Abstract
A reliable and cost-effective scaffold for tissue-engineered vascular graft that would not only support cell proliferation and growth but also maintain cell phenotype has been a long-term challenge. In this study, we propose a biodegradable and biomimetic copolymer of gelatin with vinyl acetate synthesized via a graft copolymerization technique to generate tubular scaffolds for vascular tissue engineering. Two fabrication techniques, freeze drying and electrospinning, were used to generate the differing architectures for the scaffolds and characterized. The electrospun scaffolds were found to have a faster rate of mass loss in physiological saline of 81.72% within 4 months compared with 60% mass loss for the freeze-dried samples, though the materials were more crystalline. Vascular (v) smooth muscle cells (SMCs) were seeded on these tubes, which were then subjected to dynamic pulsatile stimulation on a vascular bioreactor for a week. Gross examination of the tissue-engineered constructs revealed that the cells secreted extensive extracellular matrix, with the dynamically conditioned samples exhibiting well-orientated SMCs and collagenous fibers in comparison with growth in static conditions. In addition, the alignment of cells in the direction of strain was greater in the electrospun constructs. The electrospun scaffolds maintained the characteristic contractile phenotype of SMCs, which was confirmed by higher gene expression rates of contractile protein markers like SM22α and calponin. A significant increase in the total matrix components (collagen and elastin) in the electrospun constructs compared with the freeze-dried samples was confirmed by biochemical analysis. The results of this study indicate that a combination approach involving a biomimetic scaffold with the nanofibrillar architecture and good mechanical strength conducive to the growth of SMCs and the use of the pulsatile forces to modulate the cell morphology and phenotypic plasticity of vSMCs helps in the successful engineering of a medial layer of blood vessel.
Key words: biomaterials, cardiology, extra cellular matrix, regeneration, tissue engineering
Introduction
The overwhelming increase of cardiovascular diseases worldwide is creating a demand for more effective therapeutic approaches. Tissue engineering may serve as a regeneration therapy in this capacity, especially for small diameter vascular grafts. This treatment option would be an alternative to the use of autologous vessels from the body, which becomes limited after repeated surgeries. This option may also provide a better alternative to synthetic grafts like Dacron and expanded polytetrafluoroethylene (ePTFE), which have had limited success as small diameter vessel grafts.1
To successfully engineer a vascular graft, scaffold properties should closely mimic the extracellular environment. Since the first attempt by Weinberg and Bell in 1986 using collagen scaffolds, several scaffold materials, both natural and synthetic, and their modifications have been explored.2,3 Although synthetic scaffolds like polyglycolic acid (PGA) and polylactic acid (PLA) and its copolymers are easy to process and possess good mechanical strength, achieving the necessary cell proliferation and matrix synthesis is still a challenge. In addition, polymer degradation products can alter the local cellular environment and thus cell function, preventing the development of a proper vascular construct. Of the natural materials, collagen-based scaffolds have been favored for preparing tissue-engineered constructs. However, the huge cost and their poor mechanical properties, along with the problem of antigenicity, have limited their use as scaffolds. We selected gelatin–vinyl acetate copolymer as the scaffold material in this study because gelatin is less immunogenic and more cost effective compared with collagen; further, the copolymerization of vinyl acetate moieties as grafts imparts mechanical stability to the polymer.
Various fabrication techniques for the scaffold fabrication have been reported,4 and the major challenge is in replicating the native in vivo environment of the cells as closely as possible. In natural tissues, cells are surrounded by extracellular matrix (ECM), which has nanofibrillar structural features ranging from the nanometer scale to the micrometer scale. With recent development in electrospinning, both synthetic and natural polymers can be produced as nanofibers that have controlled morphology and function, with diameters ranging from tens to hundreds of nanometers.5 In this article we report the influence of the nanofibrillar structure of scaffolds produced by the technique of electrospinning on the smooth muscle cell (SMC) proliferation and phenotype in comparison with a conventionally fabricated scaffold via the process of freeze drying.
Vascular SMCs (vSMCs) in vivo typically reside in mechanically dynamic environments, align in a specific direction, and exist in a contractile, differentiated phenotype, which is critical for contractile functions of vascular smooth muscle. Vascular smooth muscle tissues engineered in vitro using conventional tissue-engineering techniques usually does not lead to cell alignment and does not revert from a synthetic, nondifferentiated phenotype to a contractile, differentiated phenotype. However, both phenotypes are needed for tissue engineering in which the shift at the appropriate developmental stage would provide an appropriate functional vascular media. Several studies have shown that mechanical stimulation significantly regulates the phenotype of vSMCs in three-dimensional culture systems.6
This study investigates the effect of the scaffold architecture on the mechanical and biological properties of engineered vascular constructs based on a biodegradable synthetic polymer, a copolymer of natural biodegradable gelatin with vinyl acetate (GeVAc). vSMCs were cultured in the scaffolds in a bioreactor providing pulsatile distentions, and the resultant constructs were analyzed to determine their protein synthesis and content as well as their various mechanical properties, including suture retention strength. Ultimately, we demonstrated that a combination approach involving a biomimetic scaffold with the appropriate mechanical strength conducive to the growth of SMCs and the use of the pulsatile forces to modulate the cell morphology and phenotype would help in the successful engineering of a medial layer of blood vessel.
Materials and methods
Synthesis of GeVAc copolymer
GeVAc copolymer was synthesized as described in our earlier study.7 Briefly, gelatin type A Bloom ∼ 300 (Sigma Life Sciences, St. Louis, MO) was dissolved in de-ionized water and copolymerized with vinyl acetate (Merck, Hohenbrunn, Germany) via a free radical mechanism in the weight ratio of 71.5:28.5 using AIBN (N,N′-azobis isobutyronitrile; Spectrochem Pvt. Ltd, Mumbai, India; 0.1 % w/w) as the initiator. The reaction was carried to completion at 60°C for 4 h in an inert atmosphere of nitrogen. The copolymer (GeVAc) was then precipitated out using acetone and dried overnight.
Fabrication of scaffolds
Freeze drying
The fabrication protocol according to previously published method used a tubular mold with an annular metal rod.7 A 0.1 g/mL concentration of GeVAc was prepared in distilled water and subjected to high speed stirring at 1500 rpm using a Eurostar Power Basic Mechanical Stirrer (IKA, Staufen, Germany) with a three-blade propellor stirrer (stirrer diameter, 55 mm). The slurry was poured into polypropylene vials, which were placed in a freeze-dryer (VirTis Co., Warminster, PA). The temperature was then lowered at a constant cooling rate of 1°C/min to the final freeze-drying temperature of −80°C, which was held for 24 h before lyophilizing to produce porous scaffolds. The tubes were then cross-linked using 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride and washed several times with water to remove traces of unreacted compounds. These tubes were vacuum dried and sterilized using ethylene oxide gas. The porosity and pore sizes of the freeze-dried GeVAc copolymer scaffold was evaluated using the liquid extrusion porosimetry.
Electrospinning
The electrospinning apparatus consisted of a high-voltage power supply that can provide as much as 40 kV, with two electrodes, a syringe pump, and a collector (Rotating mandrel of diameter 3 mm at 500 rpm speed (Holmarc, India)). One of the two electrodes was attached to the needle of the syringe, and the other was attached to the collector. In the initial experiments the flow rate was varied from 0.3 to 3 mL/h, and the strength of the electric field was varied from 8 to 14 kV so as to produce uniform fibers deposited on aluminum foil (Fig. 1). For electrospinning of GeVAc to obtain nanofibrillar architectures, we used HFIP (1,1,1,3,3,3-hexafluoro isopropanol) as the solvent; solution concentration of 0.075 g/mL was optimal to give uniform fibers. The other optimum conditions for obtaining uniform fibrous scaffolds for tissue engineering included use of a 21-G needle, a voltage supply of 14 kV, flow rate of 2 mL/h, a tip-to-collector distance of 14 cm, and a mandrel speed of 500 rpm using an IKA Eurostar Power Basic Mechanical Stirrer with a three-blade propellor stirrer (stirrer diameter, 5 mm). After electrospinning, the GeVAc copolymer fibers were cross-linked in glutaraldehyde (50%) vapor for 3 h at 25°C. It is believed that cross-linking by this method could minimize the toxicity of the cross-linked materials by limiting unreacted or incompletely reacted chemical species. Liquid extrusion porosimetry measurements were difficult for the electrospun sample because the pores of electrospun scaffolds are not solid like those of hydrogels or ceramics; instead they expand due to fiber flexibility on liquid extrusion. Further, the liquid used often cannot enter pores smaller than 4 μm without pressures that can cause the scaffold to collapse, thereby potentially causing an underestimate of scaffold porosity. Hence the porosity of the electrospun copolymer was estimated by measuring the apparent density of the electrospun scaffold using the density bottle method. An average of three measurements was taken for each sample. The porosity of electrospinning nanofibrous scaffold was calculated by using the following equation:
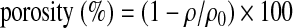 |
FIG. 1.
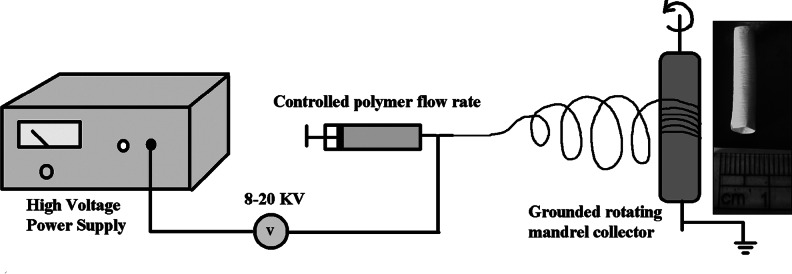
Fabrication route for obtaining electrospun tubular scaffold structure.
where ρ is the density of the electrospun scaffold and ρ0 is the density of the bulk polymer.
Biodegradation assay
An in vitro degradation profile of the materials was determined by immersing the samples in phosphate-buffered saline (PBS) and measuring mass loss with respect to time. The GeVAc copolymer samples were incubated in PBS at 37°C for 1–4 months. Four samples were incubated for all the monthly studies. The respective samples after each week were removed, frozen, and lyophilized. The mass loss was calculated by comparing the initial mass (W0) with that at a given time point (Wt). The results were presented as means±standard deviation.
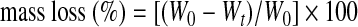 |
Media uptake ability
The ability of the scaffold to absorb the medium was studied to understand the diffusion of medium and nutrients into the scaffold, which are essential for cell viability. Previously weighed scaffolds were immersed in PBS pH 7.4 in preweighed containers for known intervals of time. The medium was carefully withdrawn at intervals and wet weight measured. Percentage swelling was calculated using the following formula:
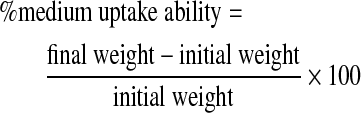 |
Bioreactor culture
SMCs obtained from rat aorta was cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% (v/v) fetal bovine serum 1% antibiotic solution. Nine scaffolds (4-mm-diameter tubular scaffolds of 30-mm length) were placed into polypropylene seeding tubes containing a vented cap. Seeding solution was added to each tube to yield a seeding density of approximately 1×108 cells/scaffold. The seeding efficiency was found to be approximately 85% for both the scaffold structures. The tubes were then placed on slotted racks in an incubator operating at 37°C and 5% CO2 for a period of 7 days in static culture. For dynamic conditioning, the scaffolds were cultured for 2 days in static culture, and for the subsequent 5 days mechanical conditioning was done in a bioreactor, during which the culture medium was changed every day.
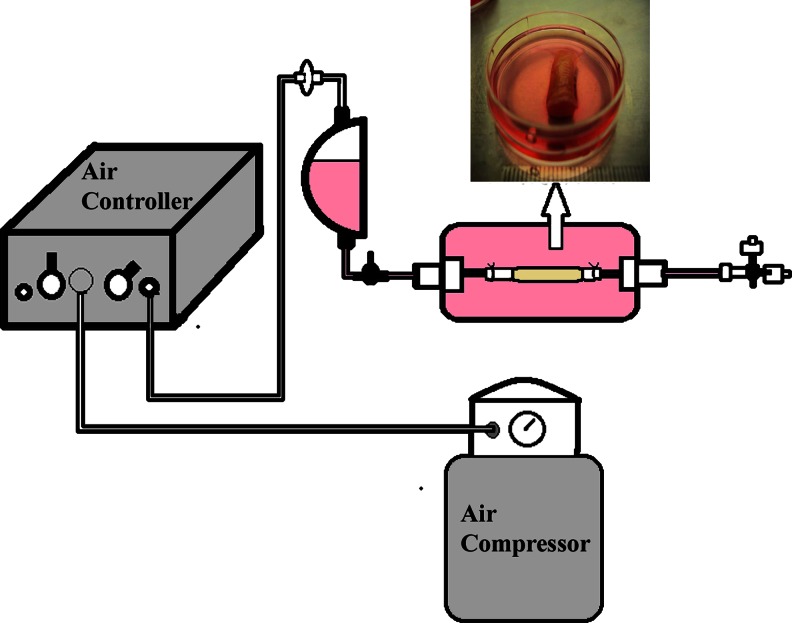
The bioreactor system imparted precise and repeatable strains of 10% strain, mimicking the rate experienced by the cardiac wall at pulsatile frequencies close to 1 Hz (i.e., with every heart beat). We used a silicon sleeve to impart the strain to the construct. A pressure of ∼155 kPa (22.5 p.s.i.) was pulsed to the system in a repeatable fashion of 1 Hz to obtain a uniform distention of 10% strain. The application of mechanical strain to tubular GeVAc constructs was achieved using this system (Fig. 2).
FIG. 2.
Bioreactor setup showing the electrospun gelatin with vinyl acetate (GeVAc) construct retrieved after mechanical stimulation.
Cell proliferation assay by DNA quantification
Assessment of cell number in constructs and their proliferation after the culture period was performed using a fluorometric dye assay. The nonadherent or dead cells were washed off before the constructs were digested in the extraction medium. For analysis, samples were lyophilized for 4–8 h and then digested in Proteinase K (Sigma Chemical Co.) lysis buffer. Hoechst 33258 dye (Sigma Chemical Co.) solution was added to each well, and fluorescence was measured on a plate reader (HIDEX Chameleon Plate Reader, Turku, Finland). DNA standards were used to calculate the DNA concentration in the initial samples. Actual cell counts were then calculated on the basis of 7.6 pg DNA/cell.8
Biochemical analysis of constructs
Collagen and elastin were assayed by techniques adapted from Brown et al.9 For each assay, samples were weighed (average wet weight 0.03 g) prior to extraction. Following the extraction steps, the collagen and elastin were assayed according to the guidelines provided with the Sircol and Fastin assay kits, respectively (Biocolor Ltd., Newtownabbey, Ireland) using a plate reader (ASYS UVM 340, ASYS Hitech GmBH, Eugendorf, Austria). The unseeded GeVAc constructs were used as the control.
Gene expression study
Total RNA was isolated from cells that were seeded and cultured on scaffolds for the study. Total cellular RNA was extracted from the scaffold using Trizol-reagent kit and quantified using spectrophotometry. cDNA was prepared using 2 μL of RNA, 1 μL of oligo-T (dT15; 100 μM) primer, 0.5 μL of RNase Inhibitor (40 U/μL; Fermentas International Inc., Burlington, Canada), 2 μL of dNTP mix (10 mM; Fermentas International Inc.), and 1 μL of reverse transcriptase enzyme (200 U/μL) at 42°C for 60 min, followed by enzyme inactivation at 70°C for 10 min. The molecules probed as part of this study were GAPDH, collagen type I, tropoelastin, α–smooth muscle actin, SM-22α, and calponin. The codon sequences for the primers are provided in Supplementary Table S1.
For quantitative determination, real-time polymerase chain reaction (PCR) was performed using a DNA Engine Opticon System (Bio-Rad Laboratories, Inc., Hercules, CA). For each gene, the quality and specificity were assessed by examining PCR melt curves after real-time PCR. The results were normalized by being expressed relative to the amount of GAPDH mRNA determined in each sample. The results of real-time PCR were represented as fold increase or decrease of gene expression with respect to control rat aorta SMCs at 1 week of culture.
Histological analysis of the constructs
In order to evaluate the cell distribution and eventual cell/matrix alignment, histology was performed. Appropriate samples were cut from the construct, carefully rinsed with PBS, fixed in 3.7% formaldehyde for 72 h, embedded in paraffin, and then sliced into 4-μm-thick sections that were stained with either hematoxylin and eosin or Verhoeff's elastic stain for elastin.
Scanning electron microscopy imaging of the constructs
For ultrastructural analysis, unseeded and seeded scaffold samples were processed for characterization by scanning electron microscopy (SEM). After culture, samples were fixed with 3% glutaraldehyde for 2 h. After thorough washing with water, the specimens were dehydrated using graded ethanol changes, critical point dried (Hitachi HCP-2, Hitachi, Koki Co., Ltd., Tokyo, Japan), gold sputtered in a vacuum (Hitachi E101), and examined by means of secondary imaging under a 15-kV scanning electron microscope (Hitachi S 2400) for observation of cell and surface morphology.
Mechanical studies on ring samples
Mechanical properties of the samples were assessed through uniaxial tensile testing apparatus. Tubular constructs were cut into 3-mm rings. The rings were then loaded to failure at 1 mm/s, while force and displacement data were recorded simultaneously through load cell signal acquisition. To calculate stress from the measured force, the area of force application was approximated as two rectangles with sides equal to the width and wall thickness of the ring. Each sample was run in triplicate and the stress–strain data were used to obtain the tensile strength and percentage of elongation at break. The estimated burst pressure (BP) was calculated from ultimate tensile strength (UTS) measurements by rearranging the law of Laplace for a pressurized thin-walled hollow cylinder:10
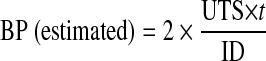 |
where t is the thickness and ID is the unpressurized internal diameter of the vascular constructs.
Suture retention strength
For suture retention strength testing, 3-0 polypropylene sutures (Ethicon Inc., Bridgewater, NJ) were placed in four quadrants of the vessel wall (n=4) at 1 mm from the vessel edge. A constant elongation (1 mm/min) was applied along the longitudinal axis of the vessel until the suture was torn through the vessel edge, and the maximum suture retention strength was recorded.
Statistical analysis
For all experiments, statistical significance of differences between groups was determined using t-test for two treatments and one-way ANOVA for more than two treatments with the Tukey post hoc test. Data were expressed as a mean±SD. Any reference to a difference implies statistical significance at the level p<0.05 unless otherwise mentioned.
Results
Synthesis of GeVAc copolymer
Vinyl acetate was grafted onto gelatin to improve the strength and stability of native gelatin via a free radical mechanism, and the grafting efficiency was found to be 50.37±9.39% with a grafting ratio of 0.5. A weight ratio of 71.5:28.5 (gelatin to vinyl acetate) was used for the synthesis for which only about 50% grafting efficiency was seen to take place. This ratio was optimized based on the easy solubility of this sample in water, which aided in the further fabrication of tubular samples.
Fabrication of scaffolds
The GeVAc polymer could be easily fabricated to form microporous structures. The range of pore sizes and the distribution attained for the freeze-dried GeVAc scaffolds with a porosity percentage of 75.7% and an average pore size of 90.2 μm were found to be optimal for the growth of cells, which was confirmed in culture studies (Supplementary Fig. S1).
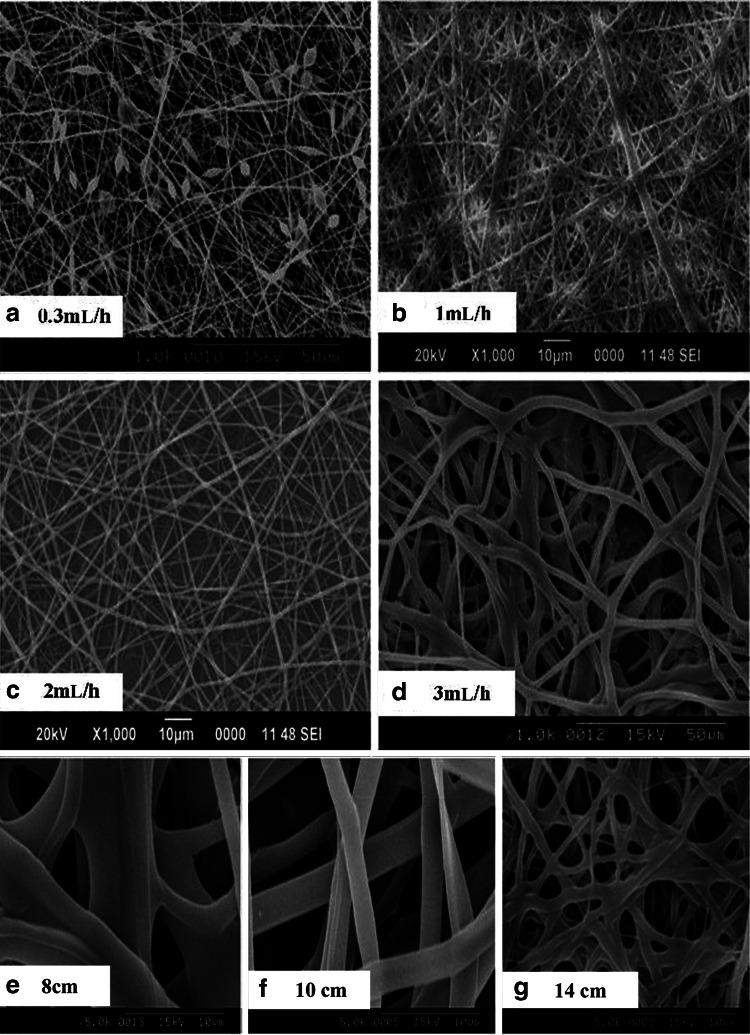
For electrospinning of GeVAc to obtain nanofibrillar architectures, we used HFIP as the solvent. Variables accounted for in the determination of spinning conditions were concentration of polymer solutions, voltage, flow rate, and distance of tip and collector. The diameter of the fibers was measured using the ImageJ software, and it was observed that by decreasing the concentration of gelatin in the solvent HFIP from 10% to 2%, the average size of gelatin fibers was reduced from 1.47±0.507 to 0.141±0.021 μm (p<0.01). Although the reduction in GeVAc concentration reduced the fiber size to 100 nm, this reduction was accompanied by a significant formation of beads. More uniform distribution of fibers was obtained for the GeVAc solution of concentration 0.075 g/mL, which showed an optimum fiber diameter of 1.004±0.171 μm while maintaining an optimum pore size between the fibers. Next, the effect of process variables was studied for the 7.5% concentration at different voltage conditions to see if any change in the fiber diameter was attained. With an increase in the voltage from 8 to 14 kV the fiber diameter decreased significantly from 1.76±0.76 μm to 0.68±0.19 μm (p<0.01; Fig. 3). We then systematically varied the delivery rates of the protein solutions between 0.3 and 3 mL/h in order to optimize the conditions that yield bead-free and uniform fibers (Fig. 4). Increasing the delivery rate from 0.3 to 3 mL/h yielded a significant increase (p<0.01) in fiber diameter from 0.93±0.80 μm to 2.19±0.54 μm. Flow rate at 0.3 mL/h showed beaded fibers. Flow rate at 2 mL/h showed uniform fiber formation, with a fiber diameter of 0.77±0.25 μm. The effect of tip-to-collector distance was also studied, and as the tip-to-collector distance increased from 8 to 14 cm, the fiber diameter decreased significantly from 3.65±2.14 μm to an optimum range of 0.67±0.21 μm (p<0.01; Fig. 4).
FIG. 3.
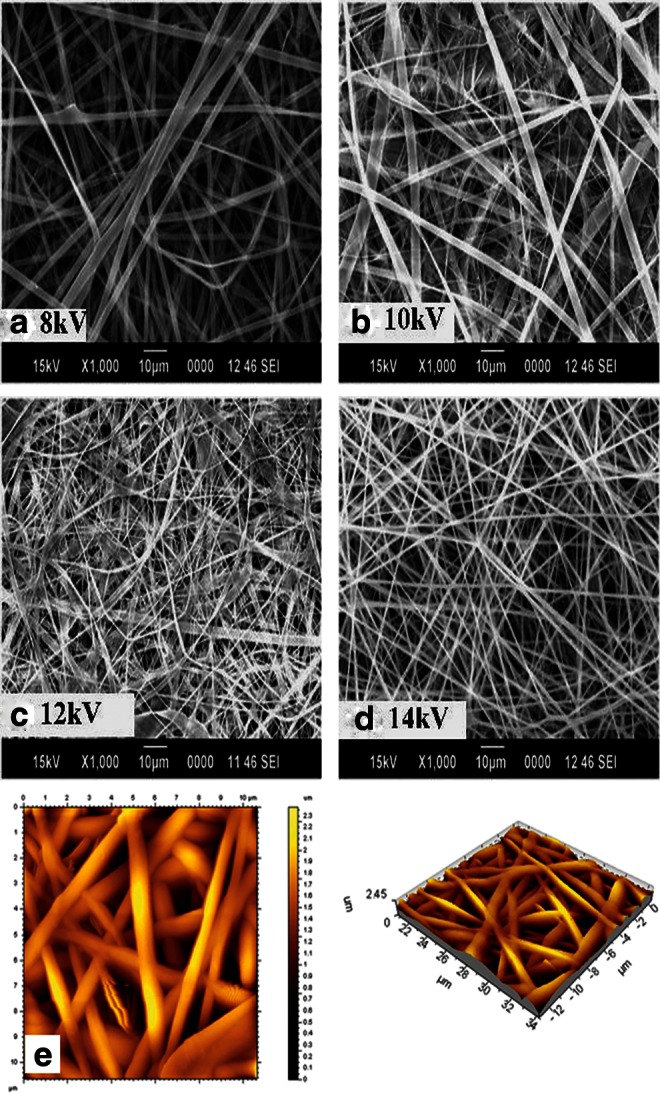
Representative scanning electron microscopy (SEM) images of the electrospun GeVAc using different voltage conditions: (a) 8 kV, (b) 10 kV, (c) 12 kV, and (d) 14 kV. (e) Noncontact atomic force microscopy image of the optimized GeVAc electrospun fibers showing height data and three-dimensional image showing the interconnectivities and pore structures on the scaffold.
FIG. 4.
Representative SEM images of the electrospun GeVAc using different flow rate conditions: (a) 0.3 mL/h, (b) 1 mL/h, (c) 2 mL/h, and (d) 3 mL/h, and at different tip-to-collector distances: (e) 8 cm, (f) 10 cm, and (g) 14 cm.
The optimum fiber diameter obtained on standardization was found to be in the range of 600–1000 μm. The atomic force microscopy (AFM) images as well as the SEM images supported our findings, and good open fibrous structures were obtained as evident in the AFM images (Fig. 3). The porosity measurement results showed a porosity of 73.5% indicating that the fibers were porous, with the percentage of porosity similar to that of the freeze-dried samples, which was beneficial for the adherence and proliferation of the cells.
Biodegradation assay
The percentage weight loss of the freeze-dried gelatin copolymer after the nonenzymatic degradation study in PBS was found to be almost 60% after 4 months of incubation in PBS at 37°C (Fig. 5a). The degradation was found to increase significantly after the third month. However, a significantly greater increase in the mass loss was observed for the electrospun samples, which showed a mass loss of about 81.72% after 4 months' incubation.
FIG. 5.
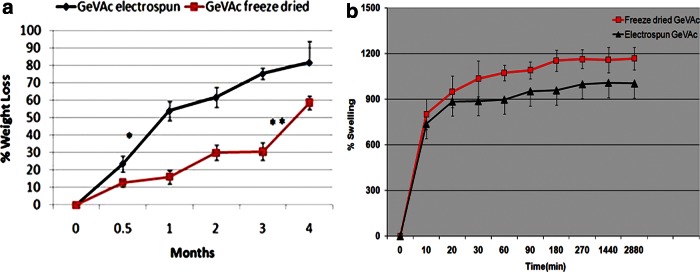
(a) Biodegradation study of electrospun and freeze dried GeVAc in phosphate-buffered saline (PBS, pH 7.4) for a period of 4 months (n=6); *p<0.01). (b) Media uptake ability of the freeze dried and electrospun GeVAc.
Media uptake ability
The percentage swelling of GeVAc scaffolds as a function of time is represented in Figure 5b. The freeze-dried scaffold was seen to swell within the first 5 min to 300%. The swelling equilibrated at 1150% after 2 h, which was indicative of good medium uptake ability. Similar results were obtained for the electropsun GeVAc, which was seen to equilibriate at approximately 1000% at 5 h. No further swelling was observed up to 48 h.
Mechanical stimulation studies in vascular bioreactor
The SMC-seeded scaffolds placed on silicon sleeves were cultured for a total period of 7 days and given mechanical stimulation of 10% strain at 1 Hz for 5 days. A glossy tubular structure was obtained for all the scaffolds after the culture period as shown in Figure 6. All scaffolds maintained their lumen diameter of 4 mm. The electrospun scaffolds, however, showed denser tissue growth when compared with the freeze-dried samples.
FIG. 6.
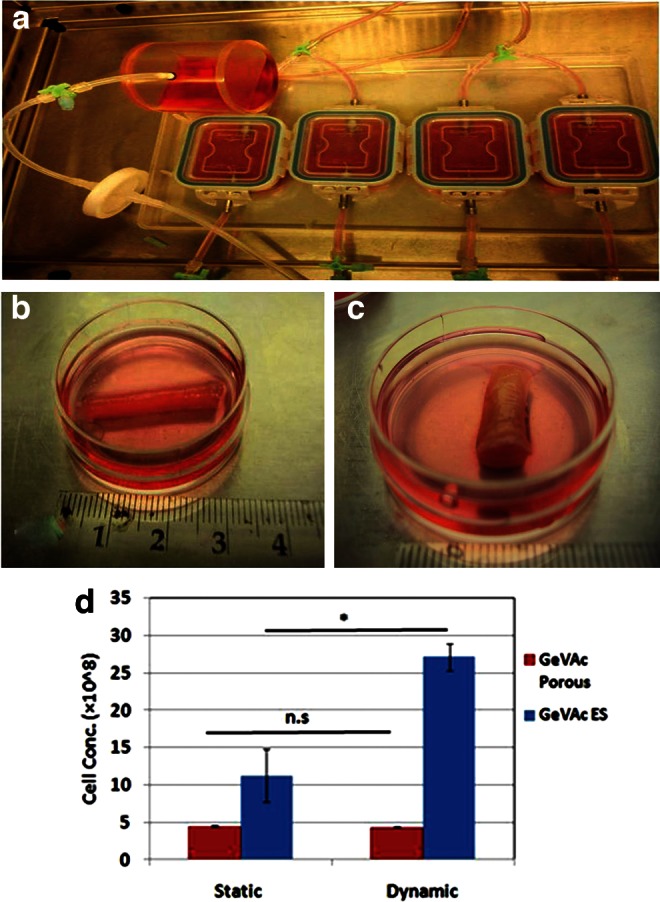
(a) Bioreactor set up used for the study. (b) Freeze-dried GeVAc construct retrieved after mechanical stimulation. (c) Electrospun GeVAc construct retrieved after mechanical stimulation. (d) DNA proliferation assay of scaffolds in static and dynamic culture. *p<0.05.
Cell proliferation assay
After 7 days of culture, the degradable constructs exposed to physiological pulsatile flow conditioning were analyzed for their proliferation rates and compared with constructs cultured under static conditions (Fig. 6d). The cellularity in the electrospun GeVAc dynamic constructs was significantly higher than that in the static constructs (p<0.01), indicating that pulsatile conditioning enhanced cell survival and proliferation. The freeze-dried GeVAc construct, however, showed no significant change in the proliferation rate on providing mechanical stimulation.
Biochemical analysis of constructs
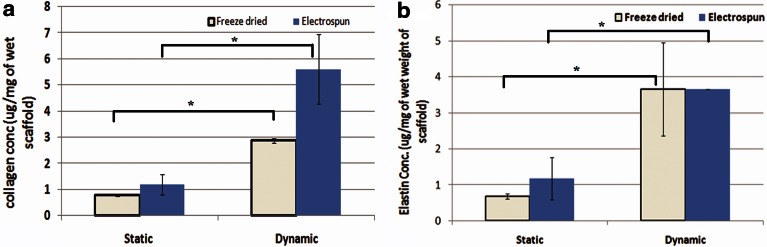
Collagen was quantitatively measured in the engineered vessel wall after it was subjected to static and dynamic culture. As shown in Fig. 7a, collagen content in the dynamic cultured GeVAc freeze-dried sample was 2.87±0.10 μg/mg of wet weight of scaffold, which was found to be significantly higher than the amount of total collagen quantified in the static cultured constructs (0.77±0.02 μg/mg of wet weight of scaffold, p<0.05). The electrospun GeVAc samples also showed a significant increase in the total collagen content in the mechanically stimulated samples (5.66±1.33 μg/mg of wet weight of scaffold) when compared with the static cultured electrospun samples (1.2±0.39 μg/mg of wet weight of scaffold; p<0.05). It was also observed that the total collagen content of the mechanically stimulated GeVAc electrospun scaffolds after 7 days of culture reached about 75% of the values that were obtained for the 3-cm segment of native rat abdominal aorta (7.392±0.506 μg/mg of wet weight).
FIG. 7.
(a) Collagen and (b) elastin content of cell–polymer constructs in static and dynamic culture. Values represent the mean and standard deviation; n=3, *p<0.05.
Quantification of elastin revealed a significant increase in the elastin deposition in the dynamic constructs when compared with the static constructs of GeVAc in both the freeze-dried and electrospun fabricated forms (Fig. 7b). The elastin content in the 7-day static culture for the freeze-dried GeVAc was found to be 0.68±0.07 μg/mg of wet scaffold, which is significantly lower than that of the dynamic constructs of 3.66±1.29 μg/mg of wet scaffold (p<0.05). The elastin content for the electrospun GeVAc in static culture was also found to be significantly lower (p<0.05) compared with the electrospun GeVAc constructs that underwent mechanical stimulation (static culture=1.18±0.18 μg/mg of wet scaffold; dynamic culture=3.65±0.004 μg/mg of wet scaffold). These values were compared with native 30-mm segments of rat abdominal aortae, which showed a value of 4.567±0.18 μg/mg of elastin per wet weight of tissue. These results showed that our findings for 7-day culture for the GeVAc scaffolds was almost reaching 80% of the values as shown by the native vessel. The tropoelastin gene expression was also confirmed by the real-time PCR data.
SEM imaging of constructs
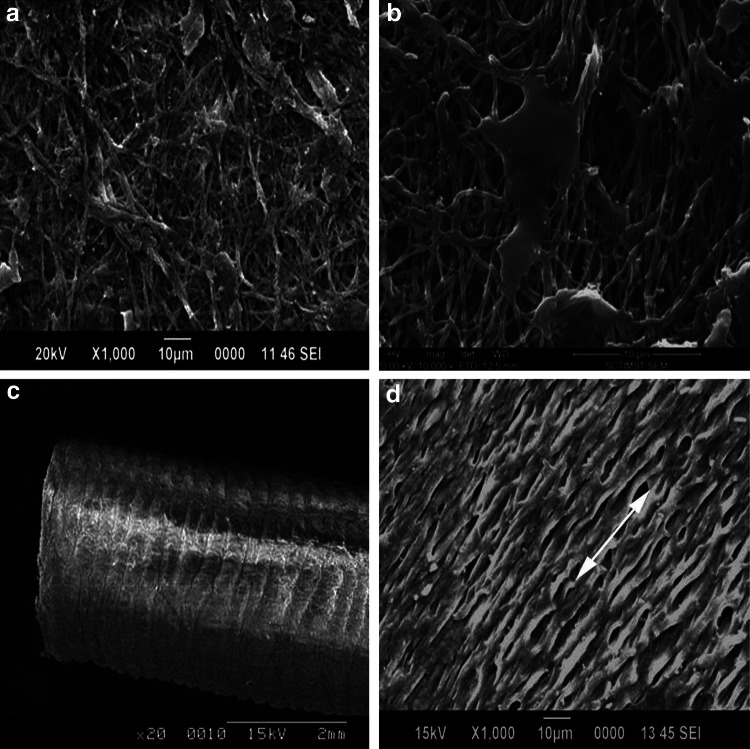
To study the growth of SMCs on the tubular constructs SEM was performed on dry specimen samples. The retrieved GeVAc freeze-dried scaffolds subjected to static and dynamic culturing were evaluated, and on mechanical stimulation the cells were seen to align themselves in the direction of pulsatile strain.7 The cells were also seen to create a sheath or canopy of ECM and cells on the surface covering up the pores of the construct. The scanning electron micrographs of the GeVAc electrospun (Fig. 8) scaffolds revealed that greater alignment of cells in the circumferential direction occurred on the scaffolds with mechanical stimulation compared with the static cultured scaffolds.
FIG. 8.
SEM analysis of the electrospun GeVAc constructs. (a, b) Electrospun constructs in static culture. (c) Electrospun GeVAc tube. (d) Alignment of rat aorta smooth muscle cells in electrospun constructs after dynamic culture.
Histological analysis of constructs
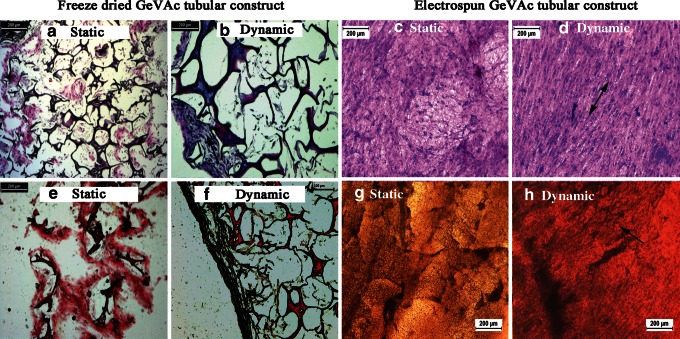
Micrographs of sections stained with hematoxylin and eosin show that, while cells were dispersed throughout the entire thickness of the constructs, a large concentration of cells migrated to or proliferated on the outer wall in the freeze-dried GeVAc samples. However, the electrospun samples showed an even distribution of cells on the scaffold (Fig. 9). The cell alignment on application of mechanical stimulation was also evident with the staining.
FIG. 9.
Distribution and alignment of cells and collagen and elastin deposition on static and dynamic conditioning of freeze dried and electrospun GeVAc constructs. (a–d) Hematoxylin and eosin staining done on static and dynamic samples (arrow depicts the cell alignment). (e–h) Verhoeff's elastic stain to show the elastin fibrils formed. Arrows point to the elastin fibers.
The Verhoeff's elastic stain was used to detect the deposition of collagen and elastin ECM components. In walls of both the static and dynamic cultured constructs, collagen deposition was observed within the freeze-dried scaffolds. The electrospun samples were seen to have more even deposition of collagen throughout the structure. However, the elastin fiber formation was observed in the peripheral region of the GeVAc freeze-dried constructs in both static and dynamic conditioning. The elastin fibers were also evident in the electrospun GeVAc scaffolds where the elastin fragments appeared as dark, geometric particles throughout the walls of the constructs.
Gene expression studies
Real-time PCR analysis revealed a significant increase in the rate of collagen and elastin expression in all the groups in a comparison of the static culture with the dynamic culture (Fig. 10). Based on a comparison of the nanofibrillar and freeze-dried GeVAc scaffolds in dynamic culture, there was a significant increase of ∼2-fold in the collagen gene expression for the nanofibrillar GeVAc; however, no pronounced fold increase in tropoelastin gene expression was observed between the two scaffolds.
FIG. 10.
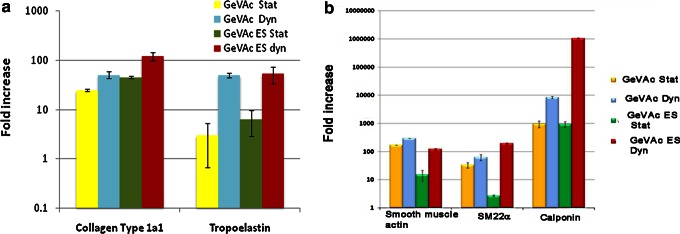
Real-time PCR analysis of the expression of (a) collagen type 1α1 and tropoelastin gene and (b) contractile phenotype marker expression. The results are represented as fold increase of constructs with respect to the two-dimensional cultured rat aorta smooth muscle cells (n=3).
The expression data for α-SMA indicated a significant fold increase in gene expression of cells in constructs harvested after mechanical stimulation or dynamic culture. However, the cells seeded on the electrospun GeVAc was found to show a fold decrease in the expression of SMA (p<0.01) when compared with the freeze-dried samples.
The cells in the electrospun scaffolds undergoing dynamic culture were found to have a higher fold increase in SM22α expression and calponin expression, the late intermediate marker for contractile phenotype, when compared with the same scaffolds fabricated in the freeze-dried form. The nanofibrillar nature of the scaffolds and the application of mechanical stimulation were also seen to influence the modulation of cell phenotype to a contractile nature, which is a prerequisite for the successful regeneration of the medial layer.
Mechanical testing of the constructs
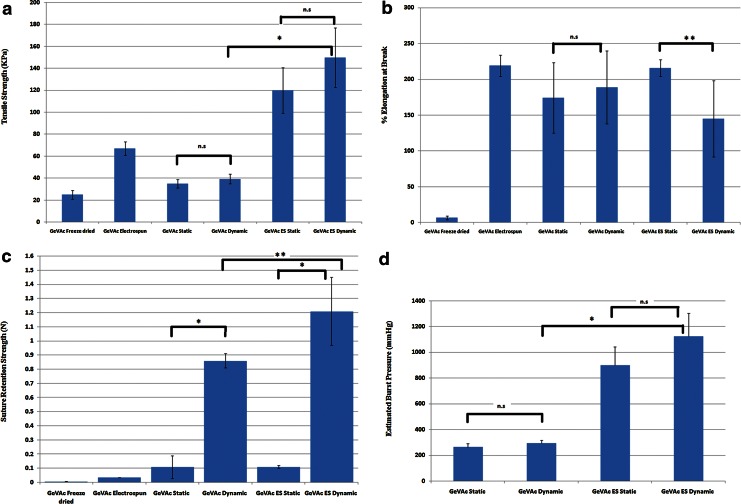
Biomechanical testing of ring samples taken from the constructs was done to ascertain the strength and elasticity of the vessels after the 7-day culture (Table 1). It was found that all the constructs showed an increase in the tensile strength following mechanical stimulation. The tensile strength of the GeVAc freeze-dried samples showed a slight increase from 34.99±3.86 kPa to 39.28±4.32 kPa. The electrospun GeVAc cell-seeded constructs showed the highest tensile strength among the samples (static=119.99±45.83 kPa and dynamic culture=149.82±37.07 kPa). The percentage of elongation at break was also performed, and the results indicated that there was no significant difference for the elongation characteristics for the freeze-dried GeVAc scaffolds in static and dynamic culture. However, the electrospun GeVAc was found to have a decrease in the elongation for the constructs grown in dynamic culture (p<0.05; static=215.65±11.82% and dynamic=144.87±53.29%). The estimated burst pressure values were significantly higher for the GeVAc electrospun samples, with a burst pressure value of 1123.72±178.03 mm Hg in the dynamic cultured samples (Fig. 11d).
Table 1.
Mechanical Properties of the Cultured Tubular Constructs
| Sample name | Tensile strength (kPa) | Elongation at break (%) | Suture retention strength (N) |
|---|---|---|---|
| GeVAc freeze-dried without cells | 25.03±10.70 | 6.79±2.7 | 0.0063±0.0002 |
| GeVAc electrospun without cells | 67.15±6.14 | 219.13±15 | 0.034±0.001 |
| GeVAc static culture | 34.99±3.86 | 174.28±48.97 | 0.11±0.08 |
| GeVAc dynamic culture | 39.28±4.32 | 188.80±91.00 | 0.86±0.05 |
| GeVAc electrospun static culture | 119.99±45.83 | 215.65±11.82 | 0.11±0.01 |
| GeVAc electrospun dynamic culture | 149.82±37.07 | 144.87±53.29 | 1.21±0.24 |
FIG. 11.
Graph depicting the (a) Tensile strength (kPa), (b) % elongation at break, (c) suture retention strength (N), and (d) estimated burst pressure (mm Hg) values for the freeze-dried and electrospun samples in static and dynamic culture. n=3; *p<0.01; **p<0.05; n.s., non significant.
Suture retention test
The suture-holding retention strength was examined for all the scaffolds in both static and dynamic culture. It was observed that the suture retention strength of the dynamic group in all the scaffolds was significantly higher compared with the static groups (Table 1). The suture retention strength of the nanofibrillar GeVAc under mechanical stimulation for 7 days was 1.21±0.24 N, which was significantly higher than the strength obtained for the freeze-dried GeVAc (0.86±0.05 N; p<0.05; Fig. 11c).
Discussion
Tissue engineering of a blood vessel construct basically calls for a biodegradable scaffold that can maintain the vasoactivity of the native vessel and the chemistry and structure that support cell adhesion and growth while creating and maintaining a space for tissue development. Natural ECM molecules possess ligands, which are recognizable by living cells, that aid their adherence and proliferation and are therefore more appropriate than synthetic surfaces that lack natural binding sites.
In this study our aim was to generate a copolymer based on the natural polymer gelatin. Gelatin is proangiogenic and nonimmunogenic, exhibits low levels of cytotoxicity, and is approved by the U.S. Food and Drug Administration. Vinyl acetate was successfully grafted onto, or copolymerized with, gelatin for enhancing mechanical properties of native gelatin, thereby increasing its stability and permitting its peptide sequences to be used for better adhesion of cells as in natural ECM, thereby creating ideal smart materials for cardiovascular tissue regeneration.
The synthesized scaffold could be tailored via the process of freeze drying and electrospinning to get porous foamed structures. The pore structure of three-dimensional scaffolds used in tissue engineering has been observed to significantly affect cell binding and migration in vitro and to influence the rate and depth of cellular in-growth in vitro and in vivo.11,12 Importantly, appropriate pore size provides structural advantages to allow cells to spread into the pores through “bridges” from adjacent cells, and there is an optimum size range for supporting cell in-growth. Zeltinger et al.13 found that vSMCs showed equal cell proliferation and ECM formation for pore sizes in the 38- to 150-μm range. Studies have also shown that nanometer-sized elements can also affect cellular behavior.14 Given that fiber diameter can affect cell attachment, proliferation, migration, and cytoskeletal organization, it is likely that optimal conditions will need to be determined for each application.15 Since the fibrous meshes that were electrospun were not joined at the fiber junctions, pore size determination was not relevant, and it may be hypothesized that each fiber of the scaffold may exist in a dynamic nature that is free to move by means of external force (amoeboid movement of the living cell). This dynamic architecture may provide the cells an opportunity to optimally adjust the pore diameter and grow into the scaffold.
Since the sample has more carboxyl, hydroxyl, and amido groups on account of it being a biological natural polymer, the sample may be expected to undergo hydrolytic or enzymatic degradation. Higher degradation rates were also observed for the electrospun GeVAc, which may be attributed to the highly ordered crystalline structures with higher surface area where the hydrolytic cleavage is much easier and directed. The higher rate of mass loss is expected to release more peptide fragments, which could increase cell proliferation rate. The higher rate of neotissue formation that took place in the study supports this hypothesis.
All the scaffolds retained their structural stability after mechanical stimulation in culture. Researchers in recent years have shown that electrospun scaffold materials made from natural materials have demonstrated promising results as blood conduits, but SMC response has been suboptimal, with poor infiltration and limited study of SMC phenotype and functional capacity.16,17 In this study, following mechanical stimulation, the proliferation of SMCs on the GeVAc electrospun scaffolds increased, suggesting that these scaffolds present a feasible surface with cell signaling cues and the ECM-like architecture favoring cell adhesion and growth. However, there was no pronounced increase in the proliferation rate for the freeze-dried sample in dynamic culture when compared with the static control. This can be attributed to the lower surface area of the freeze-dried scaffold in comparison with the electrospun scaffolds. The decreased proliferation rate might have resulted from cells becoming overgrown all through the pore area and the ECM produced by the cells covering the pores and hence limiting the diffusion of nutrients. Thus even upon application of strain, cells were not able to infiltrate through the pores that were covered with ECM. This is also evident from the SEM images that showed that the pores were completely covered by the ECM secreted by the cells. Consequently the strain induced on these scaffolds produced little effect on the proliferation because there was limited surface area for growth. However, the electrospun constructs presented a higher surface area owing to the electrospun ECM-like fiber surface where the cells had a higher surface area to proliferate. The SEM images showed the greater alignment of cells in the electrospun samples that underwent pulsatile distention, which mimicked the native ECM-like structure. This was also confirmed from the histology analysis. Mills et al.18 reported that SMCs exposed to a high strain (7%–24%) demonstrated alignment perpendicular to the strain gradient, whereas SMCs subjected to lower strain (0%–7%) remained randomly aligned. In our study perpendicular alignment was observed on application of 10% strain.
Elastin is a critical structural and regulatory matrix protein that plays a dominant role in conferring elasticity to the vessel wall.19–21 Only a few scaffolds are reported to have been able to promote elastin biosynthesis. Tranquillo et al.22 studied the elastogenesis on collagen and fibrin gel scaffolds and found that more elastin was secreted on fibrin gels than on collagen gels. Another study was done by Ramamurthi and Vesely,23 who studied hyaluron gels cross-linked with divinyl sulfone as scaffolds for culturing neonatal rat aortic SMCs and their ability to promote elastogenesis and found increasing amounts of elastin on these scaffolds, which were organized into smooth fenestrated sheets composed of fibers visible at the sheet edges. The SMCs cultured on GeVAc scaffolds in both the freeze-dried and electrospun forms also shows promising results with increased elastin production after mechanical stimulation.
Collagens type I and III are the major fibrillar collagens in blood vessels where they represent 60% and 30% of vascular collagens, respectively. Our study on collagen type I gene expression and quantification of total collagen using the Sircol assay showed that maximum collagen deposition is found in 1 week for the GeVAc electrospun samples. These results can also be correlated with the scaffold chemistry and structure. It has been shown by Peyton et al.24 that SMCs attached to RGD-bearing polyethylene glycol (PEG)–based hydrogels express markers of a contractile phenotype. Beamish et al.25 also proved that RGD-bearing hydrogels with highly specific cell–matrix interactions can support a robust, quantitative re-expression of contractile marker mRNA and proteins. Therefore, our GeVAc construct with a structural chemistry that contained RGD sequences was seen to support the proliferation and secretion of ECM components of SMCs maintaining the contractile nature.
Synthetic SMCs do not possess vasoactivity, and their continued proliferation in the implanted graft causes vessel wall thickening and narrowing of the vessel lumen. Hence SMCs should modulate between the synthetic phenotype and contractile phenotype for the regeneration of the vessel tissue. Expression of the early intermediate contractile marker SM22α and the late intermediate contractile marker calponin is also indicative of the electrospun GeVAc favoring the phenotypic modulation of cells under mechanical stimulation. It has been reported that cyclic mechanical strain applied to cultured SMCs induces increased production of growth factors, cell proliferation, ECM production, and contractile protein expression.26 Mooney and coworkers27 used collagen Type I sponge as scaffolds and found more elastin and more contractile SMCs in constructs subjected to cyclic strain than in control tissue (no strain) in which SMCs appear to be of a synthetic phenotype. Stegemann and Nerum28 showed that 4 days of exposure to periodic mechanical strain at a rate similar to in vivo (∼10% strain at 1-Hz frequency) could increase rat aortic SMC proliferation.
In order for the cyclic strain to effectively promote tissue formation and organization, the tissue-engineered vascular graft scaffold must exhibit elastic characteristics that allow mechanotransduction of SMCs seeded inside the scaffold. A previous report by Girton et al.29 showed that glycated medial collagen equivalents cultured for 13 weeks with low passage adult rat aortic SMCs had a circumferential tensile strength of only 14.6±1 kPa. Cummings et al.30 evaluated a 6-day period dynamic culture and found that the UTS of a pure collagen construct was 36.1 kPa and that of pure fibrin was 15.6 kPa. Seliktar et al.31 also found that 8 weeks of mechanical stimulation increased the strength of three-dimensional SMC-seeded collagen gels, with ultimate stress and material modulus of 58 and 142 kPa, respectively. Our 1-week dynamic culture results for the GeVAc electrospun scaffold showed that the tensile strength of the constructs had values similar to those obtained by Seliktar et al.31 in an 8-week culture period. Hence we propose GeVAc as an optimum matrix that would support the growth of cells and maintain the mechanical integrity of the tissue and therefore aid in the formation of the neotissue.
The percentage of elongation at break was also found to increase on mechanical conditioning. The values for tensile strength for the nanofibrillar GeVAc reached almost 96% of the native rat abdominal aorta in a 1-week culture period (400±10 kPa). According to the ANSI/AAMI VP20-1994 standards for vascular graft prosthesis, an arterial construct must possess mechanical properties at least comparable to the saphenous vein, that is, able to withstand a normal physiological pressure in the 80–120 mm Hg range and must have burst strength of 1680 mm Hg and suture retention strength of 3 N. The nanofibrillar GeVAc presented suture retention strength values that were significantly higher when compared to the values obtained for the native rat abdominal aorta (0.25±0.16 N), although not as high as the ANSI standards requirement. However, the burst pressure values for the GeVAc electrospun samples were nearly in agreement with the standard requirement. Moreover, the tensile strength of many of the synthetic polymers (e.g., polyglycolic acid–polyhydroxyalkanoate [PGA-PHA] blends, polyglycolic acid–poly-4-hydroxybutyrate [PGA-P4HB] blends) represent values that are much higher than those of the GeVAc scaffolds. But most of these synthetic polymers have not shown the presence of ECM components, especially elastin, which is responsible for the mechanical integrity and elastic nature of the scaffold. Further, the effective modulation of smooth muscle phenotype has not been studied. According to Owens,32 in vivo, vSMC phenotype is regulated by a complex set of signals that include cell–cell interactions, ECM components, humoral factors, local chemical conditions, and mechanical forces. The maintenance of the synthetic phenotype of cells is taken advantage of while seeding cells onto the scaffolds on which cell proliferation and secretion of ECM components take place. After the appropriate level of tissue formation is acquired the shift is reversed to a more nonproliferative phenotype to maintain the contractility of the vessels before transplantation. All these factors in combination with mechanical conditioning would help in the regeneration of the neotissue with cell contractility modulation and growth.
Conclusion
We have been able to show that for the successful regeneration of the medial layer not only is the local chemical conditions an important factor, but the architecture also plays a major role. Hence a combination approach involving a biomimetic scaffold with the appropriate mechanical strength conducive to the growth of SMCs and the use of the pulsatile forces to modulate the cell morphology and phenotype helps in the successful engineering of a medial layer of blood vessel. A more complete understanding of SMC phenotype and its control is also warranted, which will lead to important insights into the development of fully functional tissue engineered vascular graft by providing a biological model to study the phenotype shifts in the three-layered tissue construct.
Supplementary Material
Acknowledgments
The authors acknowledge the support provided by the Director and the Head of the Biomedical Technology Wing at Sree Chitra Tirunal Institute for Medical Sciences and Technology, and the funding provided partly by the Department of Biotechnology and the Council of Scientific and Industrial Research, Government of India. We would also like to thank Mr. C.V. Muraleedharan (Scientist G, SCTIMST) for helpful discussions with the mechanical testing of ring samples.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zilla P. Bezuidenhout D. Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–5027. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg CB. Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 3.Stegemann JP. Kaszuba SN. Rowe SL. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13:2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seunarine K. Gadegaard N. Tormen M, et al. 3D polymer scaffolds for tissue engineering. Nanomedicine. 2006;1:281–296. doi: 10.2217/17435889.1.3.281. [DOI] [PubMed] [Google Scholar]
- 5.Cui W. Zhou Y. Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 2010;11:014108. doi: 10.1088/1468-6996/11/1/014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beamish JA. He P. Kottke-Marchant K, et al. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas LV. Nair PD. Influence of mechanical stimulation in the development of a medial equivalent tissue-engineered vascular construct using a gelatin-g-vinyl acetate co-polymer scaffold. J Biomat Sci Polym Ed. 2012;23:2069–2087. doi: 10.1163/092050611X607148. [DOI] [PubMed] [Google Scholar]
- 8.Kim BS. Mooney DJ. Engineering smooth muscle tissue with a predefined structure. J Biomed Mater Res. 1998;41:322–332. doi: 10.1002/(sici)1097-4636(199808)41:2<322::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Brown AN. Kim BS. Alsberg E, et al. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6:297–05. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 10.Berglund JD. Nerem RM. Sambanis A. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Eng. 2004;10:1526–35. doi: 10.1089/ten.2004.10.1526. [DOI] [PubMed] [Google Scholar]
- 11.Lee M. Wu BM. Dunn JCY. Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A. 2008;87:1010–1016. doi: 10.1002/jbm.a.31816. [DOI] [PubMed] [Google Scholar]
- 12.Van Tienen TG. Heijkants RGJC. Buma P, et al. Tissue ingrowth and degradation of two biodegradable porous polymers with different porosities and pore sizes. Biomaterials. 2002;23:1731–1738. doi: 10.1016/s0142-9612(01)00280-0. [DOI] [PubMed] [Google Scholar]
- 13.Zeltinger J. Sherwood JK. Graham DA, et al. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557–572. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 14.Flemming R. Murphy C. Abrams G, et al. Effects of synthetic micro-and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 15.Deitzel J. Kleinmeyer J. Harris D, et al. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–272. [Google Scholar]
- 16.Tillman BW. Yazdani SK. Lee SJ, et al. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30:583–588. doi: 10.1016/j.biomaterials.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Hashi CK. Zhu Y. Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills I. Cohen CR. Kamal K, et al. Strain activation of bovine aortic smooth muscle cell proliferation and alignment: study of strain dependency and the role of protein kinase A and C signaling pathways. J Cell Physiol. 1997;170:228–234. doi: 10.1002/(SICI)1097-4652(199703)170:3<228::AID-JCP2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Karnik SK. Brooke BS. Bayes-Genis A, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 20.Li DY. Brooke B. Davis EC, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 21.Midwood KS. Schwarzbauer JE. Elastic fibers: building bridges between cells and their matrix. Curr Biol. 2002;12:R279–281. doi: 10.1016/s0960-9822(02)00800-x. [DOI] [PubMed] [Google Scholar]
- 22.Long JL. Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 23.Ramamurthi A. Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials. 2005;26:999–910. doi: 10.1016/j.biomaterials.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Peyton SR. Raub CB. Keschrumrus VP, et al. The use of poly (ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Beamish JA. Fu AY. Choi A, et al. The influence of RGD-bearing hydrogels on the re-expression of contractile vascular smooth muscle cell phenotype. Biomaterials. 2009;30:4127–4135. doi: 10.1016/j.biomaterials.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birukov KG. Shirinsky VP. Stepanova OV, et al. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol Cell Biochem. 1995;144:131–139. doi: 10.1007/BF00944392. [DOI] [PubMed] [Google Scholar]
- 27.Kim BS. Mooney DJ. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J Biomech Eng. 2000;122:210–215. doi: 10.1115/1.429651. [DOI] [PubMed] [Google Scholar]
- 28.Stegemann JP. Hong H. Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 29.Girton T. Oegema T. Grassl E, et al. Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J Biomech Eng. 2000;122:216–223. doi: 10.1115/1.429652. [DOI] [PubMed] [Google Scholar]
- 30.Cummings CL. Gawlitta D. Nerem RM, et al. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699–3706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 31.Seliktar D. Black RA. Vito RP, et al. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351–362. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 32.Owens JK. Role of mechanical strain in regulation of differentiation of vascular smooth muscle cells. Circ Res. 1996;79:1054–1055. doi: 10.1161/01.res.79.5.1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.