Abstract
It has been demonstrated previously that the synthesis of amino acids from photosynthetically fixed carbon in leaves of Capsicum annuum L. cv. California Wonder occurs in the middle of the photoperiod. This paper reports experiments which identify control points regulating the carbon flow in these leaves.
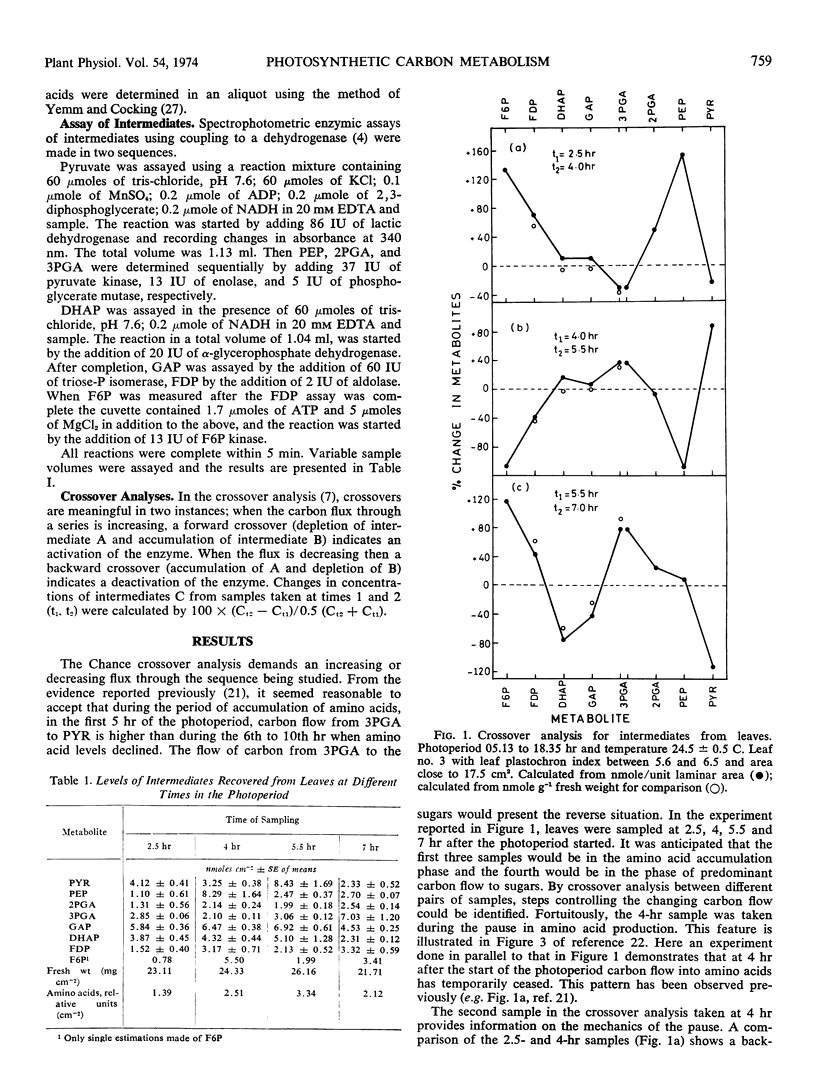
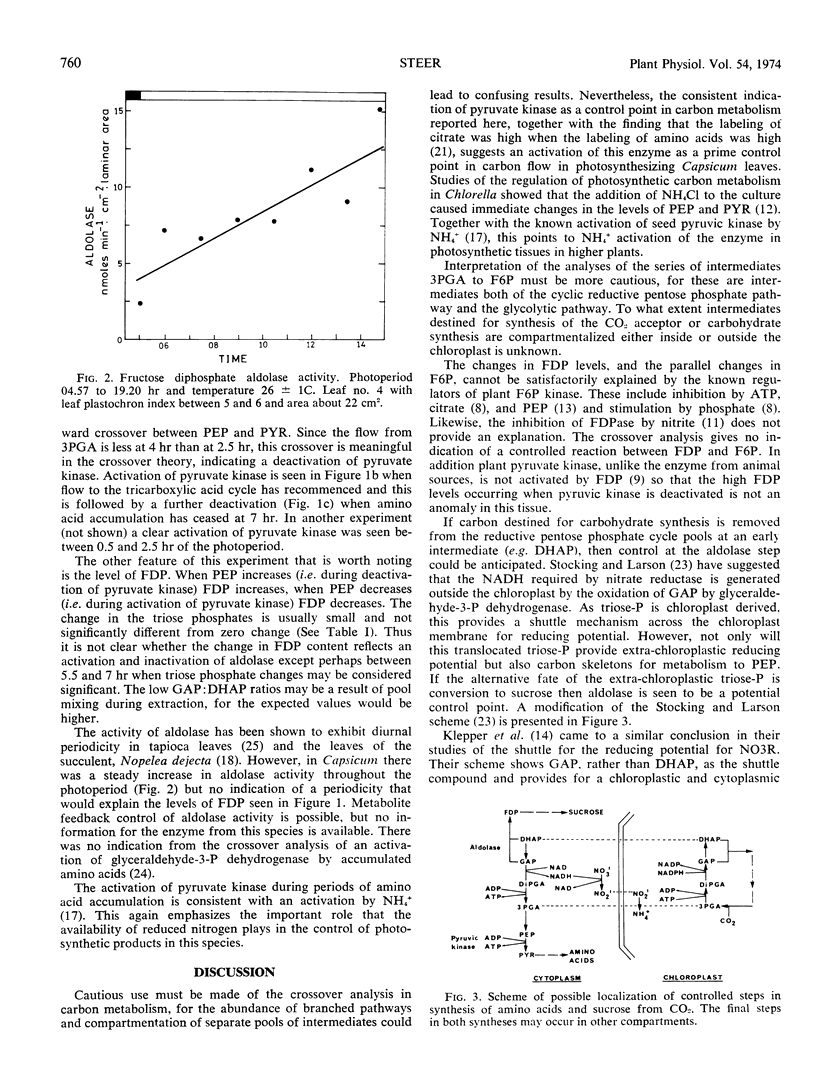
Estimations have been made of the levels of intermediates between 3-phosphoglycerate and pyruvate and between 3-phosphoglycerate and fructose 6-phosphate in leaves at different times in the photoperiod. Application of the Chance crossover analysis indicates that during periods of amino acid synthesis, pyruvate kinase is activated, possibly by ammonium ions. Fructose diphosphate aldolase could possibly be an additional control point, showing activation when amino acid synthesis has ceased. There was no indication of diurnal periodicity in the activity of fructose diphosphate aldolase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Pacold I. Chloroplast and Cytoplasmic Enzymes: IV. Pea Leaf Fructose 1,6-Diphosphate Aldolases. Plant Physiol. 1972 Mar;49(3):393–397. doi: 10.1104/pp.49.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner R., Lahat N. Changes in aldolase isoenzymes of adipose tissue induced by diabetes and ATP. Nat New Biol. 1973 Aug 8;244(136):177–178. doi: 10.1038/newbio244177a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. The regulatory properties of a plant phosphofructokinase during leaf development. Biochim Biophys Acta. 1967 Sep 12;146(1):129–137. doi: 10.1016/0005-2744(67)90079-4. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Pyruvate kinase, a possible regulatory enzyme in higher plants. Plant Physiol. 1973 Oct;52(4):312–317. doi: 10.1104/pp.52.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Chance B. Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun. 1964 Jun 1;16(2):174–181. doi: 10.1016/0006-291x(64)90357-2. [DOI] [PubMed] [Google Scholar]
- Hiller R. G., Bassham J. A. Inhibition of CO2 fixation by nitrous acid. Biochim Biophys Acta. 1965 Nov 29;109(2):607–610. doi: 10.1016/0926-6585(65)90187-1. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. Cooperativity in pea-seed phosphofructokinase. Biochim Biophys Acta. 1971 Sep 22;242(3):559–565. doi: 10.1016/0005-2744(71)90149-5. [DOI] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Analysis of adenine nucleotides and metabolic intermediates in mature and senescent leaf tissue. Anal Biochem. 1972 Feb;45(2):624–633. doi: 10.1016/0003-2697(72)90224-2. [DOI] [PubMed] [Google Scholar]
- Macnicol P. K. Metabolic Regulation in the Senescing Tobacco Leaf: II. Changes in Glycolytic Metabolite Levels in the Detached Leaf. Plant Physiol. 1973 Apr;51(4):798–801. doi: 10.1104/pp.51.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOLTER P. D., ADELMAN R. C., WEINHOUSE S. DISTINCTIVE PROPERTIES OF NATIVE AND CARBOXYPEPTIDASE-TREATED ALDOLASES OF RABBIT MUSCLE AND LIVER. J Biol Chem. 1965 Mar;240:1327–1337. [PubMed] [Google Scholar]
- Steer B. T. Control of Diurnal Variations in Photosynthetic Products: II. Nitrate Reductase Activity. Plant Physiol. 1974 Nov;54(5):762–765. doi: 10.1104/pp.54.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T. Diurnal variations in photosynthetic products and nitrogen metabolism in expanding leaves. Plant Physiol. 1973 Apr;51(4):744–748. doi: 10.1104/pp.51.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]
- Viswanathan P. N., Srivastava L. M., Krishnan P. S. Diurnal Variations in Some Enzymes of Carbohydrate Metabolism in Tapioca Leaves. Plant Physiol. 1962 May;37(3):283–287. doi: 10.1104/pp.37.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Role of Aldolase in Photosynthesis. II Demonstration of Aldolase Types in Photosynthetic Organisms. Plant Physiol. 1968 May;43(5):793–798. doi: 10.1104/pp.43.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]


