Abstract
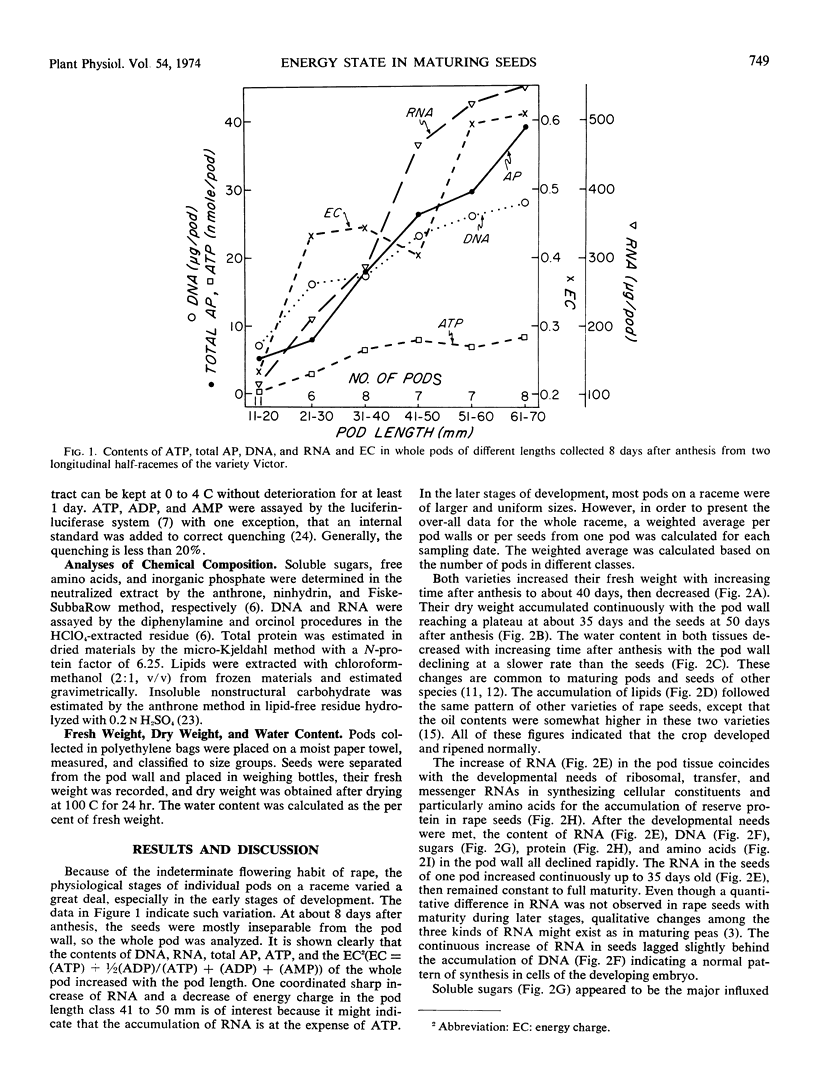
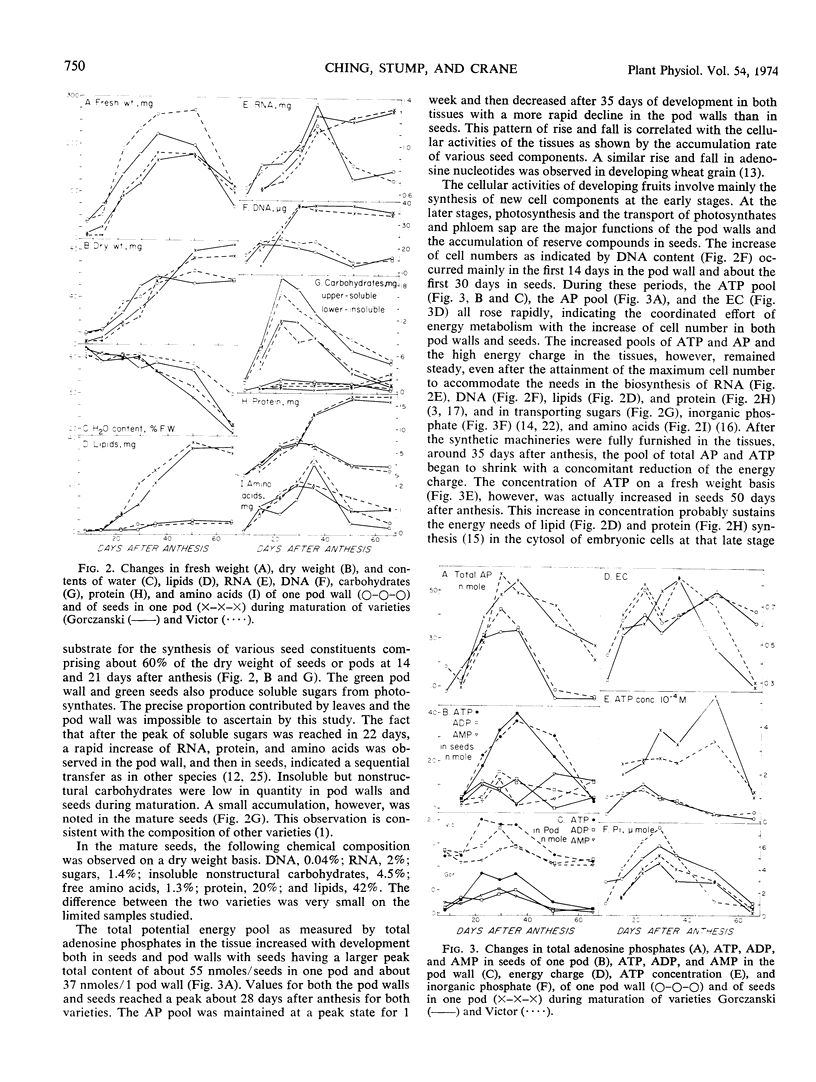
A study of energy state and chemical composition of pod walls and seeds of maturing rape (Brassica napus L.) was conducted on two varieties, Victor and Gorczanski. Total adenosine phosphates, ATP, and adenylate energy charge increased with increasing cell number and cellular synthesis during the early stages, remained high at maximum dry weight accumulation and maximum substrate influx time, and decreased with ripening. A temporal control of energy supply and ATP concentration is evident in developing tissues with determined functions; whereas the association of a high energy charge and active cellular biosynthesis occurs only in tissues with a stabilized cell number.
Full text
PDF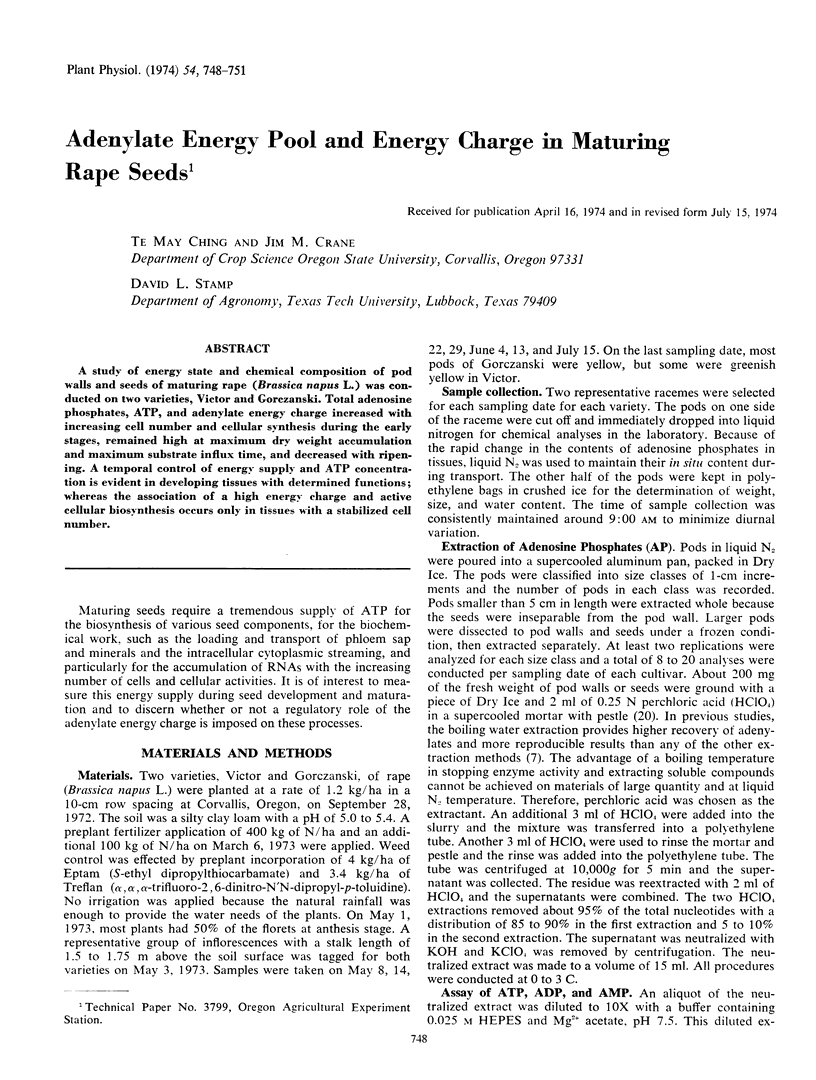

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M., Ching K. K. Content of adenosine phosphates and adenylate energy charge in germinating ponderosa pine seeds. Plant Physiol. 1972 Nov;50(5):536–540. doi: 10.1104/pp.50.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Compositional changes of douglas fir seeds during germination. Plant Physiol. 1966 Oct;41(8):1313–1319. doi: 10.1104/pp.41.8.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss W. E. Control of the adenylate charge in Novikoff ascites cells. Cancer Res. 1973 Jan;33(1):57–64. [PubMed] [Google Scholar]
- Criss W. E. Control of the adenylate charge in the Morris "minimal-deviation" hepatomas. Cancer Res. 1973 Jan;33(1):51–56. [PubMed] [Google Scholar]
- Ingle J., Beitz D., Hageman R. H. Changes in Composition during Development and Maturation of Maize Seeds. Plant Physiol. 1965 Sep;40(5):835–839. doi: 10.1104/pp.40.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner C. F. The composition of soluble nucleotides in the developing wheat grain. Plant Physiol. 1968 Jan;43(1):41–49. doi: 10.1104/pp.43.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. F., Thompson J. E. Effects of Germination on NA-K-stimulated Adenosine 5'-Triphosphatase and ATP-dependent Ion Transport of Isolated Membranes from Cotyledons. Plant Physiol. 1972 Oct;50(4):452–457. doi: 10.1104/pp.50.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth. Science. 1974 Jun 7;184(4141):1043–1050. doi: 10.1126/science.184.4141.1043. [DOI] [PubMed] [Google Scholar]
- Obendorf R. L., Marcus A. Rapid Increase in Adenosine 5'-Triphosphate during Early Wheat Embryo Germination. Plant Physiol. 1974 May;53(5):779–781. doi: 10.1104/pp.53.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Energy charge control of the Calvin cycle enzyme 3-phosphoglyceric acid kinase. Biochem Biophys Res Commun. 1973 Mar 5;51(1):139–143. doi: 10.1016/0006-291x(73)90519-6. [DOI] [PubMed] [Google Scholar]
- Pell E. J., Brennan E. Changes in respiration, photosynthesis, adenosine 5'-triphosphate, and total adenylate content of ozonated pinto bean foliage as they relate to symptom expression. Plant Physiol. 1973 Feb;51(2):378–381. doi: 10.1104/pp.51.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Paulsen G. M., Raguse C. A. Extraction of Total Available Carbohydrates from Grass and Legume Tissue. Plant Physiol. 1964 Nov;39(6):960–962. doi: 10.1104/pp.39.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John J. B. Determination of ATP in Chlorella with the luciferin-luciferase enzyme system. Anal Biochem. 1970 Oct;37(2):409–416. doi: 10.1016/0003-2697(70)90066-7. [DOI] [PubMed] [Google Scholar]
- Walbot V., Brady T., Clutter M., Sussex I. Macromolecular synthesis during plant embryogeny: rates of RNA synthesis in Phaseolus coccineus embryos and suspensors. Dev Biol. 1972 Sep;29(1):104–111. doi: 10.1016/0012-1606(72)90047-4. [DOI] [PubMed] [Google Scholar]


