Abstract
BACKGROUND AND OBJECTIVES:
Recent public health efforts focus on reducing formula use for breastfed infants during the birth hospitalization. No previous randomized trials report the effects of brief early formula use. The objective of the study was to determine if small formula volumes before the onset of mature milk production might reduce formula use at 1 week and improve breastfeeding at 3 months for newborns at risk for breastfeeding problems.
METHODS:
We randomly assigned 40 exclusively breastfeeding term infants, 24 to 48 hours old, who had lost ≥5% birth weight to early limited formula (ELF) intervention (10 mL formula by syringe after each breastfeeding and discontinued when mature milk production began) or control (continued exclusive breastfeeding). Our outcomes were breastfeeding and formula use at 1 week and 1, 2, and 3 months.
RESULTS:
Among infants randomly assigned to ELF during the birth hospitalization, 2 (10%) of 20 used formula at 1 week of age, compared with 9 (47%) of 19 control infants assigned during the birth hospitalization to continue exclusive breastfeeding (P = .01). At 3 months, 15 (79%) of 19 infants assigned to ELF during the birth hospitalization were breastfeeding exclusively, compared with 8 (42%) of 19 controls (P = .02).
CONCLUSIONS:
Early limited formula may reduce longer-term formula use at 1 week and increase breastfeeding at 3 months for some infants. ELF may be a successful temporary coping strategy for mothers to support breastfeeding newborns with early weight loss. ELF has the potential for increasing rates of longer-term breastfeeding without supplementation based on findings from this RCT.
Keywords: breastfeeding, lactation, infant formula
What’s Known on This Subject:
Public health policy focuses on reducing formula use for breastfed infants during the birth hospitalization. Observational evidence supports this approach, but no previous studies have examined the effect of early use of small volumes of formula on eventual breastfeeding duration.
What This Study Adds:
Use of limited volumes of formula during the birth hospitalization may improve breastfeeding duration for newborns with high early weight loss. Reducing the use of formula during the birth hospitalization could be detrimental for some subpopulations of healthy term newborns.
Because breastfeeding reduces the risk of the most common infectious and allergic diseases in infancy1–3 and because longer duration of breastfeeding is associated with greater health benefits,1 the World Health Organization,4 the Centers for Disease Control and Prevention,5 and the American Academy of Pediatrics6 recommend breastfeeding for at least 1 year and exclusive breastfeeding for at least 6 months. Currently, public health efforts to improve breastfeeding duration in the United States include a strong emphasis on reducing the use of formula during the birth hospitalization. The Baby Friendly Hospital Initiative4 and the Joint Commission’s Perinatal Care Core Quality Measures7–10 both encourage eliminating formula use during the birth hospitalization for healthy breastfeeding infants. However, although 74% of US infants initiate breastfeeding, only 30% maintain exclusive breastfeeding through 3 months and only 21% are still breastfeeding at 12 months.11,12 Optimizing clinical and public health approaches to improving breastfeeding duration in the United States might have a large beneficial effect on infant and maternal health.
At birth, mothers do not immediately produce copious volumes of mature milk, but instead begin with the secretion of 1 to 5 mL of colostrum per feeding.13,14 Although providers may reassure mothers that these small volumes are normal, mothers may note that infants appear fussy and hungry. In combination with the observed small volumes of colostrum, mothers may begin to develop a concern that their milk supply is insufficient,15 and this concern has been demonstrated to be the most common reason given by mothers for discontinuing breastfeeding before 3 months.16–20 Our group hypothesized that adding the early use of limited volumes of formula in addition to breastfeeding before the onset of mature milk production would have high potential for reducing breastfeeding discontinuation for some mothers by ameliorating milk supply concern. A volume of 10 mL after each breastfeeding was chosen for study because this volume would not interfere with breastfeeding 8 to 12 times per day as recommended.4,6,21
This early, limited formula (ELF) approach is controversial for 2 reasons. First, studies have shown that mothers who feed their infants both by breast and with formula during the birth hospitalization discontinue breastfeeding earlier than mothers who breastfeed exclusively during the birth hospitalization.22–25 However, these studies have been observational and might have been confounded by weak prenatal intention to breastfeed. Similarly, mothers who experience problems with initiating breastfeeding, such as poor latch, delayed onset of mature milk production, or nipple pain, might be more likely to use formula and less likely to continue breastfeeding. The 1 quasi-randomized trial in this area found no benefit to breastfeeding duration from a hospital policy restricting the use of formula during the birth hospitalization.26
The second reason the ELF approach may be controversial is that the introduction of even small volumes of early formula might reduce some of the health benefits of exclusive breastfeeding. No studies have compared the health outcomes associated with brief early formula use followed by resumption of exclusive breastfeeding to the health outcomes associated with exclusive breastfeeding from birth. A recent systematic review by the Cochrane collaboration found no previous studies examining the effect of using small amounts of formula for a limited time on eventual breastfeeding duration or other health outcomes.27 Even if brief early formula use reduced some of the health benefits of exclusive breastfeeding, brief early formula use might still have an overall health benefit for some newborns if it prolonged total breastfeeding duration and permitted an overall longer duration of breastfeeding without formula.
Our group hypothesized that early limited formula might be most effective among infants who were at higher risk of eventual formula supplementation. In a retrospective cohort study, we found that newborns who lost ≥5% of their birth weight in the first 36 hours are at increased risk of developing excess weight loss of ≥10% of their birth weight and may therefore be at increased risk of maternal milk supply concern.28 We undertook a randomized controlled trial to determine the effect of a small amount of formula delivered for a limited time on the outcome of breastfeeding duration for newborns with ≥5% weight loss at <36 hours. To avoid exposing newborns to intact cow’s milk protein, we chose an extensively hydrolyzed formula.
Methods
We enrolled healthy, exclusively breastfeeding term (≥37 week) infants born at the University of California San Francisco Medical Center and Lucille Packard Children’s Hospital who had lost ≥5% of their birth weight before 36 hours of age and were 24 to 48 hours old at enrollment. Infants were excluded if they had lost ≥10% of their birth weight, had received formula or water, required a higher level of care than a Level 1 nursery or had mothers who were <18 years old, could not speak English or Spanish, or were making mature milk as assessed by a previously validated technique.29 Infants were weighed per hospital routine rather than for the purpose of study enrollment. Informed consent was obtained from all mothers by a study doctor or nurse. This study was approved by the University of California San Francisco Committee on Human Research and the Stanford University Administrative Panel on Human Subjects in Medical Research.
We randomly assigned 40 mother-infant pairs either to receive limited amounts of formula after each breastfeeding (intervention) or to continue exclusive breastfeeding (control). This trial was registered at clinicaltrials.gov, identifier NCT00952328. The sample size was chosen as a pilot to demonstrate feasibility. The allocation sequence for randomization was generated by an independent biostatistician stratified on location; assignments were placed into sealed opaque envelopes by an independent administrative assistant. Immediately after enrollment, a study investigator opened the sequential envelope in the presence of a second investigator and revealed the randomization arm. A blinded research assistant assessed outcomes at 1 week and 1, 2, and 3 months. Thus we had complete allocation concealment and blinded outcome assessment, although blinding of the mother and the enrolling study investigators was not possible.
Immediately after enrollment, all mothers breastfed with support from a study doctor or nurse. After this breastfeeding, mothers randomly assigned to ELF (intervention group) were taught to feed their infants 10 mL of extensively hydrolyzed formula (Nutramigen, Mead Johnson, Inc., Evansville, IN) using a feeding syringe. They were instructed to syringe-feed 10 mL of formula after each breastfeeding until mature milk production began. After the supervised breastfeeding, mothers randomly assigned to continue exclusive breastfeeding (control group) were taught infant soothing techniques for 15 minutes. This teaching session was designed to control for the amount of time the investigator spent with mothers in the intervention group teaching syringe feeding.
Immediately after these procedures, the research assistant verbally administered a questionnaire to all mothers that assessed breastfeeding self-efficacy using a modified Breastfeeding Self-Efficacy Scale—Short Form30 and maternal pain using a modified Holdcroft scale.31 Subsequently, infants received usual care from their individual physicians. To assess compliance with assigned randomization group, and assess when mature milk production began, a research assistant called mothers daily using a previously validated technique.29
A research assistant blinded to group allocation assessed outcomes by telephone at 1 week and 1, 2, and 3 months. Our primary outcomes was formula use at 1 week. Our secondary outcomes included breastfeeding and exclusive breastfeeding prevalence at 1 week and 1, 2, and 3 months.
Using an intent-to-treat approach, we used χ2 testing to compare the effect of randomization arm on dichotomous outcomes, including breastfeeding and exclusive breastfeeding at 1 week and at 3 months. We used Student’s t test to compare the effect of group assignment on infant weight and breastfeeding self-efficacy and to compare infant age at onset of mature milk production by exclusive breastfeeding at 3 months. For infants whose mothers had delayed onset of mature milk production, we used StatXact (Cytel, Inc, Cambridge, MA) to calculate the exact binomial 95% confidence intervals (CIs) for risk differences on the outcomes of exclusive breastfeeding at 1 week among infants. We used Stata 9.2 (Stata Corp, College Station, TX) for all other analyses.
Results
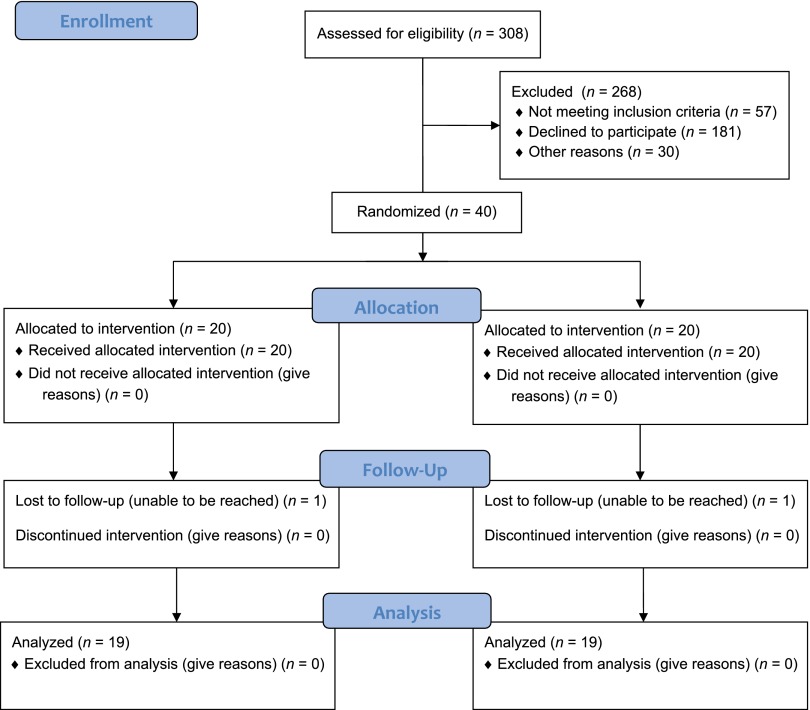
Overall, 20 (50%) infants were assigned to receive ELF and 20 (50%) infants were assigned to continue exclusive breastfeeding (Fig 1). At enrollment, weight loss was 6.0% ± 0.9% (mean ± SD) of birth weight and did not differ by randomized group allocation. Most (62%) mothers planned to breastfeed exclusively and there was no group difference. Other clinical and demographic characteristics of the baseline cohort were also similar between groups (Table 1).
FIGURE 1.
Flow diagram for randomized trial of early limited formula.
TABLE 1.
Demographic and Clinical Characteristics of the Cohort
| Characteristic | Early Limited Formula Group (Intervention) (n = 20) | Continued Exclusive Breastfeeding (Control) (n = 20) | P Value |
|---|---|---|---|
| Gestational age, wk, mean ± SD | 40.0 ± 0.8 | 39.8 ± 1.1 | .48 |
| Vaginal delivery, n (%) | 17 (85) | 17 (85) | 1.0 |
| Small-for-gestational age,a n (%) | 0 (0) | 0 (0) | NS |
| Infant age at enrollment, h, mean ± SD | 39.2 ± 6.1 | 37.6 ± 6.4 | .42 |
| Infant gender, % male | 9 (45) | 12 (60) | .34 |
| Maternal age, y, mean ± SD | 31.1 ± 5.3 | 32.5 ± 8.0 | .51 |
| Maternal race-ethnicity, n (%) | |||
| White Hispanic | 5 (20) | 9 (45) | .19 |
| White non-Hispanic | 7 (35) | 5 (25) | .49 |
| Asian | 7 (35) | 6 (30) | .74 |
| Black non-Hispanic | 1 (5) | 0 (0) | .31 |
| College graduate, n (%) | 12 (60) | 11 (55) | .75 |
| Multiparous, n (%) | 14 (70) | 10 (50) | .20 |
| Income >$50 000/y, n (%) | 7 (35) | 11 (55) | .20 |
| Percent weight loss at enrollment, mean ± SD | 6.2 ± 1.0 | 5.8 ± 0.7 | .13 |
| Plan to use formula,b n (%) | 7 (47) | 6 (32) | .37 |
NS, not significant.
Defined as <10th percentile for gestational age according to World Health Organization Growth Charts.
Available for 34 subjects.
As seen in Table 2, breastfeeding self-efficacy and maternal pain did not differ by study group. Lactogenesis II occurred at a mean of 3.1 ± 1.2 days in both groups. At their nadir, the infants’ mean weight loss was 6.8% ± 1.5% in the ELF group and 8.1% ± 2.3% in the control group (P = .10). Five cohort infants lost ≥10% of their birth weight, including 1 (5%) of 20 in the ELF group and 4 (21%) of 19 in the control group (P = .15). One control infant had missing data.
TABLE 2.
Breastfeeding Prevalence and Related Outcomes by Randomization Arm
| Outcome | Early Limited Formula Group (Intervention) (n = 20) | Continued Exclusive Breastfeeding (Control) (n = 20) | P Value |
|---|---|---|---|
| Modified breastfeeding self-efficacy score immediately after initial intervention, mean ± SDa | 3.6 ± 0.6 | 3.5 ± 0.8 | .50 |
| Infant age at onset of mature milk production, d, mean ± SD | 3.1 ± 1.0 | 3.1 ± 1.5 | 1.00 |
| Weight loss at nadir, % birth weight, mean ± SD | 6.8 ± 1.5 | 8.1 ± 2.3 | .10 |
| Excess weight loss, ≥10% of birth weight, n (%) | 1 (5) | 4 (20) | .15 |
| Modified breastfeeding self-efficacy score at 1 wk, mean ± SD | 4.0 ± 0.7 | 3.9 ± 0.7 | .84 |
| Exclusive breastfeeding at 1 wk,b n (%) | 18 (90) | 10 (53) | .01 |
| Breastfeeding at 1 mo,b n (%) | 20 (100) | 16 (84) | .06 |
| Exclusive breastfeeding at 1 mo,b n (%) | 14 (70) | 8 (42) | .08 |
| Breastfeeding at 2 mo,c n (%) | 19 (95) | 14 (82) | .22 |
| Exclusive breastfeeding at 2 mo,c n (%) | 16 (80) | 8 (47) | .04 |
| Breastfeeding at 3 mo,d n (%) | 18 (95) | 13 (68) | .04 |
| Exclusive breastfeeding at 3 mo,d n (%) | 15 (79) | 8 (42) | .02 |
Items rated on a scale from 1 (“Strongly Disagree”) to 5 (“Strongly Agree”), with positive scores associated with increased breastfeeding self-efficacy.
Available for 39 infants.
Available for 37 infants.
Available for 38 infants.
At 1-week assessment, all 39 infants with follow-up were still breastfeeding. However, in the ELF group, 2 (10%) of 20 infants had received formula in the preceding 24 hours, compared with 9 (47%) of 19 infants in the control group (risk difference 37%, 95% CI 3.4%–71.0%; P = .01). During the first week after birth, newborns assigned to ELF received 116 ± 110 mL formula, and controls received 262 ± 411 mL. Longer time until onset of lactogenesis II was associated with increased likelihood of use of formula at 1 week, with an odds ratio for formula use at 1 week of 2.0 (95% CI 1.02–3.88) for each additional day until onset of lactogenesis II. Eleven study infants had onset of mature milk production after 72 hours of age (delayed onset of lactation). Among these 11 infants, 5 (83%) of 6 randomly assigned during the birth hospitalization to continue exclusive breastfeeding used formula at 1 week, compared with 1 (20%) of 5 infants with delayed onset of lactation who had been randomly assigned to ELF during the birth hospitalization (risk difference 63%, 95% CI –2% to 96%; P = .06).
Final outcome at 3 months was obtained for 38 (95%) infants, with 2 (5%) infants unable to be contacted. Fifteen (79%) of 19 infants randomly assigned to ELF at enrollment were breastfeeding exclusively at 3 months, compared with 8 (42%) of 19 controls (P = .02). Additionally, 18 (95%) of 19 ELF infants were breastfeeding to some extent at 3 months, compared with 13 (68%) of 18 infants in the control group (P = .04). Two (10%) of the infants in the intervention group and 3 (15%) of the infants in the control group reported a febrile illness (P > .30). There were no reports of allergic disease among study infants.
Delayed onset of lactation did not affect rates of breastfeeding at 3 months but had a strong impact on exclusive breastfeeding at 3 months (Table 3). Among the 11 infants with delayed onset of lactation, 8 (73%) were using formula at 3 months, compared with 7 (27%) of 26 infants who did not have delayed onset of lactation (P < .01). No receipt of formula at 1 week strongly predicted any breastfeeding and exclusive breastfeeding at 3 months. Among 11 infants who received formula at 1 week, only 2 (18%) were exclusively breastfeeding at 3 months, whereas among 26 infants who did not receive formula at 1 week, 21 (81%) were exclusively breastfeeding at 3 months (P < .001).
TABLE 3.
Demographic and Clinical Factors: Association With Exclusive Breastfeeding at 3 mo
| Clinical and Demographic Factors | Exclusive Breastfeeding at 3 mo (n = 23) | Using Formula at 3 mo (n = 15) | P Value |
|---|---|---|---|
| Gestational age, wk, mean ± SD | 40.1 ± 0.8 | 39.5 ± 1.2 | .09 |
| Infant age at enrollment, h, mean ± SD | 39.1 ± 7 | 37.5 ± 5 | .45 |
| Infant gender, % male | 13 (57) | 7 (47) | .55 |
| Maternal age, y, mean ± SD | 32.0 ± 5.3 | 31.2 ± 8.6 | .71 |
| Multiparous, n (%) | 18 (78) | 5 (33) | .006 |
| Maternal race-ethnicity, n (%) | |||
| White Hispanic | 8 (35) | 6 (40) | .74 |
| White non-Hispanic | 7 (30) | 4 (27) | .80 |
| Asian | 7 (30) | 5 (33) | .85 |
| Black non-Hispanic | 1 (4) | 0 (0) | .41 |
| College graduate, n (%) | 14 (61) | 8 (53) | .65 |
| Income >$50 000/y, n (%) | 9 (39) | 8 (53) | .39 |
| Vaginal delivery, n (%) | 19 (83) | 13 (87) | .74 |
| Percent weight loss at enrollment, mean ± SD | 6.1 ± 0.9 | 6.1 ± 0.9 | .62 |
| Planned to give formula,a n (%) | 7 (58) | 5 (42) | .85 |
| Infant age at onset of mature milk production, d, mean ± SD | 2.6 ± 0.8 | 3.8 ± 1.5 | .004 |
| Excess weight loss, n (%) | 1 (4) | 3 (20) | .12 |
| Formula use at 1 wk, n (%) | 2 (9) | 9 (64) | <.0005 |
Determined at enrollment, available for 34 subjects.
Discussion
In this randomized trial, newborns with ≥5% early weight loss who received small amounts of formula beginning at 24 to 48 hours and ending at onset of mature milk production (ELF group) were more likely to be breastfeeding and to be breastfeeding without formula at 3 months than controls who were instructed at 24 to 48 hours to breastfeed exclusively. Our intervention set clear boundaries for the duration of supplementation by discontinuing formula at the onset of mature milk production. This approach resulted in less formula use at 1 week of age, which may have resulted in the observed improvement in breastfeeding rates at 3 months. Contrary to the current public health emphasis on reducing formula use during the birth hospitalization, our results suggest that early supplementation of limited volumes of formula before mature milk production may help support long-term breastfeeding for infants with early weight loss.
Our findings contrast with existing research in this area, which has demonstrated that use of formula for breastfed newborns during the birth hospitalization is associated with shorter breastfeeding duration.22–25 There are 2 reasons why our results may differ from previous work. First, our intervention incorporated 3 key structured techniques to reduce any negative impact of formula on breastfeeding: (1) using small, carefully measured volumes of formula, so an infant would not be satiated and demand for breastfeeding would be maintained; (2) using a syringe to prevent the nipple confusion that is associated with a bottle’s nipple; and (3) establishing a clear time frame for terminating formula use. Thus, the effect of our intervention might differ from that of unstructured formula supplementation using a bottle. Second, our randomized study design differs from previous study results based on observational evidence, which might have residual confounding both from maternal intention to breastfeed and from early difficulty establishing breastfeeding. A previous cluster-randomized trial found that formula restriction in conjunction with 9 other areas of change in breastfeeding management improved breastfeeding rates.2 However, the design of that study did not allow the authors to report the randomized effect of formula restriction alone,27 and they recently published a new analysis identifying residual confounding in their data if analyzed using a per-protocol approach.32
The ELF protocol might improve breastfeeding by 1 of 2 mechanisms. First, by improving newborn weight and hydration before the onset of mature milk production, ELF may prevent formula use after the onset of mature milk production, and formula use at this later time point might impact breastfeeding much more negatively than ELF. No use of formula at 1 week of age was the strongest predictor of exclusive breastfeeding at 3 months in this study. Second, seeing a newborn with weight loss appear fussy and hungry may exacerbate maternal milk supply concern, which is highly associated with breastfeeding discontinuation. By partially ameliorating weight loss and signs of fussiness and hunger, ELF may provide mothers with a strategy to allay their milk supply concern and continue with their desire to breastfeed for a longer duration.
Our results are important because of the current public health emphasis on reducing formula use during the birth hospitalization. Some quality measures, specifically the new Joint Commission quality measure for exclusive breastfeeding,7–10 could unintentionally reduce breastfeeding duration for some segments of the population and have a detrimental effect on maternal and infant health outcomes. Reducing unstructured, unnecessary formula use during the birth hospitalization in hospitals that currently have high rates of formula use would likely improve breastfeeding duration. However, it is possible that current efforts to reduce formula use might have the inadvertent effect of eliminating helpful formula supplementation in hospitals with low rates of unstructured, casual formula supplementation. It may be possible to identify infants at increased risk of breastfeeding discontinuation because of factors such as weight loss, and offer ELF as a supportive measure for mothers with high intention to breastfeed but high concern about weight loss. ELF provides a strategy for using limited volume and duration of supplement.
Our study has some important limitations. First, our sample size was small, leading to wide confidence intervals and inability to do any subgroup analysis or multivariate regression. If the sample size had permitted it, adjustment for parity would have been important, because there was a trend for higher parity in the intervention group and multiparity was a strong predictor of exclusive breastfeeding at 3 months. Also, in our cohort, 50% of mothers were college graduates, and most were White or Asian. Further research is needed to confirm our results in populations with increased diversity and with a sample size large enough to allow subgroup and multivariate analysis. Second, participants in our study were recruited in the San Francisco Bay Area, and overall breastfeeding duration was high in our cohort. It is possible that our intervention might be less effective among populations in which breastfeeding duration is shorter, because mothers in populations with shorter average breastfeeding duration might discontinue breastfeeding for reasons different from mothers in our cohort. Third, mothers who chose to enroll in our study were open to either exclusive breastfeeding or supplementation with formula. Therefore, our results may not be generalizable to mothers with either a strong intention to breastfeed exclusively or a strong intention to use formula. Many mothers have a strong intention regarding feeding type, and the effect of ELF on the infants of such mothers cannot be inferred from this study. Fourth, we did not include a detailed assessment of all infectious and allergic infant health outcomes. Therefore, we are unable to say whether the small volumes of formula used in ELF may have affected later infant health outcomes, and if so, whether any detrimental effect of ELF on later infant health outcomes might be counterbalanced by a beneficial effect of ELF on total breastfeeding duration. Further research, including long-term infectious and allergic outcomes, is needed to answer these important questions.
Conclusions
Our results suggest that in infants with ≥5% weight loss in the first 36 hours, supplementation with small volumes of formula in a structured manner may benefit exclusive breastfeeding at 1 week and 3 months. Further research is needed to confirm this in a larger and more diverse population, and to determine whether any such reduction in total formula use is associated with improved health outcomes. If borne out by future studies, ELF could be a strategy to manage the use of infant formula in hospital nurseries for a specific population of infants, and at the same time, increase the likelihood of longer-term breastfeeding without supplementation.
Glossary
- CI
confidence interval
- ELF
early limited formula
Footnotes
Dr Flaherman conceptualized and designed this study, obtained funding, oversaw all aspects of the data collection and analysis, and drafted the initial manuscript; Dr Aby contributed to the study design, assisted in designing the data collection instruments, coordinated and supervised data collection at 1 of the 2 data collection sites, and critically revised the manuscript for important scientific content; Dr Burgos contributed to the study design, coordinated data collection at 1 of the 2 data collection sites, and critically revised the manuscript for important scientific content; Dr Lee contributed to the study design, participated in data analysis, and critically revised the manuscript for important scientific content; Drs Cabana and Newman contributed to the study design, participated in participant enrollment at 1 of the 2 study sites, and critically revised the manuscript for important scientific content; and all authors approved this manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00952328).
FINANCIAL DISCLOSURE: Dr Cabana has served as a paid consultant for the following companies: Abbott Nutrition (Abbott Park, IL), Mead-Johnson (Evansville, IN), Nestle SA (Vevey, Switzerland), and Pfizer Consumer Products (Madison, NJ). The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants 5 K12 HD052 and 1K23HD059818-01A1 from the National Institute of Children Health and Human Development. Funded by National Institutes of Health (NIH).
COMPANION PAPER: A companion to this article can be found on page 1182, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2013-0635.
References
- 1.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1–186 [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer MS, Guo T, Platt RW, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78(2):291–295 [DOI] [PubMed] [Google Scholar]
- 3.Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr. 1995;126(2):191–197 [DOI] [PubMed] [Google Scholar]
- 4.UNICEF/WHO. Baby-friendly hospital initiative: revised, updated and expanded for integrated care, section 1, background and implementation, preliminary version. 2006. Available at: www.who.int/nutrition/topics/BFHI_Revised_Section1.pdf. Accessed January 17, 2008
- 5.US Department of Health and Human Services. Developing Healthy People 2020–maternal, infant and child health. 2010. Available at: www.healthypeople.gov/hp2020/Objectives/ViewObjective.aspx?Id=177&TopicArea=Maternal%2c+Infant+and+Child+Health&Objective=MICH+HP2020%e2%80%9312&TopicAreaId=32. Accessed April 6, 2010
- 6.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. Mar;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e827
- 7.Joint Commission. Perinatal care. 2010. Available at: www.jointcommission.org/perinatal_care/. Accessed December 8, 2010
- 8.Joint Commission. Perinatal core measures. 2010. Available at: www.jointcommission.org/assets/1/6/Perinatal%20Care.pdf. Accessed December 8, 2010
- 9.Joint Commission. Specifications manual for Joint Commission National Quality Measures (v2011A), reason for not exclusively feeding breast milk. 2011. Available at: http://manual.jointcommission.org/releases/TJC2011A/DataElem0274.html. Accessed June 17, 2011
- 10.Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2011a), measure information form. 2011. Available at: http://manual.jointcommission.org/releases/TJC2011A/MIF0170.html. Accessed June 17, 2011
- 11.Centers for Disease Control. Racial and ethnic differences in breastfeeding initiation and duration, by state - National Immunization Survey, United States, 2004-2008. MMWR Morb Mortal Wkly Rep. Mar 26;59(11):327–334 [PubMed]
- 12.Centers for Disease Control and Prevention (CDC) . Breastfeeding trends and updated national health objectives for exclusive breastfeeding—United States, birth years 2000-2004. MMWR Morb Mortal Wkly Rep. 2007;56(30):760–763 [PubMed] [Google Scholar]
- 13.Flaherman VJ, Gay B, Scott C, Avins A, Lee KA, Newman TB. Randomised trial comparing hand expression with breast pumping for mothers of term newborns feeding poorly. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F18–23 [DOI] [PMC free article] [PubMed]
- 14.Slusher T, Slusher IL, Biomdo M, Bode-Thomas F, Curtis BA, Meier P. Electric breast pump use increases maternal milk volume in African nurseries. J Trop Pediatr. 2007;53(2):125–130 [DOI] [PubMed] [Google Scholar]
- 15.Flaherman VJ, Hicks KG, Cabana MD, Lee KA. Maternal experience of interactions with providers among mothers with milk supply concern. Clin Pediatr (Phila). 2012;51(8):778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewallen LP, Dick MJ, Flowers J, et al. Breastfeeding support and early cessation. J Obstet Gynecol Neonatal Nurs. 2006;35(2):166–172 [DOI] [PubMed] [Google Scholar]
- 17.Rempel LA. Factors influencing the breastfeeding decisions of long-term breastfeeders. J Hum Lact. 2004;20(3):306–318 [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Wen SW, Dubois L, Chen Y, Walker MC, Krewski D. Determinants of breast-feeding and weaning in Alberta, Canada. J Obstet Gynaecol Can. 2004;26(11):975–981 [DOI] [PubMed] [Google Scholar]
- 19.Colin WB, Scott JA. Breastfeeding: reasons for starting, reasons for stopping and problems along the way. Breastfeed Rev. 2002;10(2):13–19 [PubMed] [Google Scholar]
- 20.Amir LH, Cwikel J. Why do women stop breastfeeding? A closer look at ‘not enough milk’ among Israeli women in the Negev Region. Breastfeed Rev. 2005;13(3):7–13 [PubMed] [Google Scholar]
- 21.Academy of Breastfeeding Medicine Protocol Committee . ABM clinical protocol #5: peripartum breastfeeding management for the healthy mother and infant at term revision, June 2008. Breastfeed Med. 2008;3(2):129–132 [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2002; (1):CD003517. [DOI] [PubMed] [Google Scholar]
- 23.Petrova A, Hegyi T, Mehta R. Maternal race/ethnicity and one-month exclusive breastfeeding in association with the in-hospital feeding modality. Breastfeed Med. 2007;2(2):92–98 [DOI] [PubMed] [Google Scholar]
- 24.DiGirolamo AM, Grummer-Strawn LM, Fein SB. Effect of maternity-care practices on breastfeeding. Pediatrics. 2008;122(suppl 2):S43–S49 [DOI] [PubMed] [Google Scholar]
- 25.Bolton TA, Chow T, Benton PA, Olson BH. Characteristics associated with longer breastfeeding duration: an analysis of a peer counseling support program. J Hum Lact. 2009;25(1):18–27 [DOI] [PubMed] [Google Scholar]
- 26.Gray-Donald K, Kramer MS, Munday S, Leduc DG. Effect of formula supplementation in the hospital on the duration of breast-feeding: a controlled clinical trial. Pediatrics. 1985;75(3):514–518 [PubMed] [Google Scholar]
- 27.Becker GE, Remmington S, Remmington T. Early additional food and fluids for healthy breastfed full-term infants. Cochrane Database Syst Rev. 2011;12(12):CD006462. [DOI] [PubMed] [Google Scholar]
- 28.Flaherman VJ, Bokser S, Newman TB. First-day newborn weight loss predicts in-hospital weight nadir for breastfeeding infants. Breastfeed Med. 2010;5(4):165–168 [DOI] [PMC free article] [PubMed]
- 29.Chapman DJ, Pérez-Escamilla R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J Nutr. 2000;130(12):2972–2980 [DOI] [PubMed] [Google Scholar]
- 30.Dennis CL. The breastfeeding self-efficacy scale: psychometric assessment of the short form. J Obstet Gynecol Neonatal Nurs. 2003;32(6):734–744 [DOI] [PubMed] [Google Scholar]
- 31.Holdcroft A, Snidvongs S, Cason A, Doré CJ, Berkley KJ. Pain and uterine contractions during breast feeding in the immediate post-partum period increase with parity. Pain. 2003;104(3):589–596 [DOI] [PubMed] [Google Scholar]
- 32.Kramer MS, Fombonne E, Matush L, Bogdanovich N, Dahhou M, Platt RW. Long-term behavioural consequences of infant feeding: the limits of observational studies. Paediatr Perinat Epidemiol. 2011;25(6):500–506 [DOI] [PMC free article] [PubMed]