Abstract
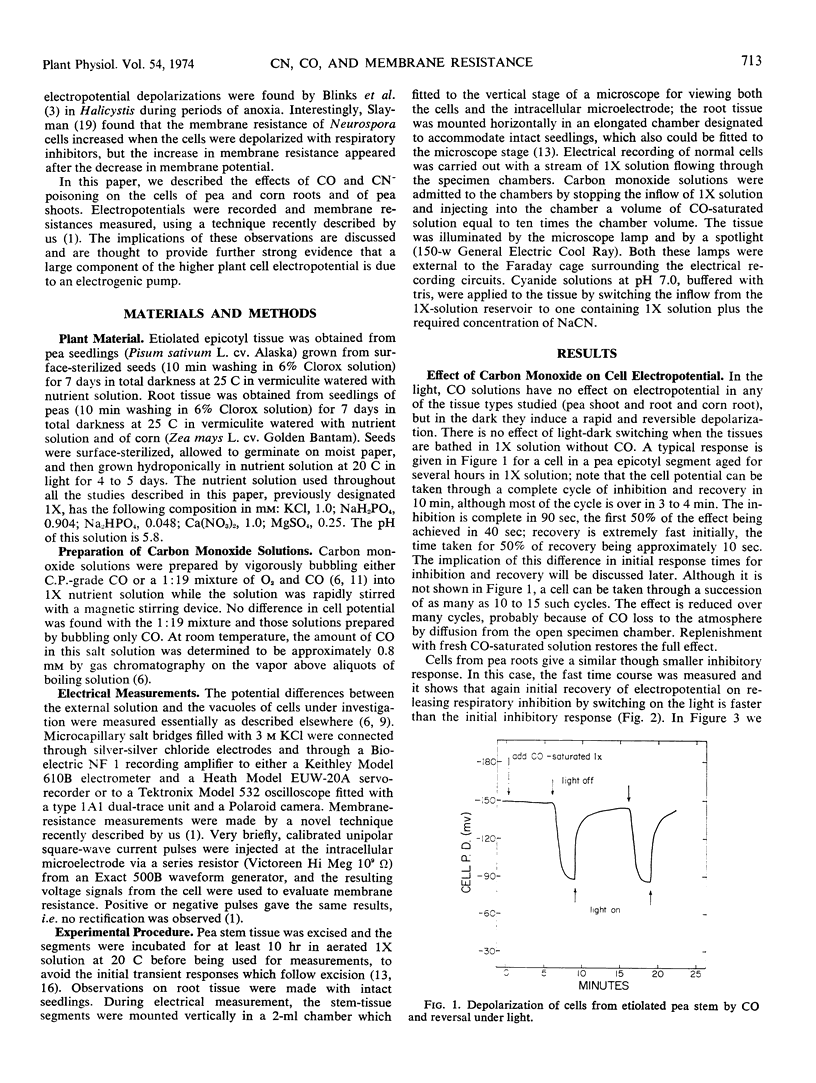
The rapid reduction in cell electropotentials induced by metabolic inhibitors is strong evidence for an electrogenic ion pump. According to Ohm's law, such a depolarization might be explained by a reduction in electric current, I, with unidirectional transport of a given ion, or an increase in permeability (decrease in resistance). With cells of etiolated seedlings of Pisum sativum L. cv. Alaska and Zea mays cv. Golden Bantam, carbon monoxide inhibition, which occurs only in the dark and is readily reversed by light, allows repeated cycling of depolarization and repolarization; there is no effect on cell membrane resistance. In contrast, cyanide inhibition results in a marked increase in membrane electrical resistance; with cyanide following repeated pulses of current used in measuring cell membrane resistance, the resistance eventually (about 10 minutes) shows an abrupt drop as in the “punch-through” effect reported by H. G. L. Coster (1965. Biophys. J. 5: 669-686).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. P., Hendrix D. L., Higinbotham N. Higher plant cell membrane resistance by a single intracellular electrode method. Plant Physiol. 1974 Jan;53(1):122–124. doi: 10.1104/pp.53.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster H. G. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of "punch-through". Biophys J. 1965 Sep;5(5):669–686. doi: 10.1016/S0006-3495(65)86745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Jacobson L. Inhibition of Ion Absorption and Respiration in Barley Roots. Plant Physiol. 1955 Jan;30(1):21–27. doi: 10.1104/pp.30.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Mertz S. M., Graves J. S., Pierce W. S., Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971 Jan;47(1):76–80. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON R. N., WILKINS M. J., WEEKS D. C. Studies in the metabolism of plant cells. IX. The effects of 2,4-dinitrophenol on salt accumulation and salt respiration. Aust J Sci Res B. 1951 Aug;4(3):248–264. doi: 10.1071/bi9510248. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Respiration and the intracellular potential. J Gen Physiol. 1965 Sep;49(1):93–116. doi: 10.1085/jgp.49.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Lu C. Y., Shane L. Correlated changes in membrane potential and ATP concentrations in Neurospora. Nature. 1970 Apr 18;226(5242):274–276. doi: 10.1038/226274a0. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]


