Abstract
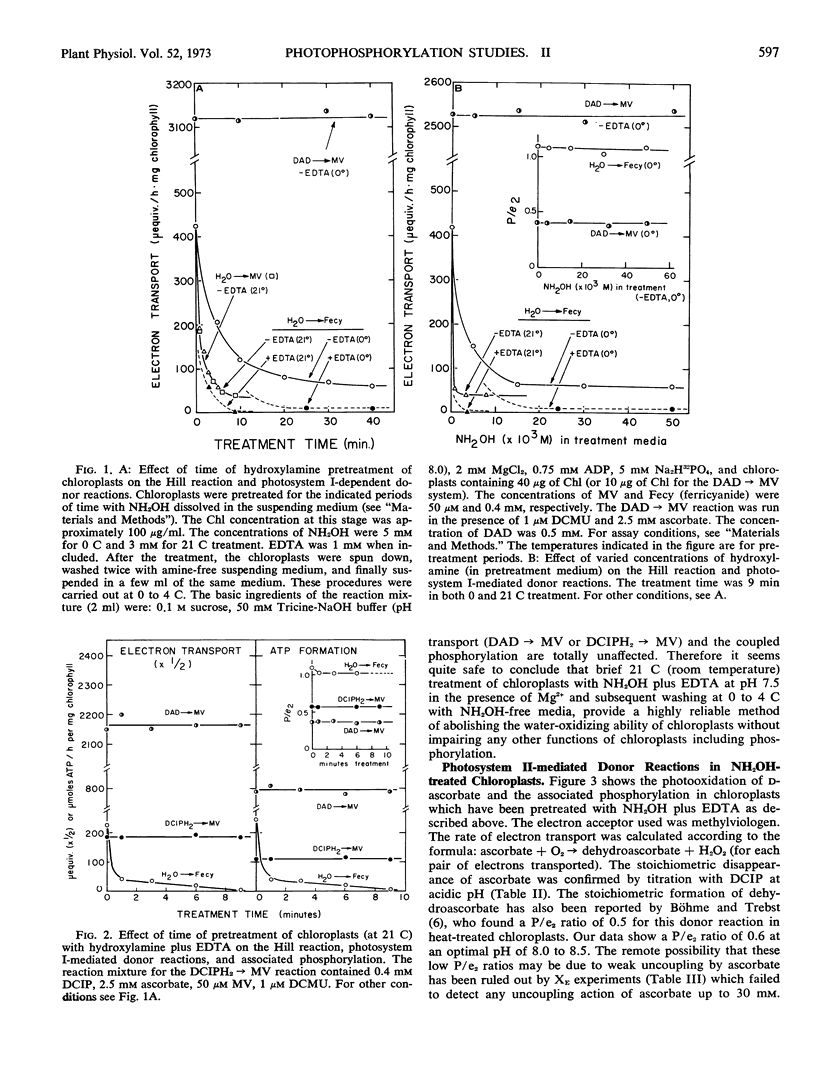
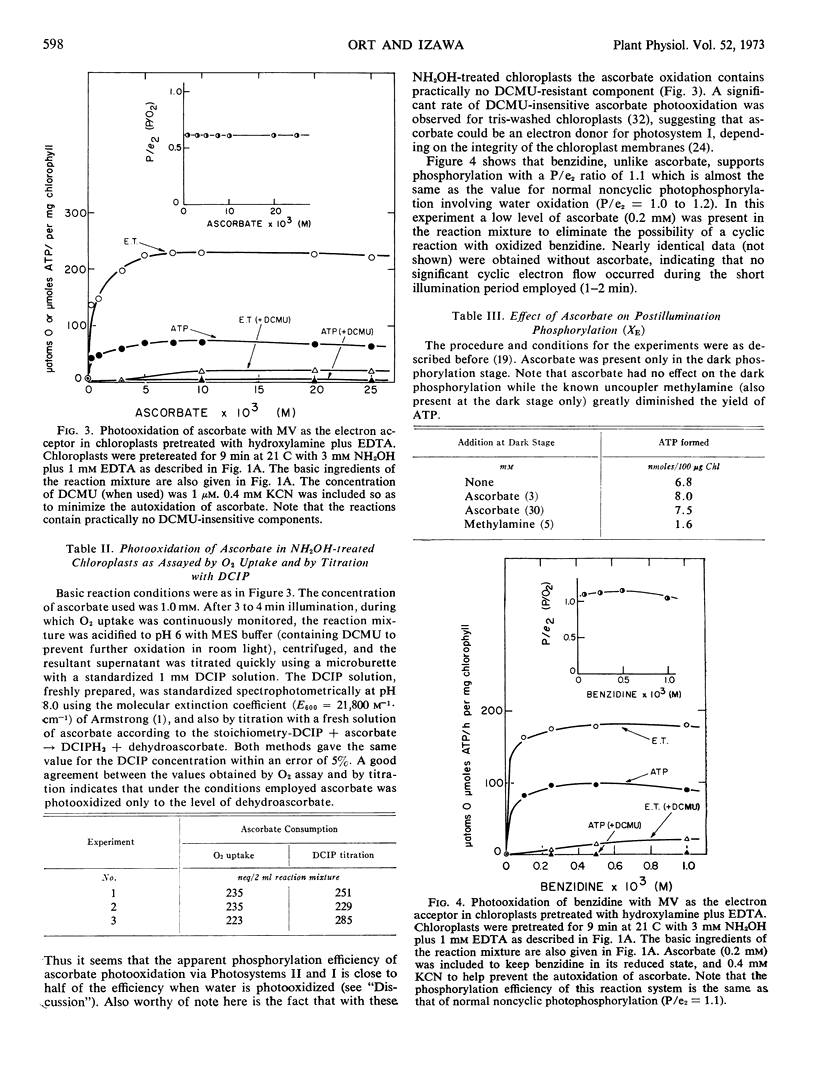
Artificial electron donors to photosystem II provide an important means for characterizing the newly discovered site of energy coupling near photosystem II. However, water oxidation must be completely abolished, without harming the phosphorylation mechanism, for these donor reactions and the associated phosphorylation to withstand rigorous quantitative analysis. In this paper we have demonstrated that treatment of chloroplasts with hydroxylamine plus EDTA at pH 7.5 in the presence of Mg2+ followed by washing to remove the amine is a highly reliable technique for this purpose. The decline of the Hill reaction and the coupled phosphorylation during the treatment were carefully followed. No change in the efficiency of phosphorylation (P/e2 1.0-1.1) was observed until the reactions became immeasurable. Photosystem I-dependent reactions, such as the transfer of electrons from diaminodurene or reduced 2,6-dichlorophenolindophenol to methylviologen, and the associated phosphorylation were totally unaffected. It is clear that the hydroxylamine treatment is highly specific, with no adverse effect on the mechanism of phosphorylation itself. Benzidine photooxidation via both photosystems II and I in hydroxylamine-treated chloroplasts (electron acceptor, methylviologen; assayed as O2 uptake) supports phosphorylation with the same efficiency as that observed for the normal Hill reaction (P/e2 = 1.1). An apparent P/e2 ratio of 0.6 was computed for the photooxidation of ascorbate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Chance B. Relation of phosphorylation to electron transport in isolated chloroplasts. Brookhaven Symp Biol. 1966;19:149–160. [PubMed] [Google Scholar]
- Böhme H., Cramer W. A. Localization of a site of energy coupling between plastoquinone and cytochrome f in the electron-transport chain of spinach chloroplasts. Biochemistry. 1972 Mar 28;11(7):1155–1160. doi: 10.1021/bi00757a007. [DOI] [PubMed] [Google Scholar]
- Böhme H., Trebst A. On the properties of ascorbate photooxidation in isolated chloroplasts. Evidence for two ATP sites in noncyclic photophosphorylation. Biochim Biophys Acta. 1969 May;180(1):137–148. doi: 10.1016/0005-2728(69)90201-1. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: I. Factors Affecting the Decay of O(2) Evolution. Plant Physiol. 1971 Apr;47(4):568–575. doi: 10.1104/pp.47.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Studies on the function of manganese in photosynthesis. Brookhaven Symp Biol. 1966;19:406–417. [PubMed] [Google Scholar]
- Cramer W. A., Butler W. L. Potentiometric titration of the fluorescence yield of spinach chloroplasts. Biochim Biophys Acta. 1969 Apr 8;172(3):503–510. doi: 10.1016/0005-2728(69)90146-7. [DOI] [PubMed] [Google Scholar]
- GOOD N. E. Activation of the Hill reaction by amines. Biochim Biophys Acta. 1960 Jun 3;40:502–517. doi: 10.1016/0006-3002(60)91391-3. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Izawa S. Photosystem-II electron transport and phosphorylation with dibromothymoquinone as the electron acceptor. Eur J Biochem. 1973 Aug 1;37(1):185–192. doi: 10.1111/j.1432-1033.1973.tb02974.x. [DOI] [PubMed] [Google Scholar]
- HIND G., JAGENDORF A. T. EFFECT OF UNCOUPLERS ON THE CONFORMATIONAL AND HIGH ENERGY STATES OF CHLOROPLASTS. J Biol Chem. 1965 Jul;240:3202–3209. [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- Izawa S., Heath R. L., Hind G. The role of chloride ion in photosynthesis. 3. The effect of artificial electron donors upon electron transport. Biochim Biophys Acta. 1969 Jun 24;180(2):388–398. doi: 10.1016/0005-2728(69)90123-6. [DOI] [PubMed] [Google Scholar]
- Izawa S. The relation of post-illumination ATP formation capacity (X-E) to H+ accumulation in chloroplasts. Biochim Biophys Acta. 1970 Nov 3;223(1):165–173. doi: 10.1016/0005-2728(70)90141-6. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Smith M. Uncoupling Phosphorylation in Spinach Chloroplasts by Absence of Cations. Plant Physiol. 1962 Mar;37(2):135–141. doi: 10.1104/pp.37.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraayenhof R., Izawa S., Chance B. Use of uncoupling acridine dyes as stoichiometric energy probes in chloroplasts. Plant Physiol. 1972 Dec;50(6):713–718. doi: 10.1104/pp.50.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Vaklinova S., Tomova N., Nikolova E., Secenska M. The effect of certain factors on the photooxidation of hydroxylamine in isolated chloroplasts. C R Acad Bulg Sci. 1965;18(7):659–662. [PubMed] [Google Scholar]
- WESSELS J. S. Studies on photosynthetic phosphorylation. II. Photosynthetic phosphorylation under aerobic conditions. Biochim Biophys Acta. 1958 Jul;29(1):113–123. doi: 10.1016/0006-3002(58)90151-3. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of the Hill Reaction by Tris and Restoration by Electron Donation to Photosystem II. Plant Physiol. 1969 Mar;44(3):435–438. doi: 10.1104/pp.44.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


