Abstract
The CDC25A-CDK2 pathway has been proposed as critical for the oncogenic action of human epidermal growth factor receptor 2 (HER2) in mammary epithelial cells. In particular, transgenic expression of CDC25A cooperates with HER2 in promoting mammary tumors, whereas CDC25A hemizygous loss attenuates the HER2-induced tumorigenesis penetrance. On the basis of this evidence of a synergism between HER2 and the cell cycle regulator CDC25A in a mouse model of mammary tumorigenesis, we investigated the role of CDC25A in human HER2-positive breast cancer and its possible implications in therapeutic response. HER2 status and CDC25A expression were assessed in 313 breast cancer patients and we found statistically significant correlation between HER2 and CDC25A (P = .007). Moreover, an HER2-positive breast cancer subgroup with high levels of CDC25A and very aggressive phenotype was identified (P = .005). Importantly, our in vitro studies on breast cancer cell lines showed that the HER2 inhibitor efficacy on cell growth and viability relied also on CDC25A expression and that such inhibition induces CDC25A down-regulation through phosphatidylinositol 3-kinase/protein kinase B pathway and DNA damage response activation. In line with this observation, we found a statistical significant association between CDC25A overexpression and trastuzumab-combined therapy response rate in two different HER2-positive cohorts of trastuzumab-treated patients in either metastatic or neoadjuvant setting (P = .018 for the metastatic cohort and P = .021 for the neoadjuvant cohort). Our findings highlight a link between HER2 and CDC25A that positively modulates HER2-targeted therapy response, suggesting that, in HER2-positive breast cancer patients, CDC25A overexpression affects trastuzumab sensitivity.
Introduction
Human epidermal growth factor receptor 2 (HER2) gene amplification and/or protein overexpression characterize 15% to 25% of invasive breast cancers and define a clinically important group of patients [1,2]. The HER2-positive breast cancer group has traditionally been associated with poor prognosis [1,3], but the advent of HER2-targeted therapies has changed the natural course of the disease for a significant fraction of patients. Although HER2-positive breast cancers display several genomic aberrations, they remain mainly dependent on HER2 signaling for survival and growth and, consistent with such dependency, are preferentially growth inhibited by HER2-specific drugs [4]. In particular, the humanized monoclonal antibody trastuzumab directed against the extracellular domain of HER2, when combined with chemotherapy, has a significant impact on HER2-positive breast cancer treatment, in both metastatic and adjuvant settings, decreasing the risk of relapse and prolonging patient survival [5–7]. Unfortunately, a relevant percentage of patients with HER2-positive breast cancer exhibits an initial resistance to trastuzumab-based therapy [8]. In the metastatic setting, primary resistance ranges from 44% to 64% for single-agent trastuzumab [9,10] and from 25% to 30% for combination therapy [5,11,12].
Indeed, HER2-positive breast cancer is hardly a homogeneous disease and many other key pathways involving HER2 still need to be unveiled. Moreover, the molecular mechanisms by which trastuzumab inhibits tumor cancer growth and potentiates chemotherapy are still not completely understood [13,14].
HER2 gene amplification/overexpression in breast cancer has been associated with increased cell proliferation, cell motility, tumor invasiveness, and reduced apoptosis mainly through the RAS and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathways [15]. Thus, the oncogenic effect of HER2 occurs through several mechanisms, including cell cycle perturbation. Specifically, activation of HER2 signal transduction promotes cell proliferation by shortening the G1 phase, and HER2 overexpression has been associated with both up-regulation of cyclin D1 and down-regulation of the CDK inhibitor p27 [16]. Accordingly, trastuzumab induces cell cycle G1 arrest through up-regulation of p27 [17] and decreased expression of cyclin E [18].
Recently, it has been proposed that CDC25A-CDK2 pathway is critical for the oncogenic action of HER2 in mammary epithelial cells [19]. In particular, transgenic expression of CDC25A cooperates with HER2 in promoting mammary tumors [20], whereas hemizygous loss of CDC25A attenuated the penetrance of HER2-induced tumorigenesis [21].
CDC25A phosphatase is an essential component of the cell cycle machinery and integrates the specific signals of checkpoint control in response to DNA damage [22,23]. The activation of the DNA damage response (DDR) causes cell cycle arrest and, depending on the extent of the insult, mediates either DNA repair or premature senescence/apoptosis of the damaged cell. Two fundamental component of the DDR are the checkpoint kinases CHK1 and CHK2 that target CDC25A to proteasome-dependent degradation, thus triggering cell cycle arrest [23–26]. Therefore, CDC25A misregulation can contribute to genomic instability and several lines of evidence support a role for CDC25A in cancer development [20,27]. Consistently, CDC25A is aberrantly expressed and predicts poor patient prognosis in a significant number of human cancers, including breast cancer [28,29]. Furthermore, we have previously shown that ectopic CDC25A overexpression in primary human breast cells results in impaired DDR, enhancing genomic instability at common fragile sites and consequently increasing the chances of tumorigenic conversion [30].
On the basis of the evidence of a synergism between HER2 and CDC25A in mouse model mammary tumorigenesis, we investigated the relation between HER2 and CDC25A in human breast cancer to understand whether CDC25A evaluation could contribute to a better characterization of HER2-positive breast cancer patients.
We analyzed both HER2 status and CDC25A expression in different series of human breast cancers either treated or not with trastuzumab. In particular, we found important clinical implications in terms of response to trastuzumab treatment for the HER2-positive tumors that overexpress CDC25A.
The effect of HER2 inhibition was also studied in HER2-positive breast cancer cell line models showing differences in CDC25A expression levels. Our results highlighted a strong association among HER2, CDC25A, and DDR induction, adding more evidence to the knowledge of the HER2 overexpression effects on regulation of the cell cycle checkpoints and cellular proliferation.
Materials and Methods
Patient Selections and Statistical Analysis
We analyzed both HER2 status and CDC25A expression in a cohort of 313 patients, with breast carcinoma diagnosed between 1994 and 2000, at the San Raffaele Hospital (Milan, Italy) and the Santa Chiara Hospital (Trento, Italy). Patients' clinicopathologic characteristics are summarized in Table 1. Representative tumor areas were carefully selected by experienced pathologists from formalin-fixed paraffin-embedded (FFPE) samples for tissue macroarrays. To study the statistical association between HER2 status and CDC25A expression, two-sided Pearson chi-squared test was used.
Table 1.
Patient Characteristics (n = 313).
| Age, median (range) | 59 years (27–90) |
| Histology | |
| Ductal | 271 (86.58%) |
| Lobular | 25 (7.99%) |
| Other | 17 (5.43%) |
| pT | |
| pT1 | 138 (44%) |
| pT2 | 135 (43.1%) |
| pT3 | 23 (7.3%) |
| pT4 | 17 (5.6%) |
| pN | |
| pN0 | 140 (44.73%) |
| >pN0 | 173 (55.27%) |
| LVI | |
| Yes | 96 (30.67%) |
| No | 217 (69.33%) |
| Grade | |
| 1 | 44 (14.06%) |
| 2 | 163 (52.08%) |
| 3 | 106 (33.86%) |
| Hormone receptor | |
| ER (+) and/or PgR (+) | 252 (80.5%) |
| ER (-) and PgR (-) | 61 (19.5%) |
| HER2 | |
| Pos | 74 (23.6%) |
| Neg | 239 (76.4%) |
| Ki67 | |
| Pos | 160 (51.1%) |
| Neg | 153 (48.9%) |
| CDC25A | |
| Median percentage of positive nuclei (range) | 19 (0–84) |
| Pos | 156 (49.8%) |
| Neg | 157 (50.2%) |
| Chemotherapy | |
| Yes | 179 (57.19%) |
| No | 97 (30.99%) |
| N/A | 37 (11.82%) |
| Hormone therapy | |
| Yes | 225 (71.89%) |
| No | 74 (23.64%) |
| N/A | 14 (4.47%) |
| Follow up, median (range) | 8 years (0–13) |
LVI indicates lymphovascular invasion; ER, estrogen receptor; PgR, progesterone receptor; ER cutoff = 10; PgR cutoff = 10; Ki67 cutoff = 25.
Kaplan-Meier method was used to evaluate statistical association between HER2 status/CDC25A expression and both overall survival (OS) and disease-free survival (DFS); OS was defined as the time from date of surgery to death due to any cause. Patients were censored at the date of last follow-up. DFS was defined as the time from surgery to recurrence or last follow-up (data available for 304 patients of 313). Survival curves were plotted by using the Kaplan-Meier method, and the significance of the associations was assessed using the two-sided log-rank test.
To assess whether the association between CDC25A expression and prognosis was independent of other variables, a multivariate regression analysis was performed by the Cox proportional hazards model. Age at diagnosis, histology, pT, pN, histologic grade, hormone receptor status, lymphovascular invasion, chemotherapy, hormonotherapy, and HER2 status were the variables used for the analysis. The study of the correlation between CDC25A expression and trastuzumab therapy response was performed on two cohorts of patients: a “metastatic cohort” of 16 patients with metastatic breast carcinoma diagnosed between 1998 and 2007 and treated with trastuzumab as first-line therapy at the Santa Chiara Hospital and a “neoadjuvant cohort” of 13 patients with breast carcinoma diagnosed between 2008 and 2011 and treated with trastuzumab as neoadjuvant therapy at the San Raffaele Hospital. For the metastatic cohort, response to trastuzumab therapy was evaluated on the basis of clinical and radiologic examination of the tumor before and after treatment using Response Evaluation Criteria In Solid Tumors [31]. For the neoadjuvant cohort, pathologic response at surgery was scored as follows: 1) complete, if no invasive residual disease in breast and axilla was found, 2) partial, if the residual tumor burden was inferior to the clinically estimated tumor diameter, and 3) absent, if the residual tumor burden was identical or higher than the clinically estimated tumor diameter (stable or progressive disease).
To study the statistical association between CDC25A expression and trastuzumab therapy response, two-sided Pearson chi-squared test was used.
SPSS version 17.0 was used for statistical analyses. P < .05 was considered statistically significant.
This study has been approved by the ethical committee of both the San Raffaele Hospital and the Santa Chiara Hospital.
HER2 Testing Methods
HER2 expression on both breast cancer cell lines and tissue macro-arrays was detected by immunohistochemistry (IHC) using HercepTest (Dako, Carpinteria, CA). Results were scored by intensity and percentage of staining on a scale from 0 to 3+ according to the American Society of Clinical Oncology/College of American Pathologists guidelines [2]. Fluorescence in situ hybridization (FISH) testing for HER2 was performed on the same samples using the PathVysion HER2 DNA Probe Kit (Abbott Molecular, Abbott Park, IL) according to the manufacturer's instructions. Slides were analyzed using Nikon 90i fluorescence microscope (Nikon Instruments SpA, Florence, Italy) with both a single-pass (green and orange) and a triple-pass filter band [4′,6-diamidino-2-phenylindole (DAPI)/green/orange]; images were captured by Genikon software (Nikon). A total of 100 neoplastic nuclei were observed per each sample and FISH scoring ranges were based on those determined for the US Food and Drug Administration-approved test for HER2 gene alterations in breast cancer using a combined genecentromere probe [32,33].
Immunohistochemistry
All immunostains were performed after microwave oven heat-based antigen retrieval, using citrate buffer at pH 6.0. The following anti-CDC25A antibody clones were tested: 3H2016, 144, F-6, N-15, M-191, 5H51 (Santa Cruz Biotechnology, Santa Cruz, CA), Ab991, Ab989 (Abcam, Cambridge, MA), DCS-120 (Thermo Scientific, Newington, NH), 3652 (Cell Signaling Technology, Danvers, MA), C25600-01U (USBio, Swampscott, MA), and PC733 (Merck Bio, Birmingham, United Kingdom). The best results were achieved with the primary antibody anti-human CDC25A [rabbit polyclonal (144), sc-97; Santa Cruz Biotechnology; 1:500 dilution]. The specificity of the immunostaining was also examined by a pre-absorption test. The diluted primary antibody was mixed with a fivefold (by weight) excess of the antigenic peptide (CDC25A 144 P sc-97P; Santa Cruz Biotechnology) in a small volume (500 µl) of phosphate-buffered saline and incubated for 2 hours at room temperature (RT) before being used in the immunostaining. Microscopic observation was performed using a Nikon Eclipse 80i and images were captured and analyzed using Aperio ScanScope and Aperio ImageScope Nuclear Algorithm (Nikon Instruments SpA). The median percentage of positive nuclei for CDC25A staining (19%) was considered as cutoff.
Cell Lines
SKBR3, BT474, and MCF-7 breast cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). SKBR3 were grown in McCoy's 5a Medium (Invitrogen) supplemented with 10% FBS (Invitrogen, Carlsbad, CA). BT474 and MCF-7 were grown in Dulbecco's modified Eagle's medium/F12 medium (Invitrogen) supplemented with 10% FBS. All cell lines were screened for mycoplasma using MycoAlert Mycoplasma Detection Kit (Lonza, Basel, Switzerland) and grown in a humidified atmosphere incubator with 5% CO2 at 37°C. SKBR3 and BT474 metaphase preparations were obtained by treating overnight with colcemid (0.01 µg/ml); metaphase harvests were carried on by hypotonic treatment with 0.075 M KCl/0.08% Na citrate (1:1) for 15 minutes and fixation in methanol/acetic acid (3:1).
Transient Transfection
Transient transfections were performed using the pCMV6-AC-CDC25A-GFP vector (OriGene, Rockville, MD) expressing the full-coding sequence of human CDC25A (NM_001789.2) in fusion with the C-terminal GFP tag. As a control, the pCMV6-AC-green fluorescent protein (GFP) vector (OriGene) was used. MCF-7 cells were seeded at 1 x 105 cells per well in six-well plate, 24 hours before transfection. Cells were transfected in Opti-MEM medium (Invitrogen) with Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's protocol. Six hours post-transfection, cells were switched into antibiotic-free medium containing 10% FBS for further 24 hours.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100 in phosphate-bufferedsaline, and immunostained overnight with primary antibodies: anti-human CDC25A [rabbit polyclonal (144), sc-97; Santa Cruz Biotechnology; 1:750 dilution], anti-CK 8.18 [mouse; Novocastra (Newcastle, United Kingdom); 1:20 dilution], or anti-cERBB2 (rabbit; Dako; 1:1000 dilution). The primary antibody was visualized using goat anti-rabbit IgG Alexa Fluor 555 (Invitrogen; 1:750 dilution) or goat anti-mouse IgG Alexa Fluor 488 (Invitrogen; 1:500 dilution). Samples were counterstained with DAPI-Antifade (VECTASHIELD Mounting Medium; Vector Laboratories, Burlingame, CA). Microscopic observation was performed using a Nikon 90i and images were captured by Genikon software (Nikon Instruments SpA).
Cell Line Treatments
Cell lines were grown to 70% to 80% confluence, then treated with 45 µM AG825 (Calbiochem, Darmstadt, Germany), a selective adenosine triphosphate (ATP)-competitive inhibitor of the tyrosine kinase activity of HER2, and incubated at 37°C for 16 hours. To inhibit translation and determine the stability of CDC25A, SKBR3 cells were incubated with 5 µg/ml cycloheximide (Sigma-Aldrich) at different times (15, 30, 60, 240, and 360 minutes). To inhibit proteasome-dependent degradation of proteins, SKBR3 cells were incubated with 50 µM proteasome inhibitor MG132 (Sigma-Aldrich) for 360 minutes. SKBR3 and BT474 cells were treated with 3 µM CDC25A inhibitor PM20 (Sigma-Aldrich), which competitively inhibits the activity of the phosphatase by binding to its active site, and incubated at 37°C for 16 hours. Control samples were treated with vehicle alone (DMSO).
Western Blot Analysis
Immunoblot analysis was performed essentially as reported [29]. The following antibodies were used: anti-CDC25A (monoclonal, F-6), anti-CHK1 (monoclonal, G4), anti-CHK2 (polyclonal), anti-AKT1 (polyclonal; Santa Cruz Biotechnology), anti-pCDK1/2Y15 (polyclonal; Calbiochem), anti-pAKTS473 (monoclonal, D9E), antipCHK1S345 (polyclonal), anti-pCHK2T68 (polyclonal), anti-pH2AX (monoclonal, 20E3), anti-pHER2Y1248 (polyclonal; Cell Signaling Technology), anti-CDK2 (monoclonal, 55; BD Transduction Laboratories, Franklin Lakes, NJ), anti-cERBB2 (polyclonal; DakoCytomation, Carpinteria, CA), and anti-cleaved poly(ADP-ribose) polymerase (PARP; monoclonal, E51; Epitomics, Burlingame, CA). β-Tubulin antibody (monoclonal, DM1B; Neomarkers, Fremont, CA) was used as a loading control.
Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was extracted from SKBR3 cells, either treated or not with AG825, using the RNeasy Mini Kit (Qiagen, Hilden, Germany). For reverse transcription-polymerase chain reaction, cDNA was synthesized in a 20-µl reaction volume containing 5 µg of total RNA by the Super-Script III First-Strand Synthesis System Kit (Invitrogen) according to the manufacturer's instructions. TaqMan analysis was performed in triplicate using the Applied Biosystems predesigned assays for CDC25A gene (Hs00947994_m1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1) was used as reference gene.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay
SKBR3 and BT474 were plated into a 24-well plate at a density of 4 x 104 cells per well. The next day, the cell lines were treated either with 45 µM AG825 or 3 µM PM20 for 96 hours. Each condition was performed in six replicates and control samples were treated with DMSO. Viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [34], and 250 µl of MTT (Sigma-Aldrich) solution (0.2 mg/ml in Opti-MEM; Invitrogen) was added to each well and the plates were further incubated for 2 hours. The crystals were dissolved in 400 µl of DMSO for 10 minutes. The absorbance was measured at a wavelength of 570 nm. Results were expressed as mean percentage of cell viability ± SE of six replicates from a representative experiment that was repeated three independent times.
Results
HER2 and CDC25A in Human Breast Cancer: Association with Poor Prognosis
To investigate a possible link between HER2 and CDC25A in human breast cancer, we analyzed HER2 status and CDC25A expression in 313 breast cancer patients for whom both clinical and pathologic data were available and none of them received trastuzumab treatment (Table 1).
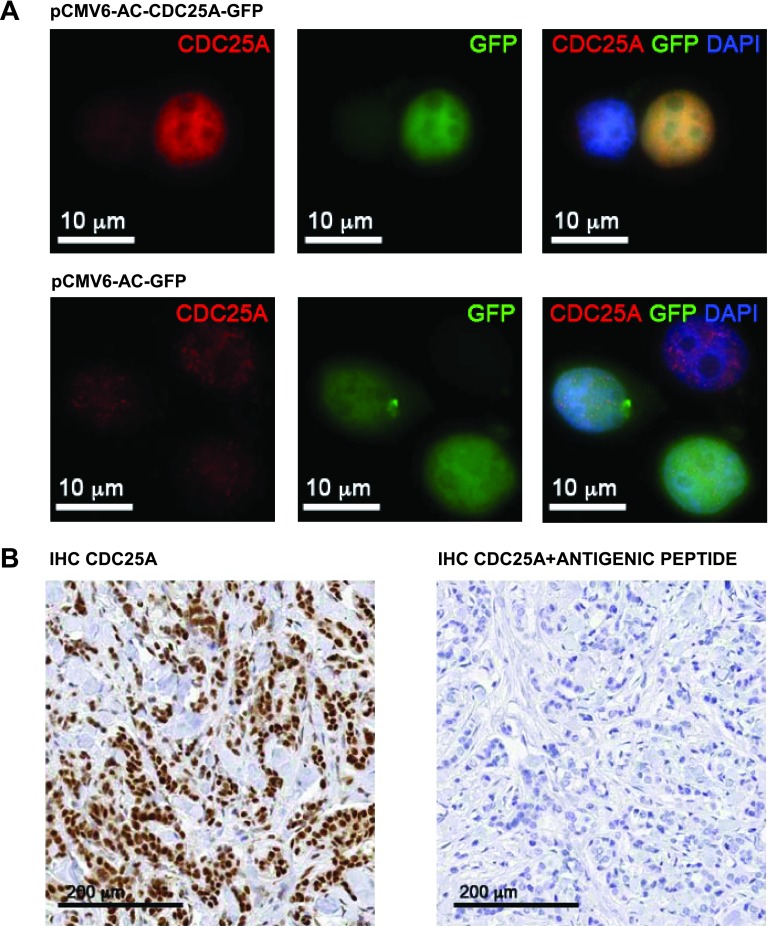
The identification of a CDC25A antibody suitable for IHC studies on FFPE breast cancer tissues was a demanding task. After a careful screen of the commercially available CDC25A antibodies, we found that the CDC25A (144) rabbit polyclonal antibody sc-97, from Santa Cruz Biotechnology, gave the best results in our assays. To validate the specificity of this antibody, we transfected MCF-7 breast cancer cells with either pCMV6-AC-GFP or pCMV6-AC-CDC25A-GFP vector. Immunofluorescence analysis highlighted the specificity of CDC25A antibody: The antibody detected a strong CDC25A expression, representing the GFP-CDC25A fusion protein, exclusively in CDC25A-GFP-MCF-7-transfected cells and not in GFP-MCF-7-transfected ones. Moreover, the immunofluorescence data underlined the expected nuclear localization of CDC25A after DAPI counterstaining (Figure 1A). CDC25A nuclear localization was observed also by IHC on FFPE breast cancer tissue samples (Figure 1B). CDC25A antibody preabsorption testing using the antigenic peptide (CDC25A 144 P sc-97P; Santa Cruz Biotechnology) completely abrogated immunoreactivity (Figure 1B), validating the antibody specificity.
Figure 1.
(A) CDC25A (144) sc-97 antibody (Santa Cruz Biotechnology) validation on the breast cancer cell line MCF-7. Immunofluorescence with CDC25A antibody in the MCF-7 breast cancer cell line transfected with either pCMV-AC-CDC25A-GFP or pCMV-AC-GFP: The antibody detected a strong CDC25A expression in the CDC25A-GFP-transfected MCF-7 cells compared to the pCMV-AC-GFP-transfected MCF-7 cells. DAPI counterstaining underlined the nuclear localization of CDC25A (red signal, CDC25A; green signal, GFP; blue signal, DAPI; yellow signal, merge). (B) CDC25A (144) sc-97 antibody IHC validation on FFPE breast cancer tissue samples. CDC25A nuclear localization was detected by IHC on FFPE breast cancer tissue sample (left); CDC25A antibody preabsorption testing using the antigenic peptide (CDC25A 144 P sc-97P; Santa Cruz Biotechnology) showed a complete block in the immunoreactivity (right).
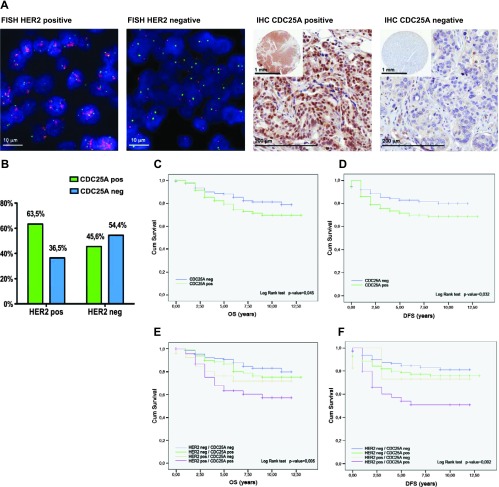
In 313 cases analyzed, nuclear CDC25A overexpression was detected by IHC in 49.8% and HER2 was scored as positive by IHC and FISH in 23.6% (Table 1 and Figure 2A). A statistically significant correlation between CDC25A expression and HER2 gene status was found (P = .007); in particular, 63.5% of HER2-positive tumors showed CDC25A overexpression (Figure 2B). No association was found between CDC25A and Ki67 expression levels (P = .529), suggesting that the correlation between CDC25A and HER2 is independent of the proliferative index.
Figure 2.
(A) HER2 status by FISH and CDC25A expression by IHC in human breast cancer. Representative example of HER2-amplified tumor (FISH HER2 positive): ratio Spectrum Orange-HER2 signals and Spectrum Green-centromere 17 signals > 2; the same case is an example of CDC25A-overexpressing tumor (IHC CDC25A positive): all infiltrating ductal carcinoma cells show strong staining with CDC25A antibody. Representative example of HER2-nonamplified tumor (FISH HER2 negative): ratio Spectrum Orange-HER2 signals and Spectrum Green-centromere 17 signals < 2; the same case is an example of CDC25A-nonexpressing tumor (IHC CDC25A negative): no expression of CDC25A is seen in the infiltrating ductal carcinoma cells. (B) HER2 gene status and CDC25A expression histogram; 63.5% of HER2-positive breast cancer patients showed CDC25A overexpression and 54.4% of patients with HER2-negative breast cancer were negative for CDC25A overexpression. (C and D) CDC25A expression significantly correlates with prognosis. Kaplan-Meier curves showed that overexpression of CDC25A was associated with the decrease of both OS (P = .045) and DFS (P = .032). (E and F) Significative stratification of mortality risk by combinations of HER2 status and CDC25A expression. The group of patients with the highest mortality was the one with HER2/CDC25A double-positive breast carcinomas in terms of either OS (P = .005) or DFS (P = .002; purple curves).
Furthermore, Kaplan-Meier curves showed that overexpression of CDC25A was associated with the decrease of both OS (P = .045) and DFS (P = .032; Figure 2, C and D). Although in the multivariate analysis of prognostic factors on survival CDC25A expression failed to show an independent influence on cancer-specific death (P = .127), a significant stratification of mortality risk was identified when HER2 status and CDC25A expression were evaluated in combination for their relative effect on survival. The group with the highest mortality was the one in which breast carcinomas showed both HER2 positivity (overexpression/amplification) and CDC25A overexpression in terms of either OS (P = .005) or DFS (P = .002; Figure 2, E and F).
HER2 Stabilizes CDC25A Protein Levels in Human Breast Cancer Cell Line SKBR3
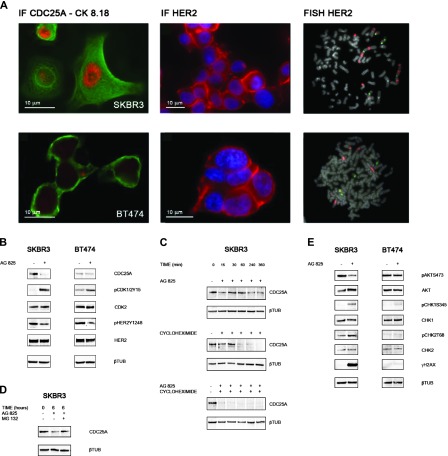
To evaluate whether HER2 signaling impinged on CDC25A, we treated with the specific HER2 inhibitor AG825 two HER2-positive human breast cancer cell lines that showed differences in CDC25A expression levels: SKBR3, positive for both CDC25A overexpression and HER2 amplification (HER2/CDC25A double-positive cells), and BT474, displaying basal level of CDC25A and HER2 amplification (Figure 3A).
Figure 3.
(A) CDC25A expression and HER2 expression/gene amplification in breast cancer cell lines. Immunofluorescence with CDC25A and cERBB2 (Dako) antibodies revealed CDC25A overexpression (nuclear red signal) and HER2 overexpression (cytoplasmic red signal) in SKBR3 cells and basal levels of CDC25A and HER2 overexpression in BT474 cells. Green signal is the immunofluorescence staining of cytokeratin pair 8 and 18 (CK8.18) highlighting the cell cytoplasm; blue signal is DAPI counterstaining for nuclei. FISH analysis showed HER2 amplification (red signals) in SKBR3 and BT474 metaphase spreads. (B) HER2 inhibition induces CDC25A down-regulation of both protein expression and functional activity. Cell lines were treated with AG825 (45 µM) for 24 hours. Control cells were treated with DMSO. Immunoblot data showed a decrease in CDC25A protein levels in HER2/CDC25A double-positive SKBR3 cells treated with AG825. SKBR3 cells showed also a decreased in CDC25A activity after HER2 abrogation as measured by the increase of the inhibitory phosphorylation of its CDK substrates, pCDK1/2Y15. No such strong effect was seen in BT474. AG825 treatment could inhibit the kinase activity of HER2 as assessed by a decrease in HER2Y1248 phosphorylation with no effect on total HER2 levels in the HER2-positive cell lines, SKBR3 and BT474. (C) HER2 inhibition affects CDC25A protein stability in SKBR3. SKBR3 cells were treated with the protein synthesis inhibitor cycloheximide (5 µg/ml) at different time points (0, 15, 30, 60, 240, and 360 minutes) in the presence or absence of AG825: Immunoblot data showed that AG825 decreased the half-life of CDC25A protein from 60 to less than 15 minutes. β-Tubulin was used as a loading control. (D) Ubiquitin/proteasome pathway is involved in the increased turnover of CDC25A after SKBR3 AG825 treatment. To inhibit proteasome-dependent degradation of proteins, SKBR3 cells, either treated or not with AG825, were incubated with 50 µM proteasome inhibitor MG132 for 6 hours. CDC25A immunoblot analysis showed that inhibition of the proteasome by treatment with MG132 rescued CDC25A down-regulation. β-Tubulin was used as a loading control. (E) HER2 inhibition leads to CDC25A down-regulation through the PI3K/AKT pathway and DDR activation. Cell lines were treated with AG825 for 24 hours. Control cells were treated with DMSO. Immunoblot data showed, in AG825-treated SKBR3 cells, a decrease in AKT activity, as measured by the decrease in its phosphorylation on serine 473 (pAKTS473) and no reduction of total AKT observed; CHK1 and CHK2 activation, through phosphorylation of their key sites, serine 345 and threonine 68, respectively (pCHK1S345, pCHK2T68), with no effect on total CHK1 and CHK2 levels; and histone H2AX gamma phosphorylation (γH2AX). No differences were observed in BT474 cells either with or without AG825 treatment. β-Tubulin was used as a loading control.
AG825 treatment efficiently reduced HER2 phosphorylation in HER2-positive SKBR3 and BT474 cell lines, as assessed by the decrease of HER2Y1248 phosphorylation without any significant effect on total HER2 levels. In HER2/CDC25A double-positive SKBR3 cells, inhibition of HER2 by AG825 resulted in a significant down-regulation of CDC25A at both protein level and functional activity, as assessed by inhibitory phosphorylation of its CDK substrates (pCDK1/2Y15). No such strong effects, but a slight induction of CDK inhibitory phosphorylation, associated to a limited reduction of CDC25A levels, was observed in BT474 after HER2 chemical inhibition (Figure 3B).
We next investigated the mechanism by which CDC25A was downregulated in response to HER2 inhibitor treatment. AG852 failed to significantly affect CDC25A mRNA level, as measured by quantitative reverse transcription-polymerase chain reaction, in SKBR3 cells (data not shown), indicating a post-transcriptional inhibition. Thus, to analyze the protein stability of CDC25A upon HER2 inhibition, we determined the CDC25A protein half-life by treating SKBR3 cells with the protein synthesis inhibitor cycloheximide at different time points, in the presence or absence of AG825. We found that AG825 treatment associated with a decrease in the half-life of CDC25A protein from 60 to less than 15 minutes. These data suggested that HER2 expression impinged on CDC25A protein stability (Figure 3C).
We then examined whether the ubiquitin/proteasome pathway was involved in the increased turnover of CDC25A observed after AG825 treatment. Inhibition of the proteasome by treatment with MG132 rescued AG825-induced CDC25A down-regulation (Figure 3D), indicating that the reduced expression of CDC25A observed in SKBR3 cells after HER2 inhibition is due to enhanced proteasome-mediated degradation.
These data suggest that the HER2 signaling may affect the stability of CDC25A in HER2-positive breast cancer cells.
HER2 Inhibition Associates with CDC25A Down-Regulation, PI3K/AKT Pathway Inhibition, and DDR Activation
HER2 overexpression induces the activation of the PI3K/AKT pathway [15]. Since activated AKT is a negative regulator of both CHK1 and CHK2 [35,36], the PI3K/AKT pathway suppresses DNA damage-induced checkpoint activation. On these grounds, we determined whether inhibition of HER2 in SKBR3 cells triggered degradation of CDC25A by relieving PI3K/AKT-mediated inhibition of the DDR.
We observed that AG825-mediated inhibition of HER2 in SKBR3 cells resulted in a marked reduction of AKT activity, as measured by the decrease in its phosphorylation at serine 473; no reduction of total AKT was detected. Furthermore, we noticed that HER2 inhibition activated the two fundamental players of DDR, CHK1 and CHK2 checkpoint kinases, through phosphorylation of their key sites, serine 345 and threonine 68, respectively. The total levels of both CHK1 and CHK2 remained unchanged. No differences were observed in BT474 cells either with or without AG825 treatment (Figure 3E).
This result promoted us to further investigate a possible induction of a DDR response following HER2 inhibition. C-terminal phosphorylation of histone H2AX (Ser139) (γH2AX) is required for the assembly of repair complexes and induction of DDR. Remarkably, we found that histone H2AX underwent gamma phosphorylation (γH2AX) after pharmacological inhibition of HER2 only in SKBR3 cells (Figure 3E).
Taken together, these results are consistent with the hypothesis that HER2 inhibition mediates cell cycle checkpoint activation and DDR induction and this associates with CDC25A degradation in the HER2/CDC25A double-positive breast cancer cell line SKBR3.
HER2 Down-Regulation Results in a Lower Viability and Increased Cell Death of the CDC25A-Overexpressing Cell Line
We next investigated whether the ability of the HER2 inhibitor AG825 to affect cell growth and viability of breast cancer cells was influenced by the levels of CDC25A expression.
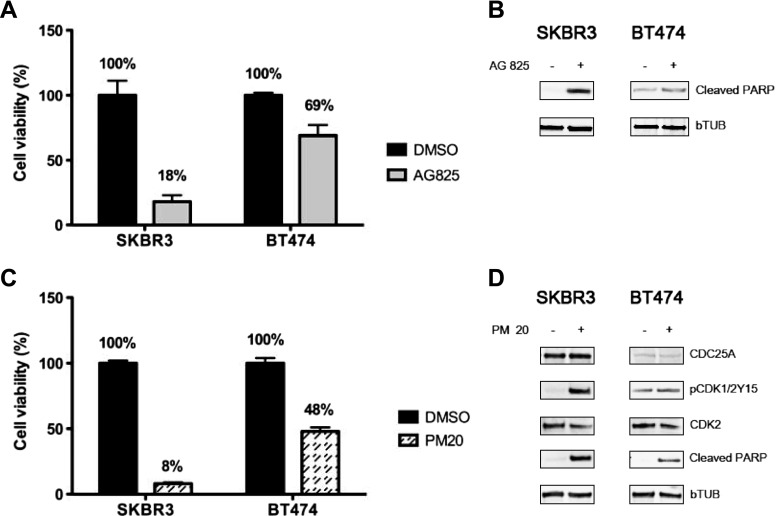
To this end, we assessed the effect of HER2 inhibition on the cell viability of the HER2-positive breast cancer cell models. We observed that AG825 efficacy in reducing cell viability was much more marked in HER2/CDC25A double-positive SKBR3 cells compared to the BT474 cells that, although HER2 positive, express basal levels of CDC25A. In particular, as determined by MTT assay, we found that after AG825 treatment the SKBR3 viable cells were 18% compared to 69% of BT474 (Figure 4A).
Figure 4.
(A and B) HER2 down-regulation affects both viability and cell death in the CDC25A-overexpressing cell line. SKBR3 and BT474 cells were treated or not with AG825 (45 µM) for 96 hours. Cell viability changes were measured by MTT assay. Results were expressed as mean percentage of cell viability ± SE of six replicates from a representative experiment that was repeated three independent times. HER2 down-regulation resulted in a lower viability of the CDC25A-overexpressing cell line (SKBR3) compared to the CDC25A basal expressing cells (BT474) (A). Cell death was evaluated by immunoblot for cleaved PARP: PARP cleavage was greater in SKBR3 compared to BT474. β-Tubulin was used as a loading control (B). (C and D) CDC25A inhibition by PM-20 induces breast cancer cell death. SKBR3 and BT474 were treated or not with PM-20 (3 µM) for 96 hours. Cell viability changes were measured by MTT assay as performed for HER2 inhibition studies: A marked reduction in SKBR3 viability was observed (C); PM-20 significantly inhibited CDC25A phosphatase activity, as evinced by increased pCDK1/2Y15 levels, and associated to increased apoptotic response, as deduced by increased PARP cleavage. β-Tubulin was used as a loading control (D).
To examine whether the marked reduction in cell viability of SKBR3 cells treated with the HER2 inhibitor was related to apoptosis, we used PARP cleavage (cleaved PARP) as a surrogate marker for apoptosis [37]. After pharmacological HER2 inhibition, the extent of PARP cleavage was greater in SKBR3 compared to BT474 (Figure 4B), supporting the notion that decreased viability of SKBR3 cells treated with the HER2 inhibitor was due to increased apoptosis.
Similarly, chemical inhibition of CDC25A activity by PM-20 resulted in increased pCDK1/2Y15 levels and associated with enhanced apoptosis (Figure 4, C and D).
CDC25A Is a Potential Predictor of Response to Trastuzumab Treatment
The data collected so far suggested that the concurrence of HER2 and CDC25A positivity hypersensitizes breast cancer cells to the pharmacological inhibition of HER2.
On these grounds, we assessed the potential clinical relevance of our findings and investigated whether CDC25A overexpression was associated to trastuzumab therapy response rate.
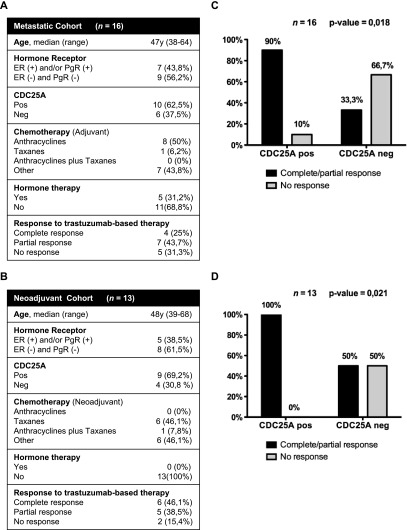
To this end, we analyzed CDC25A expression by IHC in two cohorts of HER2-positive patients that received trastuzumab either for the treatment of metastatic disease (metastatic cohort, n = 16; Figure 5A) or for neoadjuvant therapy of early-stage breast cancer (neoadjuvant cohort, n = 13; Figure 5B). In both metastatic and neoadjuvant cohorts of patients, CDC25A expression was evaluated before trastuzumab treatment: In particular, we analyzed the primary tumor sample for the metastatic cohort and the primary tumor biopsy for the neoadjuvant cohort.
Figure 5.
Statistically significant correlation between CDC25A overexpression and trastuzumab response. (A) Metastatic cohort of patients. (B) Neoadjuvant cohort of patients. Chi-squared test showed a statistically significant correlation between CDC25A overexpression and trastuzumab response in the metastatic cohort (P = .018), where 90% of patients with CDC25A overexpression showed partial or complete response to trastuzumab, while the 66.7% of CDC25A-negative patients had stable or progressive disease (C). Chi-squared test showed a statistically significant correlation between CDC25A overexpression and trastuzumab response in the neoadjuvant cohort (P = .021), where 100% of patients with CDC25A overexpression showed partial or complete response to trastuzumab (D).
In agreement with the CDC25A overexpression data obtained in the previously analyzed cohort of 313 breast cancer patients (63.5% of HER2/CDC25A double-positive tumors), we observed CDC25A overexpression in 62.5% of the metastatic cohort cases and in 69.2% of the neoadjuvant ones.
After trastuzumab treatment, HER2 and CDC25A were analyzed in the neoadjuvant cohort patients with either no response (2) or partial response (5), for whom tumor samples were available: Interestingly, these data completely overlapped with the ones obtained from the primary tumor biopsy before therapy.
Most importantly, in the two studied sets of patients, we found a statistically significant correlation between CDC25A overexpression and response to trastuzumab-combined therapy (P = .018 for the metastatic cohort and P = .021 for the neoadjuvant cohort). In particular, when patients were stratified according to CDC25A status, we observed that among the cases overexpressing CDC25A the fraction of partial/complete response was 90% in the metastatic series and 100% in the neoadjuvant series. In the same series, the response rate for CDC25A-negative cases was 33% and 50%, respectively (Figure 5, C and D).
Together, the above data, although related to a limited number of patients, support the notion of a relationship between CDC25A over-expression and trastuzumab efficacy, indicating a CDC25A prognostic relevance in the HER2-positive group of patients treated with trastuzumab-combined therapy.
Discussion
HER2 overexpression confers a more aggressive phenotype to human breast cancer and associates with poor prognosis. Two approved therapies targeting HER2, the monoclonal antibody trastuzumab and the tyrosine kinase inhibitor lapatinib, are clinically active against HER2-positive breast cancer and these anti-HER2 drugs have changed the natural history of the disease. However, therapeutic resistance to trastuzumab or lapatinib, as either single agents or in combination with chemotherapy in the metastatic setting, often occurs in a significant proportion of these patients with HER2-overexpressing tumors [38].
This suggests that some tumors either inherently possess or acquire mechanisms of resistance that allow escaping from HER2 inhibition, thus hampering the efficacy of targeted therapies.
To fulfill the promise of personalized care, it is dramatically important to improve both the understanding of the biologic features and the knowledge of the clinical behavior for each individual HER2-positive tumor. HER2 relationship with other signaling pathways has not yet been totally clarified and unveiling previously unrecognized HER2 interactions could allow a better outlining of human HER2-positive breast cancer subgroups.
This study provides the first evidence of a link between CDC25A and HER2 in breast cancer and demonstrates that the expression of CDC25A affects the response to trastuzumab of HER2-positive tumors.
In a series of 313 breast cancers, a statistically significant correlation between CDC25A expression and HER2 gene status (P = .007) was found, with 63.5% of HER2-positive tumors showing CDC25A overexpression. In addition, such evaluation highlighted a group of HER2-positive breast cancer patients characterized by CDC25A overexpression that showed the highest mortality rate and therefore a particularly aggressive phenotype (P = .005).
To better characterize this tumor subtype and the possible interaction between CDC25A and HER2, the HER2/CDC25A double-positive human breast cancer cell line SKBR3 was chosen as an in vitro model sharing a similar portrait. In these cells, HER2 inhibition was associated with proteasome-mediated degradation of CDC25A, indicating that HER2 pathway may impinge on CDC25A protein expression. Furthermore, in SKBR3 cells, CDC25A degradation triggered by HER2 inhibition was mediated by cell cycle checkpoint activation and DDR induction. This observation is in line with a previous study on a tumor-prone transgenic mouse model, showing that HER2 signaling contributes to skin tumorigenesis by activating the PI3K/AKT pathway, which in turn suppresses DNA damage-induced checkpoint activation and prevents CDC25A from degradation [39]. In fact, HER2 down-regulation in SKBR3 resulted in inhibition of the PI3K/AKT pathway and was associated with degradation of CDC25A: Treatment of SKBR3 with an HER2 chemical inhibitor resulted in a marked reduction of AKT activity and in the activation of the CHK1 and CHK2 checkpoint kinases. γH2AX was clearly detected only in SKBR3-treated cells, providing additional evidences that HER2 inhibition induced DDR in HER2/CDC25A double-positive cells.
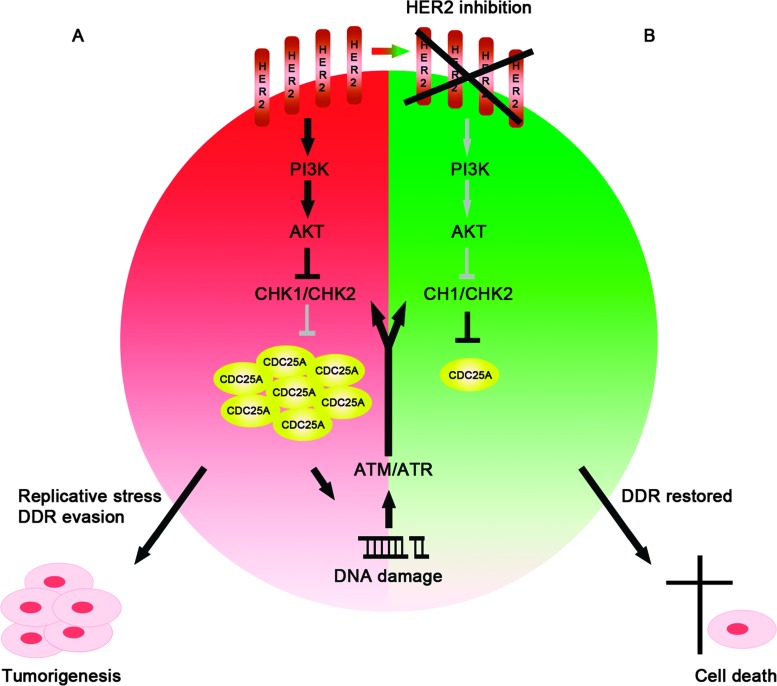
We previously showed that constitutive overexpression of CDC25A in primary human mammary epithelial cells leads to DNA replication stress and consequent checkpoint activation. Then, the prolonged exposure to high levels of CDC25A, pushing the cell throughout the cell cycle transition, may induce an impaired DDR machinery, bypassing the DNA damage cell cycle arrest [30]. The present data suggest a scenario where such DDR evasion in HER2/CDC25A double-positive breast cancer cells could be enhanced by HER2 through CDC25A stabilization. In this condition, HER2 down-regulation would lead to AKT inhibition, consequently reliving its inhibitory action on CHK1/CHK2. The activation of the checkpoint kinases would promote the degradation of CDC25A and the DDR restoration in checkpoint-proficient cells (see Figure 6).
Figure 6.
HER2 inhibition in a CDC25A overexpression context restores the DDR through a PI3K/AKT-dependent mechanism. In CDC25A-positive breast cancer cells, high levels of CDC25A, pushing the cell throughout the cell cycle transition, may induce an impaired DDR machinery, bypassing the DNA damage-induced cell cycle arrest. In HER2/CDC25A double-positive breast cancer cells, such DDR evasion could be enhanced by HER2 through CDC25A stabilization (A). In this context, HER2 down-regulation leads to AKT inhibition that stops its inhibitory action on CHK1/CHK2; the activation of the checkpoint kinases promotes CDC25A degradation and restores the DDR in checkpoint-proficient cells (B).
Consistent with such a model, HER2 chemical inhibition resulted in a much more dramatic induction of apoptosis in the CDC25A-overexpressing cell line (SKBR3) compared to the CDC25A basal expressing cells (BT474). This observation is in accord with a previous study that reported a particular sensitivity to trastuzumab-induced apoptosis by SKBR3 compared to BT474 cells [40].
The data presented here indicate that the efficacy of the pharmacological inhibition of HER2 signaling, which affects cell growth and viability of breast cancer cells, relies at least in part on CDC25A expression and the concurrence of HER2 and CDC25A positivity in breast cancer cells could hypersensitize breast tumor cells to HER2 inhibition.
Indeed, there was an association between CDC25A and trastuzumab-combined therapy response: The CDC25A expression analysis in two cohorts of HER2-positive patients that received trastuzumab either for the treatment of metastatic disease or for neoadjuvant therapy of early-stage breast cancer showed a statistically significant correlation between CDC25A overexpression and response rate to trastuzumab-based therapy (P = .018 for the metastatic cohort and P = .021 for neoadjuvant cohort).
It was recently reported that the pretreatment of a subset of triple-negative breast cancer cells with epidermal growth factor receptor inhibitors can markedly sensitize their apoptotic response to DNA-damaging chemotherapy through the reactivation of DNA damage signaling pathways [41]. Thus, the higher response to trastuzumab-combined therapy of the CDC25A-positive patients could be due to the inhibition of CDC25A that makes these tumors more susceptible to DNA damage chemotherapy.
Taken together, the presented findings identify a subtype of HER2-overexpressing breast cancer with high levels of CDC25A and a very aggressive phenotype. In particular, the HER2/CDC25A double-positive tumors may respond to HER2-targeted therapy because HER2 down-regulation results in decreased CDC25A-associated kinase activity, DDR induction, and increased apoptosis, shedding light into the mechanisms of HER2/CDC25A crosstalk.
Moreover, this study demonstrates that the interaction between CDC25A and HER2 positively modulates the response to HER2 inhibitors: CDC25A expression analysis in a larger series of HER2-positive breast tumors may validate its clinical use in identifying patients with a better response to trastuzumab-combined therapy.
Acknowledgments
We thank Marcella Mottolese, Gilda Magliacane, Alessandro Baroni, and Stefano Grassi for generously sharing expertise and ideas and Anna Sapino and Maurilio Ponzoni for critical review of the manuscript.
Footnotes
This study was supported by grants from the Italian Association for Cancer Research (AIRC) to C.D. and R.M. (MCO10016) and by the Italian Ministry of Health to C.D. and R.M. The authors declare no conflict of interest.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, Schlessinger J, Lippman ME, King CR. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Carbonell X, Castaneda-Soto NJ, Clemens M, Green M, Harvey V, Morales S, Barton C, Ghahramani P. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Schaller G, Fuchs I, Gonsch T, Weber J, Kleine-Tebbe A, Klare P, Hindenburg HJ, Lakner V, Hinke A, Bangemann N. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 over-expressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007;25:3246–3250. doi: 10.1200/JCO.2006.09.6826. [DOI] [PubMed] [Google Scholar]
- 13.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 14.Fiszman GL, Jasnis MA. Molecular mechanisms of trastuzumab resistance in HER2 overexpressing breast cancer. Int J Breast Cancer. 2011;2011:352182. doi: 10.4061/2011/352182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timms JF, White SL, O'Hare MJ, Waterfield MD. Effects of ErbB-2 overexpression on mitogenic signalling and cell cycle progression in human breast luminal epithelial cells. Oncogene. 2002;21:6573–6586. doi: 10.1038/sj.onc.1205847. [DOI] [PubMed] [Google Scholar]
- 17.Marches R, Uhr JW. Enhancement of the p27Kip1-mediated anti-proliferative effect of trastuzumab (Herceptin) on HER2-overexpressing tumor cells. Int J Cancer. 2004;112:492–501. doi: 10.1002/ijc.20378. [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf EA, Liu Y, Tucker SL, McKenzie T, Qiao N, Akli S, Biernacka A, Meijer L, Keyomarsi K, Hunt KK. A novel interaction between HER2/neu and cyclin E in breast cancer. Oncogene. 2010;29:3896–3907. doi: 10.1038/onc.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray D, Terao Y, Christov K, Kaldis P, Kiyokawa H. Cdk2-null mice are resistant to ErbB-2-induced mammary tumorigenesis. Neoplasia. 2011;13:439–444. doi: 10.1593/neo.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray D, Terao Y, Fuhrken PG, Ma ZQ, DeMayo FJ, Christov K, Heerema NA, Franks R, Tsai SY, Papoutsakis ET, et al. Deregulated CDC25A expression promotes mammary tumorigenesis with genomic instability. Cancer Res. 2007;67:984–991. doi: 10.1158/0008-5472.CAN-06-3927. [DOI] [PubMed] [Google Scholar]
- 21.Ray D, Terao Y, Nimbalkar D, Hirai H, Osmundson EC, Zou X, Franks R, Christov K, Kiyokawa H. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res. 2007;67:6605–6611. doi: 10.1158/0008-5472.CAN-06-4815. [DOI] [PubMed] [Google Scholar]
- 22.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 23.Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATMChk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- 24.Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 25.Molinari M, Mercurio C, Dominguez J, Goubin F, Draetta GF. Human Cdc25 A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO Rep. 2000;1:71–79. doi: 10.1093/embo-reports/kvd018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 27.Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 28.Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006;18:185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Cangi MG, Cukor B, Soung P, Signoretti S, Moreira G, Jr, Ranashinge M, Cady B, Pagano M, Loda M. Role of the Cdc25A phosphatase in human breast cancer. J Clin Invest. 2000;106:753–761. doi: 10.1172/JCI9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cangi MG, Piccinin S, Pecciarini L, Talarico A, Dal Cin E, Grassi S, Grizzo A, Maestro R, Doglioni C. Constitutive overexpression of CDC25A in primary human mammary epithelial cells results in both defective DNA damage response and chromosomal breaks at fragile sites. Int J Cancer. 2008;123:1466–1471. doi: 10.1002/ijc.23659. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–3251. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- 32.Masood S, Bui MM, Yung JF, Mark HF, Wong EY, Birkmeier JM, Yang SJ, Hsu P. Reproducibility of LSI HER-2/neu Spectrum Orange and CEP 17 Spectrum Green Dual Color deoxyribonucleic acid probe kit. For enumeration of gene amplification in paraffin-embedded specimens: a multicenter clinical validation study. Ann Clin Lab Sci. 1998;28:215–223. [PubMed] [Google Scholar]
- 33.Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18:3651–3664. doi: 10.1200/JCO.2000.18.21.3651. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 35.King FW, Skeen J, Hay N, Shtivelman E. Inhibition of Chk1 by activated PKB/Akt. Cell Cycle. 2004;3:634–637. [PubMed] [Google Scholar]
- 36.Hirose Y, Katayama M, Mirzoeva OK, Berger MS, Pieper RO. Akt activation suppresses Chk2-mediated, methylating agent-induced G2 arrest and protects from temozolomide-induced mitotic catastrophe and cellular senescence. Cancer Res. 2005;65:4861–4869. doi: 10.1158/0008-5472.CAN-04-2633. [DOI] [PubMed] [Google Scholar]
- 37.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 38.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 39.Madson JG, Lynch DT, Svoboda J, Ophardt R, Yanagida J, Putta SK, Bowles A, Trempus CS, Tennant RW, Hansen LA. Erbb2 suppresses DNA damage-induced checkpoint activation and UV-induced mouse skin tumorigenesis. Am J Pathol. 2009;174:2357–2366. doi: 10.2353/ajpath.2009.080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 41.Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, Yaffe MB. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]