Abstract
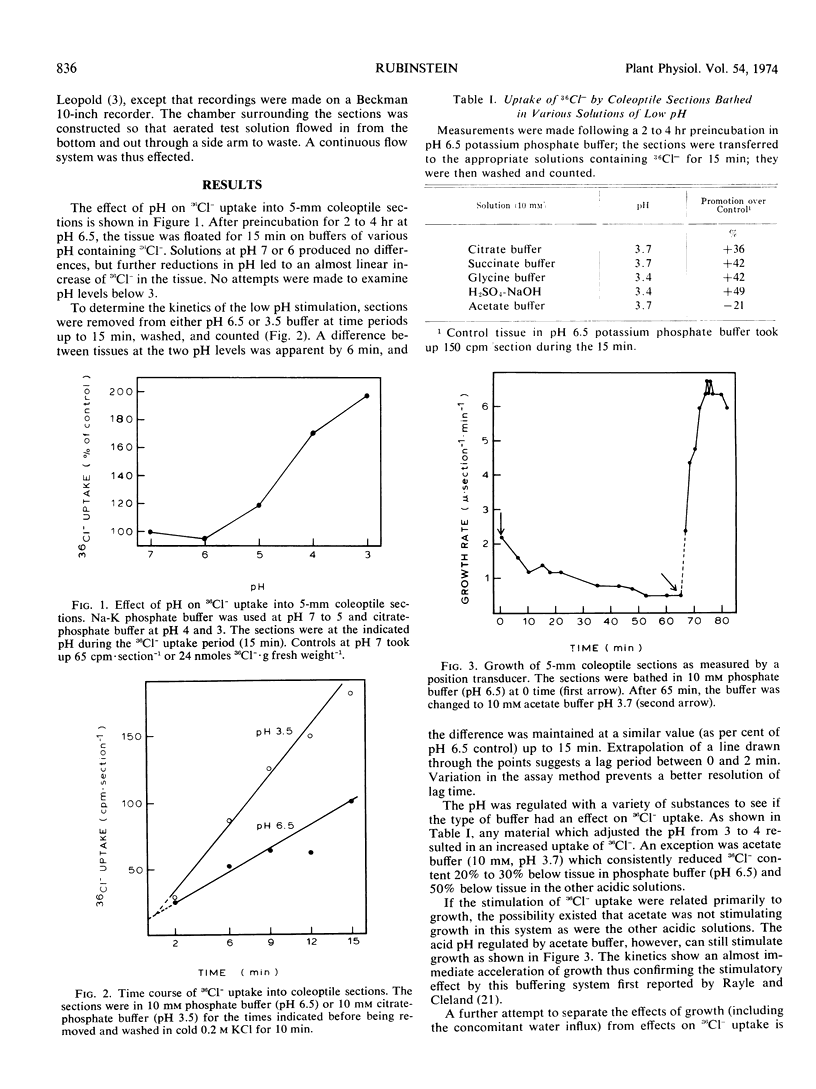
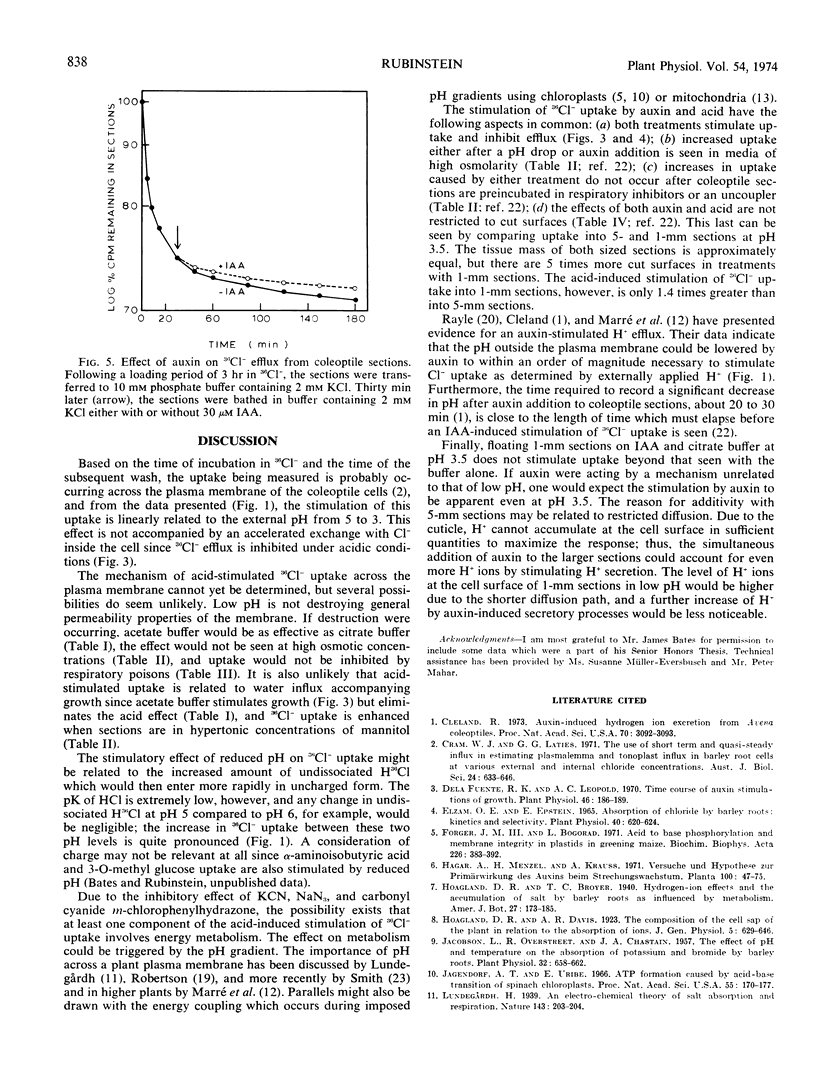
The effect of pH on 36Cl− movement into coleoptile cells (Avena sativa L. cv. Garry) was investigated and compared with effects of indoleacetic acid. 36Cl− uptake, but not efflux, is stimulated when coleoptile sections are placed in media adjusted to pH levels from 5 to 3 after a preincubation period at pH 6.5. The enhancement is seen within 2 minutes, is not correlated with growth, and is completely erased by respiratory inhibitors. In comparison to the acid-induced stimulation, the stimulatory effect of indoleacetic acid on 36Cl− uptake is also not accompanied by accelerated efflux, and indoleacetic acid does not further stimulate 36Cl− uptake into 1-millimeter sections beyond that seen at pH 3.5 without auxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dela Fuente R. K., Leopold A. C. Time course of auxin stimulations of growth. Plant Physiol. 1970 Aug;46(2):186–189. doi: 10.1104/pp.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzam O. E., Epstein E. Absorption of Chloride by Barley Roots: Kinetics and Selectivity. Plant Physiol. 1965 Jul;40(4):620–624. doi: 10.1104/pp.40.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger J. M., 3rd, Bogorad L. Acid to base phosphorylation and membrane integrity in plastids of greening maize. Biochim Biophys Acta. 1971 Mar 2;226(2):383–392. doi: 10.1016/0005-2728(71)90105-8. [DOI] [PubMed] [Google Scholar]
- Hoagland D. R., Davis A. R., Assistance of J. C. Martin THE COMPOSITION OF THE CELL SAP OF THE PLANT IN RELATION TO THE ABSORPTION OF IONS. J Gen Physiol. 1923 May 20;5(5):629–646. doi: 10.1085/jgp.5.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Overstreet R., Carlson R. M., Chastain J. A. The Effect of pH and Temperature on the Absorption Of Potassium and Bromide by Barley Roots. Plant Physiol. 1957 Nov;32(6):658–662. doi: 10.1104/pp.32.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Active H Efflux from Cells of Low-salt Barley Roots during Salt Accumulation. Plant Physiol. 1970 Jun;45(6):787–790. doi: 10.1104/pp.45.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear D. G., Barr J. K., Barr C. E. Localization of hydrogen ion and chloride ion fluxes in Nitella. J Gen Physiol. 1969 Sep;54(3):397–414. doi: 10.1085/jgp.54.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward F. C., Preston C. EFFECTS OF pH AND THE COMPONENTS OF BICARBONATE AND PHOSPHATE BUFFERED SOLUTIONS ON THE METABOLISM OF POTATO DISCS AND THEIR ABILITY TO ABSORB IONS. Plant Physiol. 1941 Jul;16(3):481–519. doi: 10.1104/pp.16.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]


