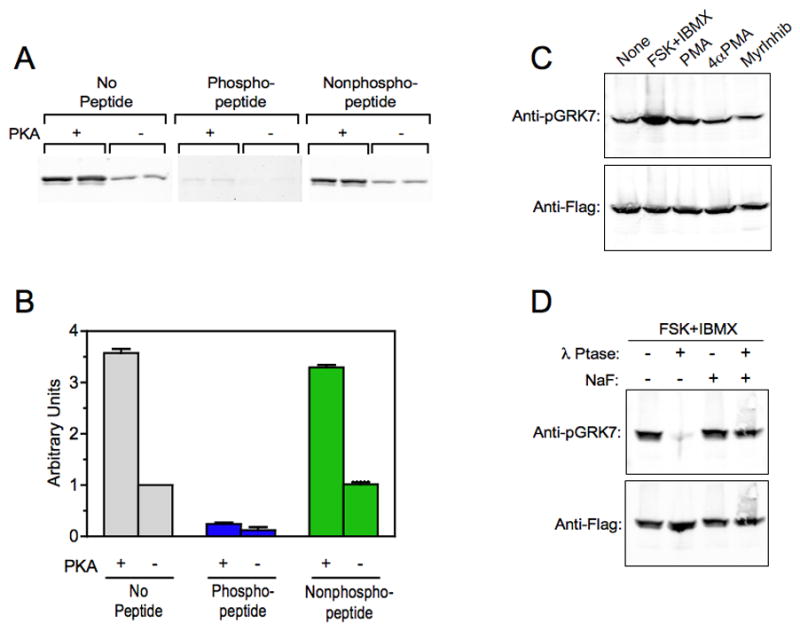
(A) Immunoblot analysis comparing the binding of the anti-pGRK7 antibody to purified, Flag-tagged human GRK7 phosphorylated by PKA (+) and untreated (−). The blots were incubated with a 1:2,000 dilution of the anti-pGRK7 antibody preincubated overnight with no peptide, phosphopeptide or nonphosphopeptide at a 10-fold excess by weight, as described in the Materials and Methods. The experiment contains duplicate samples and is representative of 2 independent experiments.
(B) Two independent experiments, including the one shown in (A), were analyzed using the Odyssey imaging system and demonstrates that the binding of anti-pGRK7 is specific for phosphorylated GRK7. Error bars represent the range between the two independent experiments.
(C) HEK-293 cells expressing GRK7 were treated with 50 μM forskolin (FSK) and 1 mM IBMX, 100 nM phorbol myristyric acid (PMA), 100 nM 4-α-myristyric acid (4αPMA), or 50 μM myristoylated PKC peptide inhibitor (MyrInhib), for 30 min at room temperature. Cell extracts were analyzed by immunoblotting using the anti-pGRK7 and anti-Flag antibodies at 1:2,000 and 1:10,000, respectively.
(D) HEK-293 cells were treated with 50 μM FSK and 1 mM IBMX for 30 min. Cell extracts were prepared with (+) or without (−) 50 mM NaF, a phosphatase inhibitor, then incubated with or without 600 units λ phosphatase (λ Ptase) for 30 min as described in the Materials and Methods, and subjected to immunoblot analysis using the same antibody dilutions as described in (C).
Phosphorylation of human GRK7 in HEK-293 cells
Human GRK7 cDNA inserted into the pFLAG-CMV2 vector (Sigma) was mixed with Opti-MEM I medium (Invitrogen) and Lipofectamine 2000 (Invitrogen). The transfection was carried out according to the manufacturer’s directions using 3 μg of DNA per 3.5 cm culture dish. One day after plating, the cells were incubated with 1 mM mevalonolactone in complete medium to ensure isoprenylation of the GRK7 carboxyl terminus. Cells were cultured for 48 hours prior to the experiments described below.
To determine the levels of phosphorylated GRK7, cells were incubated with either 50 μM forskolin and 1 mM isobutylmethylxanthine (IBMX), 100 nM phorbol myristyric acid (PMA; a PKC activator), 100 nM 4-α-myristyric acid (an inactive analog of PMA), or 50 μM myristoylated PKC peptide inhibitor, in DME/F12 for 30 min at 37 °C in a humidified CO2 incubator. After washing with PBS, the cells were solubilized in hot SDS-Laemmli buffer (Laemmli 1970) containing 50 mM NaF as a phosphatase inhibitor. The samples were heated at 95°C for 5 min, sheared with a 25G needle and centrifuged at 10,000g for 10 min at room temperature to remove insoluble material. Samples were subjected to 10% SDS-PAGE, followed by immunoblot analysis and visualization using the Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, NB).
For some experiments, cells treated with 50 μM forskolin and 1 mM IBMX as described above were washed in PBS, then harvested with chilled 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton-X-100 in the presence or absence of 50 mM NaF, as described in the legend to Fig. 2B. Cell suspensions were lysed by rapid freeze/thaw in liquid nitrogen and centrifuged at 4 °C for 20 min at 100,000g. After protein determination, 50 μg of cell lysate were incubated with or without 600 units λ phosphatase (New England Biolabs, Ipswich, MA) in buffer containing 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 20 mM MgCl2, 0.1 mM EGTA, 2 mM DTT and 0.01% Brij 35 for 30 min at 30 °C. SDS-Laemmli buffer was added to stop the reaction and aliquots were subjected to 10% SDS-PAGE followed by immunoblot analysis.
For immunocytochemical analysis, HEK-293 cells were plated on 2.2 mm coverslips coated with human fibronectin (BD Biocoat™ Cellware; BD Bioscience, San Jose, CA) and transfected with Flag-tagged human GRK7 as described above. Two days post transfection, cells were incubated with 50 μM forskolin and 1 mM IBMX in DME/F12 for 30 min at 37 °C in a humidified CO2 incubator. They were fixed in 2% paraformaldehyde/PBS for 30 min, rinsed 3 times in PBS, and permeabilized in PBS containing 0.05% saponin for 15 min. The coverslips were rinsed again in PBS and incubated with anti-Flag (1:1,500) or anti-pGRK7 (1:100) for 2 h at room temperature in PBS containing 0.05% saponin and 10% goat serum. After rinsing in PBS, the coverslips were incubated with goat anti-mouse (conjugated to AlexaFluor-594) or goat anti-rabbit (conjugated to AlexaFluor-488) secondary antibodies, respectively, at a 1:2,000 dilution for 1 h, rinsed 3 times in PBS and mounted for visualization by fluorescence microscopy. Images were acquired using a SPOT digital camera (Diagnosic Instruments, Inc., Sterling Heights, MI) attached to a fluorescent microscope (Nikon, Inc., Melville, NY). Exposure settings and post-imaging processing with Adobe Photoshop were identical for all images.
Analysis of phosphorylated GRK7 in vertebrate retinas
All animals used in these studies were treated according to the guidelines established by the Institutional Care and Use Committee for the University of North Carolina at Chapel Hill. Frogs were maintained on a 14 h dark/10 h light cycle. For some experiments, frogs were dark- or light-adapted, as described in the figure legends, prior to euthanasia with benzocaine for 20 min and decapitation. For frogs sacrificed in the dark, retinas were dissected under infrared light. Light-adapted frogs were exposed to a light intensity of approximately 1,500 lux for the indicated times. These retinas were dissected under light intensities ranging from 1,500 to 3,000 lux. In some experiments, retinas were immediately homogenized in modified SDS-Laemmli buffer containing 12.5 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol and 100 mM NaF. The homogenate was heated for 3 min at 95 °C and sheared with a 25 G needle. After centrifugation at 10,000g for 20 min at room temperature, the supernatant was collected for protein determination. For immunoblot analysis, samples were diluted 2-fold and reconstituted with β-mercaptoethanol and bromophenol blue (Laemmli 1970). For other experiments, retinas were incubated ex vivo after removal in the light or in the dark in the presence or absence of various pharmacological agents prior to homogenization, as described in the figure legends.
For immunocytochemistry, eyes from pigs, frogs or zebrafish were removed from euthanized animals, enucleated and fixed overnight at 4 °C in PBS (pH 7.5) containing 4% paraformaldehyde. After washing 3 times in PBS, eyes were cryoprotected by sequential incubation in PBS containing 10%, 20% and 30% sucrose, followed by embedding in OCT and freezing in isopentane cooled by liquid nitrogen. Eyes were cryostat-sectioned at 10 μm. Sections were dried overnight at 50 °C. For pig and zebrafish retinas, sections were incubated in PBS containing 0.2% Triton-X-100 for 10 min at room temperature, rinsed 3 times in PBS, followed by incubation for 1 h at room temperature in blocking buffer containing PBS, 5% goat serum, 5% BSA and 0.1% Triton-X-100. Sections were incubated overnight at 4 °C with primary antibodies diluted in buffer containing PBS, 5% BSA and 0.1% Triton-X-100, as described in the figure legends. Staining was visualized after washing 3 times in PBS containing 0.1% triton (PBST), followed by 3 washes in PBS and incubation with fluorescent secondary antibody in antibody dilution buffer for 2 h at room temperature. After 3 washes each in PBST and PBS, sections were mounted for visualization by fluorescence microscopy. For Xenopus retina sections, samples were placed directly in blocking buffer containing PBS, 5% goat serum, 5% BSA, 0.5% Triton-X-100 after drying overnight. Subsequent procedures were identical to those described above, except that 0.5% Triton-X-100 was used in the blocking and antibody dilution buffers. Images were collected using a SPOT digital camera attached to a fluorescent microscope as described above. In each experiment, the microscope and camera settings, as well as postimage processing using Adobe Photoshop, were identical, allowing each set of images to be compared directly.
Results
Phosphorylation of GRK7 in cultured cells
Cyclic AMP levels are known to be regulated by light and play a role in circadian fluctuations of physiological processes in vertebrate photoreceptor cells (Farber et al. 1981, Cohen & Blazynski 1990, Weiss et al. 1995, Traverso et al. 2002, Green & Besharse 2004). For example, cAMP has been implicated in light-dependent retinomotor movements in lower vertebrates, photoreceptor cell disc shedding in mammals and transcriptional regulation and the timing of the photoreceptor circadian clock (Yu et al. 2007). Despite the critical nature of these findings, the molecular mechanisms are not well-characterized and few substrates for PKA, the major effector for cAMP, are known. Previous work from our laboratory demonstrated that GRK7 is phosphorylated on 3 serines in vitro (Fig. 1). There is a single autophosphorylation site, Ser-490, in the carboxyl-terminal domain and 2 sites in the amino-terminal domain, Ser-23 and Ser-36, that are phosphorylated by PKA in vitro (Weiss et al. 1998, Horner et al. 2005). Ser-36 is conserved in GRK7 from all species examined – including the 2 paralogs of X. laevis GRK7 reported here (see below) – and is the major phosphorylation site observed both in vitro and in forskolin-treated HEK-293 cells expressing GRK7 (Horner et al. 2005). An antibody that recognizes GRK7 phosphorylated at Ser-36 (anti-pGRK7) was generated to define the conditions under which GRK7 is phosphorylated in vivo.
Fig. 1. Conservation of Ser-36 in vertebrate GRK7 orthologs.
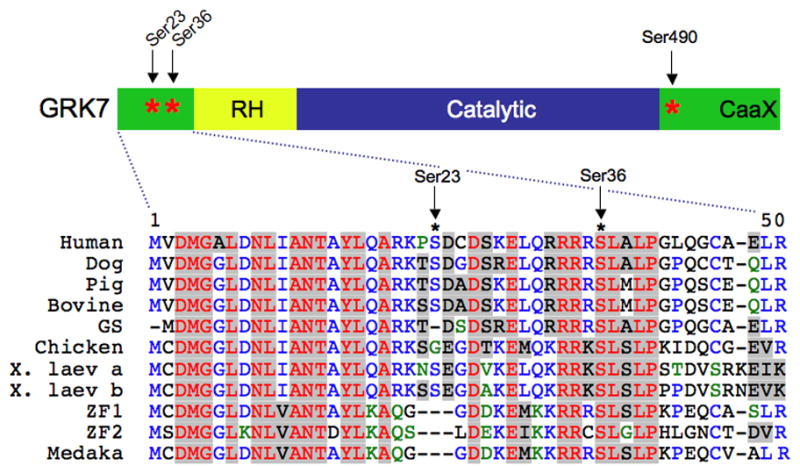
Serines phosphorylated by PKA in vitro (Ser-23 and Ser-36) and the autophosphorylation site (Ser-490) are marked with asterisks (*). RH, RGS Homology Domain; Caax, isoprenylation motif; GS, ground squirrel; X. laev, Xenopus laevis; ZF, zebrafish, Medaka, medaka fish.
To test the antibody’s specificity for phosphorylated GRK7, Flag-tagged GRK7 isolated from transfected HEK-293 cells was phosphorylated in vitro by the catalytic subunit of PKA and analyzed by immunoblot analysis (Fig. 2A). Preincubation of the antibody with the corresponding phosphopeptide, but not the nonphosphopeptide, blocks recognition of GRK7 by this antibody, indicating that the antibody is highly specific for phosphorylated GRK7. Interestingly, binding of anti-pGRK7 preincubated with phosphopeptide to unphosphorylated GRK7 is dramatically lower than binding in the absence of phosphopeptide (Fig. 2B). These results indicate that approximately 25% of the GRK7 isolated from HEK-293 was phosphorylated by an endogenous kinase, presumably PKA.
HEK-293 cells expressing Flag-tagged GRK7 were incubated with forskolin and IBMX for 30 min to raise cAMP levels. Under these conditions, the amount of phosphorylated GRK7 recognized by the anti-pGRK7 antibody increased approximately 3-fold (Fig. 2C). Only a small increase (approximately 50%) was observed when cells were incubated with PMA, a PKC activator. There was no observable effect of exposing cells to the inactive analog, 4αPMA, or a myristoylated PKC peptide inhibitor. When extracts from cells incubated with forskolin and IBMX were treated with a broad-spectrum phosphatase, λ phosphatase, phosphorylated GRK7 was no longer detected (Fig. 2D). Dephosphorylation of GRK7 was blocked by the presence of NaF, a phosphatase inhibitor, in the cell extracts. These results indicate that the major kinase in HEK-293 cells that phosphorylates GRK7 is PKA and that the anti-pGRK7 antibody can detect phosphorylated GRK7 in cells using immunoblot analysis.
To determine whether anti-pGRK7 can detect phosphorylated GRK7 by immunocytochemistry, HEK-293 cells expressing Flag-tagged GRK7 were treated with forskolin and IBMX as described above. The cells were fixed and stained with the anti-Flag and anti-pGRK7 antibodies to visualize GRK7 and phosphorylated GRK7, respectively (Fig. 3). While forskolin and IBMX had no effect on the levels of GRK7 detected using the anti-Flag antibody (Figs. 3A vs. 3D), phosphorylated GRK7 levels were increased compared to levels in untreated cells (Figs. 3B vs. 3E), as seen in the merged images (Figs. 3C vs. 3F). These results demonstrate that anti-pGRK7 is effective for localizing and evaluating levels of phosphorylated GRK7 by immunocytochemistry.
Fig. 3. Immunocytochemical detection of phosphorylated GRK7 in HEK-293 cells.
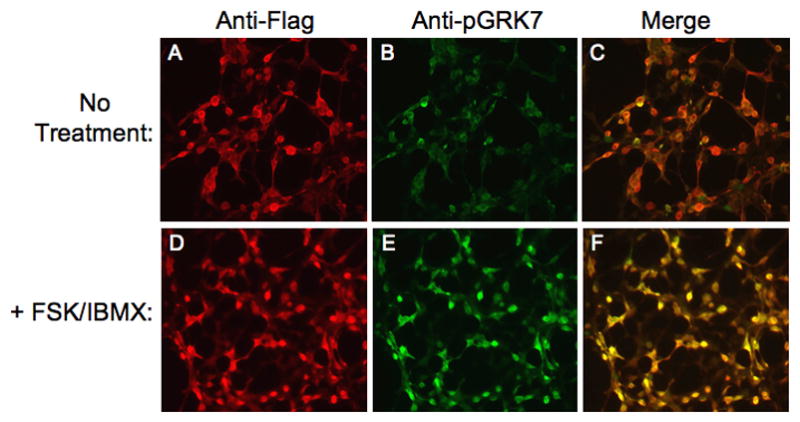
Cells were incubated in the absence (A, B, C) or presence (D, E, F) of 50 μM FSK and 1 mM IBMX for 30 min. Cells were stained simultaneously with anti-Flag (A, D) and anti-pGRK7 (B, E) antibodies at 1:1,500 and 1:100, respectively. Panel C represents the merging of images A and B. Panel F represents the merging of images D and E.
GRK7 is phosphorylated in vivo
Pig retinas are used as a model for human vision because the size of the eye and the rod/cone density are similar (Prince et al. 1960, Gerke et al. 1995). Eyes from euthanized pigs were enucleated, fixed and stained with anti-pGRK7 to determine the presence of phosphorylated GRK7 (Fig. 4). The outer and inner segments of cones were both stained, although staining is highest in the outer segment, consistent with the known distribution of GRK7 using an antibody directed against pig GRK7 (Weiss et al. 2001). A phosphopeptide corresponding to the epitope of the anti-pGRK7 antibody blocks the staining with GRK7, whereas the nonphosphorylated peptide has no effect. These data demonstrate that phosphorylated GRK7 is located in mammalian cones, in vivo.
Fig. 4. Detection of phosphorylated GRK7 in cones of pig retinas stained with anti-pGRK7.
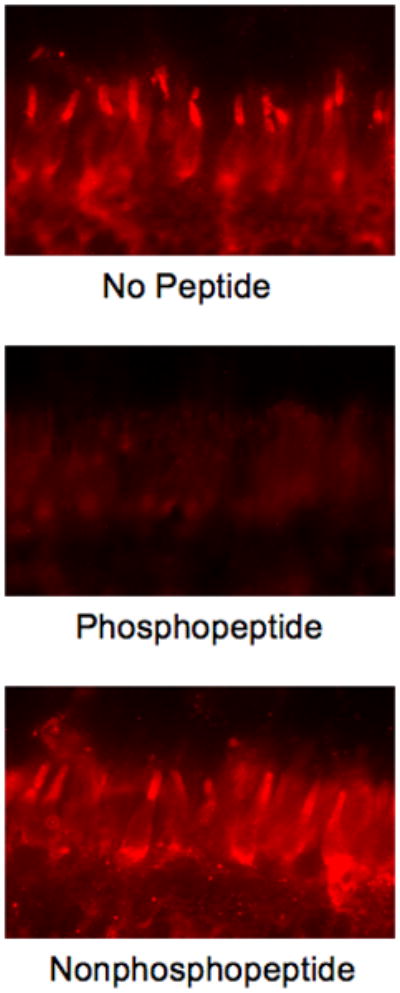
Anti-pGRK7 was preincubated at a 1:25 dilution overnight at 4 °C in the absence of peptide (upper panel), or with a 10-fold excess by weight of the phosphopeptide (middle panel), or the nonphosphopeptide (lower panel), followed by incubation with sections as described in the Materials and Methods. COS, cone outer segments, CIS, cone inner segments, ONL, outer nuclear layer.
The conservation of the consensus sequence for PKA phosphorylation at Ser-36 in a wide range of species (Fig. 1) suggests that phosphorylation is also conserved and plays an important role in the function of GRK7. To evaluate this hypothesis, we also analyzed the phosphorylation of GRK7 in nonmammalian vertebrates. Unlike mammals, zebrafish GRK7 mRNA has been detected in brain and pineal gland, as well as in the retina (Rinner et al. 2005, Wada et al. 2006), suggesting that GRK7 could have functions in multiple neuronal tissues in this fish. Immunocytochemical analysis of the zebrafish retina demonstrated expression of GRK7 in all cone cell types (Wada et al. 2006). For our studies, zebrafish were dark-adapted for 3 h prior to euthanasia to prevent the RPE from obscuring the view of the cones (Hodel et al. 2006). The anti-pGRK7 antibody detected phosphorylated GRK7 in all three cone cell types in dark-adapted zebrafish retinas – the short single cones, large single cones and the double cones (Fig. 5) – indicating the functional conservation of GRK7 phosphorylation in fish.
Fig. 5. Detection of phosphorylated GRK7 in 3 layers of cones of dark-adapted zebrafish retina using anti-pGRK7. Zebrafish were dark-adapted for 3 h prior to euthanasia and preparation of eyes for immunohistochemistry as described in the Materials and Methods.
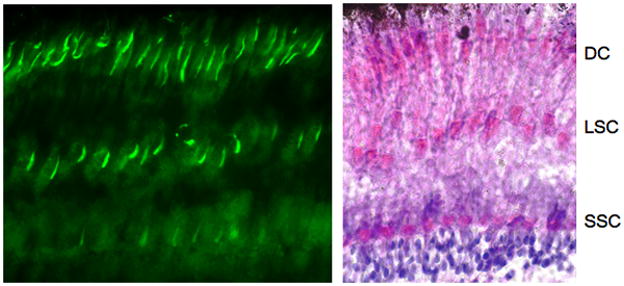
Left panel: anti-pGRK7 (1:100).
Right panel: zebrafish retina stained with hematoxylin and eosin.
DC, double cones; LSC, long single cones; SSC, short single cones.
The Xenopus laevis retina has served as a model for many aspects of vertebrate vision, including development, phototransduction, circadian regulation, photoreceptor cell physiology and trafficking (Anderson & Green 2000, Gabriel 2000, Rohlich & Szél 2000, Witkovsky 2000, Green 2003, Adler & Raymond 2008). We cloned 2 paralogs of X. laevis GRK7 (GRK7a and GRK7b), which were found to be 94% identical at the amino acid level. This is due to a duplication of the Xenopus laevis genome that occurred approximately 20–40 million years ago (Chain et al. 2008). In comparison, the 2 zebrafish GRK7 proteins, zGRK7-1 and zGRK7-2 are only 73% identical at the amino acid level, suggesting their duplication and divergence occurred much earlier. The average identity between the XGRK7 paralogs and human GRK7 is 68%, whereas the average identity between human and zebrafish GRK7 is 58–59%. The greater identity between human and frog GRK7 compared to zebrafish is consistent with the evolutionary distance reported for fish, amphibians and mammals (Futuyama 1998). As described in the Materials and Methods, the carboxyl-terminal sequence of XGRK7a was used to generate an antibody against Xenopus GRK7 (anti-XGRK7) for the experiments described below.
Frogs undergo retinomotor movements in the dark, resulting in elongation of the cones towards the retinal pigment epithelium (RPE) (Anderson & Green 2000). To prevent the outer segments from being obscured by melanin granules in the RPE, albino Xenopus frogs were used for the following experiment. Cone outer segments were clearly stained with the anti-XGRK7 antibody under both dark- and light-adapted conditions (Figs. 6A, 6F). When frogs were dark-adapted overnight, the outer segments also stained with anti-pGRK7 (Fig. 6B), indicating that GRK7 is phosphorylated under these conditions. In contrast, cones in the retinas from frogs that were light-adapted for 3 h were not visible with anti-pGRK7 (Fig. 6G). These data demonstrate that phosphorylation of GRK7 is regulated by light in Xenopus cones. Regulation of GRK7 phosphorylation by light exposure was also measured by immunoblot analysis. Frogs were dark-adapted overnight, then either euthanized immediately or exposed to light for 3 hours prior to euthanasia. The retinas were removed and subjected to immunoblot analysis using the anti-pGRK7 antibody (Fig. 7). The results confirmed that phosphorylated GRK7 levels are higher in the dark compared to the light.
Fig. 7. Phosphorylation of GRK7 on Ser-36 is regulated by light in Xenopus retinas.
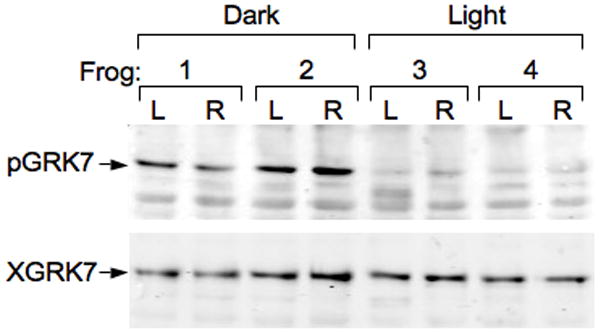
Dark adaptation elevates levels of phosphorylated GRK7 in Xenopus retinas in vivo. Four frogs were dark-adapted overnight. Frogs 1 and 2 were euthanized and the retinas removed under infrared illumination. Frogs 3 and 4 were exposed to light for 3 hours before euthanasia. Each dissected retina was immediately homogenized and protein concentration was determined as described in the Methods. Twenty-five μg of protein extract was subjected to SDS-PAGE and immunoblotting with anti-pGRK7 (1: 2,000) and anti-XGRK (1:1,000). L, left eye; R, right eye.
To determine the rates of phosphorylation and dephosphorylation in vivo, frogs were dark-adapted overnight. When these frogs were exposed to light to determine the time course of GRK7 dephosphorylation (Fig. 8A), phosphorylated GRK7 is reduced to background levels within 1 h after light exposure. Dark-adapted frogs were light-adapted for 1 h, then placed back in the dark. Within 1 hour, they recovered greater than 80% of the phosphorylation level originally observed in the dark (Fig. 8B). Therefore, phosphorylation and dephosphorylation are reversible within 60 min under these conditions.
Fig. 8. Timing of phosphorylation and dephosphorylation.
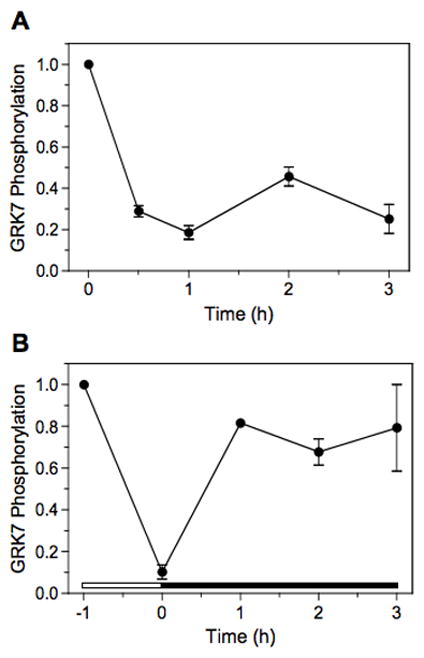
(A) Frogs were dark-adapted overnight, followed by exposure to light for 0, 0.5, 1, 2 and 3-hour intervals before euthanasia. Retinas were removed and prepared for immunoblot analysis as described in the Materials and Methods.
(B) Frogs were dark-adapted overnight, exposed to light for 1 h, then placed in the dark for varying periods of time before euthanasia and processing of the retinas for immunoblot analysis. The white horizontal bar represents the period of light exposure and the black horizontal bar represents the period of dark adaptation.
Each sample represents 25 μg of retina homogenate. Error bars represent the average of duplicate samples.
Phosphorylation of GRK7 is controlled by cAMP in cones
We have shown that PKA phosphorylates GRK7 in vitro (Horner et al. 2005) and that increasing cyclic nucleotide concentrations stimulate the phosphorylation of GRK7 in tissue culture cells (Figs. 2 and 3). Both cAMP and cGMP are regulated by light in vertebrate retinas. Therefore, it is very likely that phosphorylation is controlled by cyclic nucleotides via PKA or PKG in vivo. To determine whether cyclic nucleotides do play a role in GRK7 phosphorylation, frogs were dark-adapted overnight, followed by exposure to light for 1 h to reduce phosphorylated GRK7 to background levels prior to euthanasia. Retinas removed from these frogs were incubated in forskolin and IBMX, which is predicted to raise the levels of both cAMP and cGMP (Fig. 9A). The results demonstrate that phosphorylated GRK7 does increase in frog retinas ex vivo under these conditions. Because either cyclic nucleotide could be responsible for stimulating GRK7 phosphorylation, retinas removed from light-adapted frogs were incubated in either IBMX, which potentially raises the levels of both cyclic nucleotides by inhibiting their breakdown (Fig. 9B), or forskolin, which directly stimulates adenylyl cyclase to synthesize cAMP (Fig. 9C). Since either forskolin or IBMX elevates levels of phosphorylated GRK7, cAMP appears to be sufficient to increase the level of phosphorylated GRK7. To confirm the role of cAMP, retinas were incubated with 2 cell-permeant cyclic nucleotide analogs, dibutyryl cAMP and dibutyryl cGMP (Fig. 9D). Although dibutyryl cAMP is not as robust as either forskolin or IBMX, it does increase the levels of phosphorylated GRK7, whereas dibutyryl cGMP does not. These results implicate cAMP and its downstream kinase, PKA, in the phosphorylation of GRK7 in vivo.
Fig. 9. GRK7 phosphorylation is regulated by cAMP.
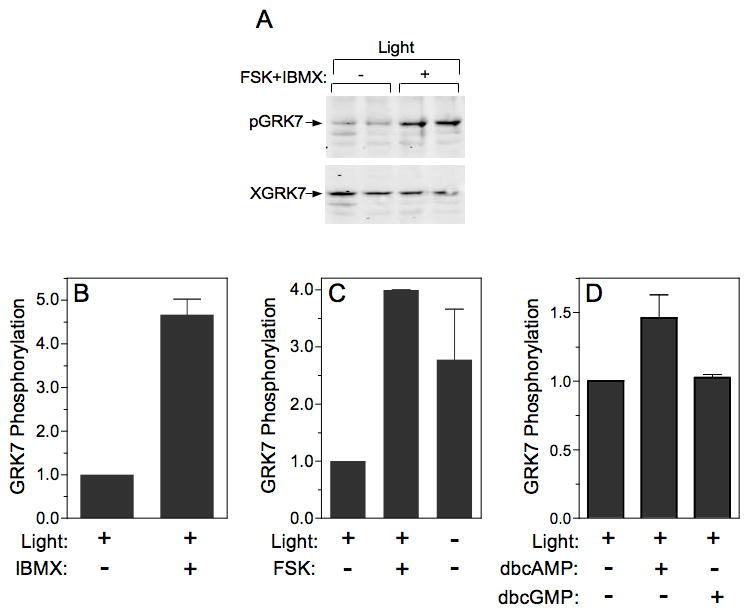
(A) Frog retinas were incubated for 30 min in the light without (−) or with (+) forskolin (FSK) and IBMX. At the end of the incubation, retinas were homogenized and subjected to immunoblot analysis as described in the legend to Fig. 7.
(B) Frogs were exposed to light for 1 h prior to euthanasia. Retinas were removed and exposed to light for 30 min in the absence (−) or presence (+) of IBMX.
(C) Frogs were dark-adapted overnight or light-adapted for 1 h, then euthanized. Retinas were incubated in the light or the dark with (+) or without (−) forskolin for 1 h.
(D) Frogs were exposed to light for 1 h before euthanasia. Retinas were incubated in the light in the presence or absence of dbcAMP or dbcGMP for 30 min.
DISCUSSION
The GRK7 gene is present in a diversity of vertebrates ranging from bony fish to mammals, with the only known exceptions being rats and mice. These rodents appear to be unique in having lost this gene during evolution, since related species, including chipmunks and ground squirrels, do express GRK7 (Weiss et al. 1998, Weiss et al. 2001). The GRK7 gene may be at least 400 million years old, based on evidence that bony fish diverged from its common ancestor with cartilaginous fish more than 400 million years ago (Futuyama 1998). Interestingly, the sequence surrounding and including Ser-36, the PKA phosphorylation site, is conserved throughout this evolutionary period, implying that phosphorylation of this amino acid plays an important role in the function of the protein. Our previous studies demonstrated that Ser-36 can be mutated to alanine without loss of enzyme activity, suggesting that it does not play an essential structural or enzymatic role, but rather a regulatory one (Horner et al. 2005). While we have not yet defined the consequences of phosphorylation in vivo, our studies in vitro suggest that phosphorylation by PKA may reduce the ability of GRK7 to phosphorylate its substrates (Horner et al. 2005). Studies in multiple vertebrate species indicate that cAMP levels are high in the dark and decline in the light in photoreceptor cells (Farber et al. 1981, Cohen & Blazynski 1990, Weiss et al. 1995, Traverso et al. 2002). Therefore, we predicted that phosphorylation of GRK7 by PKA would also be high in the dark and low in the light. This hypothesis is confirmed by the present studies. In dim light or in the dark, when PKA activity is high, increased phosphorylation of GRK7 would be predicted to reduce its activity, thereby increasing the amount and duration of cone transducin activation by the cone opsins and contributing to enhanced sensitivity to light. In contrast, under light-adapted conditions, PKA activity would be reduced, resulting in reduced phosphorylation of GRK7, increased activity of this kinase and increased phosphorylation of the cone opsins. Under these conditions, cone transducin activation would be lower and of shorter duration, possibly contributing to light adaptation and to protection of the retina from light damage.
Several proteins involved in phototransduction, including transducin, arrestin and recoverin, are transported between the inner and outer segments in rod photoreceptors as a mechanism of long-term light adaptation and protection against light damage (Sokolov et al. 2002, Nair et al. 2005, Peterson et al. 2005, Calvert et al. 2006). In cones, both cone arrestin and cone transducin have been shown to redistribute in response to light (Zhang et al. 2003a, Zhang et al. 2003b, Elias et al. 2004, Chen et al. 2007, Rosenzweig et al. 2007), although this was not observed in zebrafish (Kennedy et al. 2004). In contrast, GRK1 has not been reported to change its location in rods in response to light in rats (Strissel et al. 2005), but redistribution has been observed in the medaka fish for one of the GRK1 paralogs (Imanishi et al. 2007). In the present report, we did not observe light-driven movement of GRK7 in frog cones. These observations are consistent with the hypothesis that GRK7 is regulated directly by PKA phosphorylation, rather than via translocation to a different cellular compartment. Other potential consequences of GRK7 phosphorylation by PKA include regulation of its stability and association with other proteins. For example, phosphorylation of phosducin by PKA and CamKII prevents the binding of Gtβγ complex to phosducin and promotes the binding of 14-3-3 protein (Nakano et al. 2001, Thulin et al. 2001, Lee et al. 2004). This interaction with 14-3-3 is speculated to protect phosducin from proteosomal degradation or alter its location within the photoreceptor cell (Thulin et al. 2001). Interestingly, arylalkylamine N-acetyltransferase (AANAT), a rate-limiting enzyme in melatonin synthesis in the pineal gland and the retina (Klein 2007), is phosphorylated by PKA in a circadian fashion (Ivanova & Iuvone 2003), which also causes it to bind 14-3-3 protein (Ganguly et al. 2001, Pozdeyev et al. 2006), thereby blocking proteosomal degradation in the retina (Pozdeyev et al. 2006) and the pineal gland (Gastel et al. 1998).
Although cGMP and cAMP are both regulated by light, our data indicate that it is cAMP that induces the phosphorylation of GRK7. Forskolin alone, via stimulation of adenylyl cyclase, is sufficient to elevate levels of phosphorylated GRK7 in light-adapted retinas ex vivo. Likewise, the cell permeant cAMP analog, dbcAMP, but not dbcGMP, is effective at stimulating GRK7 phosphorylation. Therefore, it is PKA, rather than PKG, that phosphorylates GRK7. Previously, the only known mechanism for regulation of the retinal GRKs was the binding of the Ca2+ sensor protein, recoverin, to GRK1 (Gorodovikova et al. 1994, Calvert et al. 1995, Chen et al. 1995), although the influence of this interaction on signaling downstream of the photoreceptor cells is still being debated (Makino et al. 2004, Sampath et al. 2005). Recent studies in frogs indicate that GRK7 is able to bind the amphibian cone ortholog, S-modulin (Tachibanaki et al. 2005, Torisawa et al. 2008). We also find that bovine recoverin binds human GRK7 in vitro (M. Kapur, S. Osawa and E. R. Weiss, unpublished observations). Whether phosphorylation by PKA affects recoverin binding is presently unknown.
Our data indicate for the first time that cAMP can regulate the phosphorylation of proteins involved in cone phototransduction. We have shown that GRK7 is phosphorylated by PKA in vivo in both lower and higher vertebrates. The conservation of Ser-36 phosphorylation among these species is highly suggestive of its importance for GRK7 function. Otherwise, it is unlikely that this sequence would have been preserved for over 400 million years (Budovskaya et al. 2005). Phosphorylation is high in the dark and low in the light, consistent with changes in cAMP levels that occur in nature. These results illustrate a novel mechanism for light-mediated regulation of GRK7 in response to changing levels of cAMP that occur upon light- and dark-adaptation. Future studies will address the influence of phosphorylation on the photoresponse and cone cell physiology. Other interesting questions that remain to be addressed include the signaling pathways that regulate cAMP fluctuations in response to light and circadian rhythm. Given that cAMP has been found mainly in inner segments in rods and cones (Vaquero et al. 2001, Traverso et al. 2002), the association of PKA with specific substrates that are localized mainly in the outer segment, remains to be determined. Answers to these and other questions regarding the control of GRK7 and other substrates of PKA in vivo will help refine our knowledge of how cAMP modifies photoreceptor responses to light.
Acknowledgments
The authors would like to thank Dr. James Manning in the Department of Emergency Medicine at UNC-CH for the donation of pig eyes used in our studies. We would also like to thank Dr. Marc Caron (Department of Cell Biology, Duke University), and Drs. John Rawls and Suk-Won Jin of the Zebrafish Facility at UNC-CH for zebrafish eyes. ERW is supported by R01 EY072224 from the NIH and the Duke University Eye Center Core grant, P30 EY 05722.
References
- Adler R, Raymond PA. Have we achieved a unified model of photoreceptor cell fate specification in vertebrates? Brain Res. 2008;1192:134–150. doi: 10.1016/j.brainres.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FE, Green CB. Symphony of rhythms in the Xenopus laevis retina. Microsc Res Tech. 2000;50:360–372. doi: 10.1002/1097-0029(20000901)50:5<360::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Budovskaya YV, Stephan JS, Deminoff SJ, Herman PK. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 2005;102:13933–13938. doi: 10.1073/pnas.0501046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Klenchin VA, Bownds MD. Rhodopsin kinase inhibition by recoverin. Function of recoverin myristoylation. J Biol Chem. 1995;270:24127–24129. doi: 10.1074/jbc.270.41.24127. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Chain FJ, Ilieva D, Evans BJ. Duplicate gene evolution and expression in the wake of vertebrate allopolyploidization. BMC Evol Biol. 2008;8:43. doi: 10.1186/1471-2148-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- Chen CK, Zhang K, Church-Kopish J, Huang W, Zhang H, Chen YJ, Frederick JM, Baehr W. Characterization of human GRK7 as a potential cone opsin kinase. Mol Vis. 2001;7:305–313. [PubMed] [Google Scholar]
- Chen J, Wu M, Sezate SA, McGinnis JF. Light threshold-controlled cone alpha-transducin translocation. Invest Ophthalmol Vis Sci. 2007;48:3350–3355. doi: 10.1167/iovs.07-0126. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Gupta N, Osawa S, Locke KG, Weiss ER, Wright AF, Birch DG, Milam AH. G-protein-coupled receptor kinase 1 (GRK1) and GRK7 expression and cone deactivation kinetics in enhanced S-cone syndrome (ESCS) caused by mutations in NR2E3. Invest Ophthalmol Vis Sci. 2003;44:1268–1274. doi: 10.1167/iovs.02-0494. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Visual Neurosci. 1990;4:43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and α-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- Farber DB, Souza DW, Chase DG, Lolley RN. Cyclic nucleotides of cone-dominant retinas. Reduction of cyclic AMP levels by light and by cone degeneration. Invest Ophthalmol Vis Sci. 1981;20:24–31. [PubMed] [Google Scholar]
- Futuyama DJ. Evolutionary Biology. Sinauer Associates, Inc; Sunderland, MA: 1998. [Google Scholar]
- Gabriel R. Introduction: what do we owe to the frog’s eye in retinal research? A historical perspective. Microsc Res Tech. 2000;50:325–326. doi: 10.1002/1097-0029(20000901)50:5<325::AID-JEMT1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Gastel JA, Weller JL, et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc Natl Acad Sci USA. 2001;98:8083–8088. doi: 10.1073/pnas.141118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science. 1998;279:1358–1360. doi: 10.1126/science.279.5355.1358. [DOI] [PubMed] [Google Scholar]
- Gerke CG, Hao Y, Wong F. Topography of rods and cones in the retina of the domestic pig. Hong Kong Med J. 1995;1:302–308. [Google Scholar]
- Gorodovikova EN, Gimelbrant AA, Senin I, Philippov PP. Recoverin mediates the calcium effect upon rhodopsin phosphorylation and cGMP hydrolysis in bovine retina rod cells. FEBS Lett. 1994;349:187–190. doi: 10.1016/0014-5793(94)00661-x. [DOI] [PubMed] [Google Scholar]
- Green CB. Molecular control of Xenopus retinal circadian rhythms. J Neuroendocrinol. 2003;15:350–354. doi: 10.1046/j.1365-2826.2003.00999.x. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Hisatomi O, Matsuda S, Satoh T, Kotaka S, Imanishi Y, Tokunaga F. A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS Lett. 1998;424:159–164. doi: 10.1016/s0014-5793(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Hodel C, Neuhauss SC, Biehlmaier O. Time course and development of light adaptation processes in the outer zebrafish retina. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:653–662. doi: 10.1002/ar.a.20329. [DOI] [PubMed] [Google Scholar]
- Horner TJ, Osawa S, Schaller MD, Weiss ER. Phosphorylation of GRK1 and GRK7 by cAMP-dependent protein kinase attenuates their enzymatic activities. J Biol Chem. 2005;280:28241–28250. doi: 10.1074/jbc.M505117200. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Hisatomi O, Yamamoto S, Satoh T, Kotaka S, Kobayashi Y, Tokunaga F. A third photoreceptor-specific GRK found in the retina of Oryzias latipes (Japanese killifish) Zool Sci. 2007;24:87–93. doi: 10.2108/zsj.24.87. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Dunn FA, Hurley JB. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 2004;41:915–928. doi: 10.1016/s0896-6273(04)00086-8. [DOI] [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee BY, Thulin CD, Willardson BM. Site-specific phosphorylation of phosducin in intact retina. Dynamics of phosphorylation and effects on G protein βγ dimer binding. J Biol Chem. 2004;279:54008–54017. doi: 10.1074/jbc.M405669200. [DOI] [PubMed] [Google Scholar]
- Liu P, Osawa S, Weiss ER. M opsin phosphorylation in intact mammalian retinas. J Neurochem. 2005;93:135–144. doi: 10.1111/j.1471-4159.2004.03003.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Imanishi Y, Palczewski K. Rhodopsin phosphorylation: 30 years later. Prog Ret Eye Res. 2003;22:417–434. doi: 10.1016/s1350-9462(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol. 2004;123:729–741. doi: 10.1085/jgp.200308994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, et al. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Chen J, Tarr GE, Yoshida T, Flynn JM, Bitensky MW. Rethinking the role of phosducin: light-regulated binding of phosducin to 14-3-3 in rod inner segments. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4693–4698. doi: 10.1073/pnas.071067198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JJ, Orisme W, Fellows J, McDowell JH, Shelamer CL, Dugger DR, Smith WC. A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci. 2005;46:3988–3998. doi: 10.1167/iovs.05-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev N, Taylor C, Haque R, et al. Photic regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J Neurosci. 2006;26:9153–9161. doi: 10.1523/JNEUROSCI.1384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince JH, Diesem CD, Eglitis I, Ruskell GL. Anatomy and Histology of the Eye and Orbit in Domestic Animals. Charles C. Thomas; Springfield, IL: 1960. The pig; pp. 210–233. [Google Scholar]
- Rinner O, Makhankov YV, Biehlmaier O, Neuhauss SC. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron. 2005;47:231–242. doi: 10.1016/j.neuron.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Rohlich P, Szél A. Photoreceptor cells in the Xenopus retina. Microsc Res Tech. 2000;50:327–337. doi: 10.1002/1097-0029(20000901)50:5<327::aid-jemt2>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rosenzweig DH, Nair KS, Wei J, et al. Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci. 2007;27:5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shimauchi-Matsukawa Y, Aman Y, Tachibanaki S, Kawamura S. Isolation and characterization of visual pigment kinase-related genes in carp retina: polyphyly in GRK1 subtypes, GRK1A and 1B. Mol Vis. 2005;11:1220–1228. [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem. 2005;280:29250–29255. doi: 10.1074/jbc.M501789200. [DOI] [PubMed] [Google Scholar]
- Tachibanaki S, Arinobu D, Shimauchi-Matsukawa Y, Tsushima S, Kawamura S. Highly effective phosphorylation by G protein-coupled receptor kinase 7 of light-activated visual pigment in cones. Proc Natl Acad Sci USA. 2005;102:9329–9334. doi: 10.1073/pnas.0501875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulin CD, Savage JR, McLaughlin JN, et al. Modulation of the G protein regulator phosducin by Ca2+-calmodulin-dependent protein kinase II phosphorylation and 14-3-3 protein. J Biol Chem. 2001;27:23805–23815. doi: 10.1074/jbc.M101482200. [DOI] [PubMed] [Google Scholar]
- Torisawa A, Arinobu D, Tachibanaki S, Kawamura S. Amino acid residues in GRK1/GRK7 responsible for interaction with S-modulin/recoverin. Photochem Photobiol. 2008 doi: 10.1111/j.1751-1097.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- Traverso V, Bush RA, Sieving PA, Deretic D. Retinal cAMP levels during the progression of retinal degeneration in rhodopsin P23H and S344ter transgenic rats. Invest Ophthalmol Vis Sci. 2002;43:1655–1661. [PubMed] [Google Scholar]
- Vaquero CF, Pignatelli A, Partida GJ, Ishida AT. A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J Neurosci. 2001;21:8624–8635. doi: 10.1523/JNEUROSCI.21-21-08624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Sugiyama J, Okano T, Fukada Y. GRK1 and GRK7: unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J Neurochem. 2006;98:824–837. doi: 10.1111/j.1471-4159.2006.03920.x. [DOI] [PubMed] [Google Scholar]
- Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM, Osawa S. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci. 2001;21:9175–9184. doi: 10.1523/JNEUROSCI.21-23-09175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ER, Hao Y, Dickerson CD, Osawa S, Shi W, Zhang L, Wong F. Altered cAMP levels in retinas from transgenic mice expressing a rhodopsin mutant. Biochem Biophys Res Commun. 1995;216:755–761. doi: 10.1006/bbrc.1995.2686. [DOI] [PubMed] [Google Scholar]
- Weiss ER, Osawa S, Shi W, Dickerson CD. Effects of carboxyl-terminal truncation on the stability and G protein coupling activity of bovine rhodopsin. Biochemistry. 1994;33:7587–7593. doi: 10.1021/bi00190a011. [DOI] [PubMed] [Google Scholar]
- Weiss ER, Raman D, Shirakawa S, Ducceschi MH, Bertram PT, Wong F, Kraft TW, Osawa S. The cloning of GRK7, a candidate cone opsin kinase, from cone- and rod-dominant mammalian retinas. Mol Vis. 1998;4:27. < http://www.molvis.org/molvis/v4/p27>. [PubMed] [Google Scholar]
- Witkovsky P. Photoreceptor classes and transmission at the photoreceptor synapse in the retina of the clawed frog, Xenopus laevis. Microsc Res Tech. 2000;50:338–346. doi: 10.1002/1097-0029(20000901)50:5<338::AID-JEMT3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Gao Y, Willis CL, Li P, Tiano JP, Nakamura PA, Hyde DR, Li L. Mitogen-associated protein kinase- and protein kinase A-dependent regulation of rhodopsin promoter expression in zebrafish rod photoreceptor cells. J Neurosci Res. 2007;85:488–496. doi: 10.1002/jnr.21157. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cuenca N, Ivanova T, Church-Kopish J, Frederick JM, MacLeish PR, Baehr W. Identification and light-dependent translocation of a cone-specific antigen, cone arrestin, recognized by monoclonal antibody 7G6. Invest Ophthalmol Vis Sci. 2003a;44:2858–2867. doi: 10.1167/iovs.03-0072. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang W, Zhang H, Zhu X, Craft CM, Baehr W, Chen CK. Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction. Mol Vis. 2003b;9:231–237. [PubMed] [Google Scholar]


