Abstract
Introduction and hypothesis
Benign joint hypermobility syndrome may be a risk factor for pelvic floor disorders. It is unknown whether hypermobility impacts the progress of childbirth, a known risk factor for pelvic floor disorders. Our objective was to investigate the association between joint hypermobility syndrome, obstetrical outcomes, and pelvic floor disorders. Our hypotheses were: (1) women with joint hypermobility are less likely to experience operative delivery and prolonged second-stage labor; and (2) pelvic floor disorders are associated with benign hypermobility syndrome, controlling for obstetrical history.
Methods
Joint hypermobility was measured in 587 parous women (participants in a longitudinal cohort study of pelvic floor disorders after childbirth). Their obstetrical histories were obtained from review of hospital records. Pelvic floor disorders were assessed using validated questionnaires and a structured examination for prolapse. Joint hypermobility and pelvic floor disorders were evaluated at enrollment (5–10 years after first delivery). We compared obstetrical outcomes and pelvic floor disorders between women with and without joint hypermobility, defined as a Beighton score ≥4.
Results
Hypermobility was diagnosed in 46 women (7.8 %) and was associated with decreased odds of cesarean after complete cervical dilation or operative vaginal delivery [odds ratio (OR)=0.51; 95 % confidence interval (CI):0.27–0.95]. Anal sphincter laceration was unlikely to occur in women with hypermobility (OR=0.19; 95 % CI 0.04–0.80). However, hypermobility was not associated with any pelvic floor disorder considered.
Conclusions
Benign joint hypermobility syndrome may facilitate spontaneous vaginal birth but does not appear to be a risk factor for pelvic floor disorders in the first decade after childbirth.
Keywords: Benign joint hypermobility syndrome, Beighton criteria, Pelvic floor disorders, Pelvic organ prolapse
Introduction
Childbirth has a very substantial impact on a woman’s probability of developing pelvic floor disorders, such as urinary incontinence (UI) and pelvic organ prolapse (POP) [1]. However, childbirth is neither necessary nor sufficient for the development of these conditions: nulliparity is not completely protective against pelvic floor disorders [2], nor are pelvic floor disorders ubiquitous after childbirth [3]. Thus, other factors, independent of parity, influence the development of pelvic floor disorders.
Research on the epidemiology of pelvic floor disorders suggests that some women may be phenotypically or genotypically predisposed. For example, prevalence appears to differ across racial groups [4]. Also, family history of prolapse appears to increase the odds of prolapse among nulliparous women [5, 6], suggesting a hereditary component. Finally, local connective tissue factors might play a role in a women’s likelihood to develop prolapse and other pelvic floor disorders [7]. These observations provide important clues about risk factors for these conditions.
One possible risk factor is joint hypermobility. Benign hypermobility syndrome, diagnosed using physical examination criteria, is a manifestation of generalized connective tissue laxity and is seen in approximately 10 % of adult US women [8]. Several case–control studies suggest that joint hypermobility is associated with pelvic floor disorders [9–12]. However, most of these studies have not controlled for childbirth history and other known risk factors for pelvic floor disorders. It is unknown whether joint laxity might influence obstetrical outcomes. Indeed, it has long been recognized that joint laxity increases over the course of pregnancy [13, 14], allowing the bony pelvis to adapt to accommodate vaginal birth. We are unaware of studies of the influence of benign joint hypermobility syndrome on the course of the second stage of labor. It is plausible that women with hypermobility syndrome may be at lower risk for cephalopelvic disproportion. A lower rate of cesarean deliveries could potentially be the mechanism by which women with hypermobility are exposed to a higher risk of pelvic floor disorders later in life. Thus, a critical question is whether childbirth experiences are the mechanism by which hypermobility syndrome increases the later development of pelvic floor disorders.
The objective of this research was to investigate the association between joint hypermobility syndrome, childbirth outcomes, and pelvic floor disorders. Our first hypothesis was that women with joint hypermobility would experience less obstruction to childbirth in the second stage of labor, manifested by decreased rate of operative intervention and a shorter second-stage of labor. Our second hypothesis was that pelvic floor disorders would be associated with benign hypermobility syndrome, controlling for the effects of obstetrical history. The overall goal of this research was to consider whether the association between hypermobility and pelvic floor disorders might be mediated by childbirth outcomes.
Materials and methods
This was a supplementary analysis of the Mothers’ Outcomes after Delivery (MOAD) study [1], a prospective cohort study of pelvic floor outcomes among parous women. Participants were recruited from the obstetrical population at a large community hospital in suburban Maryland, USA. Details of study methods, eligibility criteria, and recruitment procedures have been previously described [1]. Women were eligible for the MOAD study if they had given birth to their first child 5–10 years before enrollment. Participants were recruited based on the mode of delivery of their first child (cesarean vs. vaginal), and groups were matched for age at the time of first delivery and years since that delivery. Exclusion criteria, based on the first delivery, included maternal age <15 or >50 years, delivery at <37 weeks of gestation, placenta previa, multiple gestation, known fetal congenital anomaly, still-birth, prior myomectomy, and abruption. Eligibility was established via review of the obstetrical hospital records and confirmed with subsequent telephone interviews. This research was approved by the Institutional Review Board, and all participants provided written informed consent.
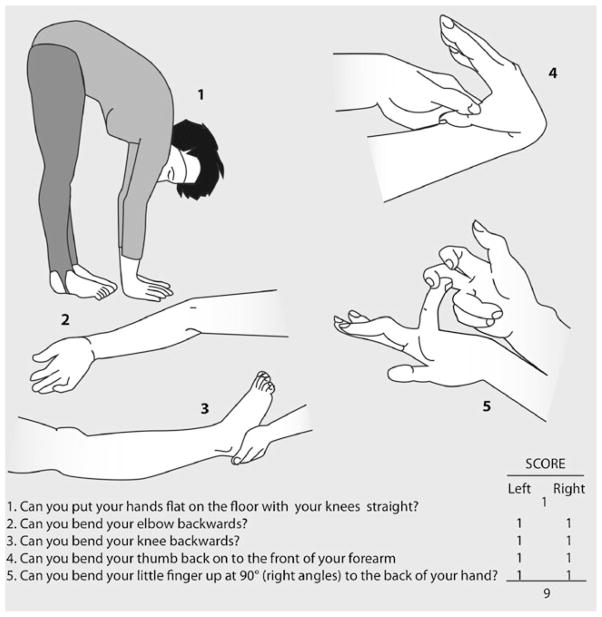
For this supplementary study, the exposure of interest was benign joint hypermobility syndrome. Joint mobility was assessed on physical examination at the time of study enrollment using five standard maneuvers known as the Beighton Modification of the Carter and Wilkinson Scoring System [15]. Each participant was asked to complete the following tasks in order to calculate their flexibility score (Fig. 1): bending at the waist and placing hands flat on the floor without bending knees (1 point), hyperextension of knees (1 point for each side), hyperextension of elbows (1 point for each side), touching thumb to forearm (1 point for each side), and bending fifth finger beyond 90°(1 point for each side). Hyperextension was gauged using a goniometer. A maximum score of 9 indicates hypermobility for each maneuver. Benign joint hypermobility syndrome is diagnosed with a Beighton score of ≥4. [15].
Fig. 1.
Beighton Modification of the Carter and Wilkinson Scoring System. (Image reproduced by kind permission of Arthritis Research, UK.)
Our first hypothesis was that hypermobility decreases operative intervention during the second stage of labor. Obstetrical outcomes were defined by a review of each participant’s obstetrical hospital records. Because our hypothesis is relevant only to women who had experienced the second stage of labor, we excluded those who delivered exclusively by cesarean prior to labor or in the first stage of labor. The three comparison groups of interest were: (a) cesarean after complete cervical dilation, (b) spontaneous vaginal birth, and (c) operative vaginal birth. Spontaneous vaginal birth was the reference group. We also considered whether each participant had experienced a prolonged second stage of labor (defined as >120 min) and whether she had experienced a third- or fourth-degree anal sphincter laceration. Obstetrical exposures were determined at the “woman level” by considering each participant’s history across all of her deliveries [1]. Because the focus of the MOAD study is the relationship between obstetrical events and pelvic floor outcomes, women were placed in the group corresponding to the delivery that was likely to be most traumatic to the pelvic floor. For instance, any woman with an operative delivery was placed in that group regardless of her other delivery types.
Standard methods for contingency tables (i.e., Fisher’s exact test) were used to describe the association of hypermobility syndrome with maternal characteristics and obstetrical outcomes. Assuming that the Beighton score measured at entry into MOAD represents the score over the time span of all deliveries for each woman, we used logistic regression methods with the obstetrical events of interest at each delivery as the outcome and hypermobility syndrome as the exposure. As each woman contributed as many records as deliveries, we used robust methods to calculate the standard errors (SE) of the regression coefficients.
Our second hypothesis was that benign hypermobility syndrome is associated with pelvic floor disorders. Each participant was evaluated for the presence or absence of pelvic floor disorders at enrollment (e.g., 5–10 years after first delivery) using two validated measures. First, symptoms of stress urinary incontinence (SUI), overactive bladder (OAB), anal incontinence (AI), and prolapse were assessed using the validated Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ) [16]. We used validated thresholds [16] for each EPIQ score to distinguish between women with and without bothersome symptoms of SUI, OAB, AI, and prolapse. In addition, objective evidence of pelvic organ support was assessed during a gynecologic exam using the Pelvic Organ Prolapse Quantification (POP-Q) examination [17]. Based on examination results, prolapse was defined as any descent of the cervix or vaginal walls to or beyond the hymen. Each pelvic floor disorder was considered separately. The primary exposure of interest was hypermobility syndrome, and univariate OR (OR) were calculated using standard methods for 2 × 2 tables. Logistic regression was used to provide OR for the association between pelvic floor disorders and hypermobility syndrome. This was accomplished through analysis of five logistic models, with one of five pelvic floor disorders as the outcome of interest for each model. The presence of hypermobility syndrome was the exposure of interest in each model. Adjusted OR were obtained by including the delivery group in each of the five models (with spontaneous vaginal delivery as the reference).
Results
Of 1,011 in the MOAD enrollment cohort [1], we excluded 420 women who had never experienced the second stage of labor, leaving 591 for analysis. Four additional women were excluded due to missing Beighton score, leaving a sample of 587 for this analysis. Forty-six of 587 (7.8 %) women met the criterion for benign joint hypermobility syndrome (Beighton score ≥4). The characteristics of women with and without hypermobility are shown in Table 1. As noted, women with hypermobility were somewhat younger at the time of enrollment and were somewhat less likely to have delivered their first child after age 35. There were no differences between groups with respect to race, multiparity, or obesity [body mass index (BMI)≥30 kg/m2].
Table 1.
Maternal characteristics of the 587 women with a history of reaching the second stage of labor by Beighton flexibility scores at enrollment (e.g., 5–10 years from first delivery)
| Characteristic | Beighton score
|
P value | |
|---|---|---|---|
| <4 N=541 |
≥4 N=46 |
||
| Age at enrollment, median (IQR), in years | 40.0 (36.4, 43.2) | 37.7 (35.3, 40.8) | 0.047 |
| Race | |||
| Caucasian | 469 (87 %) | 39 (85 %) | 0.582 |
| African American | 53 (10 %) | 4 (9 %) | |
| Other | 19 (4 %) | 3 (7 %) | |
| Maternal age >35 at 1st delivery | 158 (29 %) | 8 (17 %) | 0.091 |
| Multiparous (at enrollment) | 402 (74 %) | 33 (72 %) | 0.727 |
| Body mass index ≥30 kg/m2 (at enrollment) | 101 (19 %) | 7 (15 %) | 0.693 |
IQR Interquartile range
Obstetrical outcomes are described in Table 2. Women with hypermobility experienced less operative intervention in the second stage of labor. Specifically, women with hypermobility were significantly less likely to experience cesarean or operative vaginal birth (p=0.049). Women with hypermobility were less likely to have experienced prolonged second-stage labor, although the difference was not significant (p=0.164). Finally, women with hypermobility were significantly less likely to have experienced an anal sphincter laceration (p=0.021). In an analysis at the delivery- evel, adjusting for African American race, maternal age >35 at first delivery, multiparity, and obesity, we found that hypermobility was inversely associated with cesarean after complete cervical dilation or operative vaginal delivery [OR=0.51; 95 % confidence interval (CI): 0.27–0.95) and anal sphincter laceration (OR=0.19; 95 % CI 0.04–0.80) but not prolonged second stage of labor (OR=0.63; 95 % CI:0.35—1.13).
Table 2.
Obstetrical outcomes of the 587 study participants, by Beighton flexibility scores at enrollment. Data are presented as N (%)
| Characteristic | Beighton score
|
P value | |
|---|---|---|---|
| <4 (N=541) | ≥ 4 (N=46) | ||
| Delivery groupa | 0.049 | ||
| Cesareanb | 132 (24 %) | 8 (17 %) | |
| Spontaneous vaginal birthc | 288 (53 %) | 33 (72 %) | |
| ≥1 Operative vaginal birth | 121 (22 %) | 5 (11 %) | |
| Prolonged second stage >120 mind | 237 (44 %) | 15 (33 %) | 0.164 |
| Anal sphincter lacerationd | 93 (17 %) | 2 (4 %) | 0.021 |
Across all delivery types (see “Methods”)
After complete cervical dilation
Non-operative (reference group)
Ever present, across all deliveries before enrollment
Association between hypermobility and pelvic floor disorders is shown in Table 3. Hypermobility did not increase the relative odds for any pelvic floor disorder considered. This observation was not affected in a multivariable logistic model controlling for delivery group.
Table 3.
Pelvic floor disorders and Beighton flexibility scores at enrollment (e.g., 5–10 years after first delivery) among the 587 women with a history of reaching the second stage of labor
| Pelvic Floor Disorder | Beighton score
|
Unadjusted OR (95 % CI)a | Adjusted ORb (95 % CI)a | |
|---|---|---|---|---|
| <4 N=541 |
≥4 N=46 |
|||
| Stress urinary incontinence, n=82 (14 %) | 73 (13 %) | 9 (20 %) | 1.56 (0.72, 3.37) | 1.62 (0.74, 3.52) |
| Overactive bladder, n=54 (9 %) | 51 (9 %) | 3 (7 %) | 0.67 (0.20, 2.24) | 0.77 (0.23, 2.60) |
| Anal incontinence, n=72 (12 %) | 66 (12 %) | 6 (13 %) | 1.08 (0.44, 2.64) | 1.14 (0.46, 2.80) |
| Prolapse symptoms, n=21 (4 %) | 21 (4 %) | 0 (0 %) | -c | -c |
| Prolapse on examination, n=65 (11 %) | 60 (11 %) | 5 (11 %) | 0.98 (0.37, 2.57) | 0.95 (0.36, 2.53) |
95% Confidence interval
Odds ratio (OR) adjusted by delivery group: either cesarean, spontaneous vaginal, or operative vaginal birth
Undefined due to no symptoms among those with Beighton score ≥4
Discussion
Our results suggest that women with benign joint hypermobility syndrome are significantly more likely to deliver by spontaneous vaginal birth than women without hypermobility. More specifically, they were less likely to deliver by cesarean after complete cervical dilation and less likely to deliver by operative vaginal birth than women without hypermobility. This finding is consistent with our hypothesis. Joint laxity may allow for more easy passage of the fetal head through the pelvis. Thus, from an obstetrical perspective, joint hypermobility may be advantageous to facilitate spontaneous vaginal birth.
We were surprised to find no evidence of an association between joint hypermobility syndrome and any of the pelvic floor disorders considered. Prior research suggested an association, especially for hypermobility and prolapse. Prior studies compared women seeking treatment for prolapse with controls seeking gynecologic care, and therefore, unmeasured differences between cases and controls may have influenced results. Also, the prevalence of hypermobility reported in these prior studies is much higher than would be expected. For example, among healthy female blood donors, benign joint hypermobility syndrome was identified in 10 % [8]. The prevalence of joint hypermobility in our study population was 7.8 %, which is similar to the reported rate. In contrast, Al-Rawi [9] identified hypermobility among 66 % of 76 consecutive women with prolapse versus 18 % of 76 age- and parity-matched controls. In 1995, Norton [10] found that women 50 % of 36 women with stage 2–3 cystocele had hypermobility, in contrast to 27 % of 70 women with grade 0–1 cystocele. Finally, in 2010, Aydeniz [11] noted hypermobility in 54 % of 75 women presenting for treatment of prolapse versus 10 % in 52 volunteers. Thus, the prevalence of benign hypermobility syndrome among both cases and controls is unexpectedly high in these published studies.
A limitation of our research is that we assume that hypermobility identified at the time of MOAD study enrollment played a role in obstetrical events 5–10 years prior. It is uncertain whether hypermobility changes over a lifespan, although the prevalence of hypermobility appears to decrease with age. For example, hypermobility syndrome has been diagnosed among <1%of postmenopausal women [18] versus 7.5 % of younger women [19] versus 13 % of school children [20]. Therefore, we cannot with certainty conclude that the hypermobility observed at the time of study enrollment was present prior to childbearing. A prospective study would be necessary to confirm our observations. Additionally, our exclusion of women who never experienced the second stage of labor may have contributed to selection bias, especially with regard to women who elected to deliver via cesarean without obvious medical indication.
This study investigates associations at a single point in time (e.g., 5–10 years from first delivery). The median age of this population was close to 40 years, and it is plausible that the impact of hypermobility syndrome on pelvic floor disorders is not seen until later in life. Longitudinal follow-up of this cohort will establish whether women with hypermobility syndrome will experience a higher incidence of pelvic floor disorders over time. In addition, as this population ages and pelvic floor disorders become more prevalent, we anticipate greater statistical power to investigate less common outcomes (such as bothersome symptoms of prolapse).
In summary, our results suggest that joint hypermobility may facilitate spontaneous vaginal birth but is not a risk factor for pelvic floor disorders 5–10 years after delivery. Other local or systemic connective tissue factors may play a role in the genesis of pelvic floor disorders. Additional research is needed to identify at-risk phenotypes for the development of pelvic floor disorders so that prevention efforts can be targeted appropriately.
Acknowledgments
Acknowledgements and funding This work was supported by a grant from NICHD (R01 HD056275).
Abbreviations
- MOAD
Mothers’ Outcomes after Delivery
- EPIQ
Epidemiology of Prolapse and Incontinence Questionnaire
Footnotes
Conflicts of interest None.
Contributor Information
Leise R. Knoepp, Department of Obstetrics and Gynecology, Ochsner Medical Center, New Orleans, LA, USA
Kelly C. McDermott, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Alvaro Muñoz, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Joan L. Blomquist, Department of Obstetrics and Gynecology, Greater Baltimore Medical Center, Baltimore, MD, USA
Victoria L. Handa, Email: vhanda1@jhmi.edu, Department Gynecology and Obstetrics, Johns Hopkins School of Medicine, Baltimore, MD, USA. 4940 Eastern Avenue, 301 Building, 3rd Floor, Baltimore, MD 21224-2780, USA
References
- 1.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol. 2011;118:777–784. doi: 10.1097/AOG.0b013e3182267f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris RL, Cundiff GW, Coates KW, Bump RC. Urinary incontinence and pelvic organ prolapse in nulliparous women. Obstet Gynecol. 1998;92:951–954. doi: 10.1016/s0029-7844(98)00286-5. [DOI] [PubMed] [Google Scholar]
- 3.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend MK, Curhan GC, Resnick NM, Grodstein F. The incidence of urinary incontinence across Asian, black, and white women in the United States. Am J Obstet Gynecol. 2010;202:378, e1–7. doi: 10.1016/j.ajog.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchsbaum GM, Duecy EE, Kerr LA, Huang LS, Perevich M, Guzick DS. Pelvic organ prolapse in nulliparous women and their parous sisters. Obstet Gynecol. 2006;108:1388–1393. doi: 10.1097/01.AOG.0000245784.31082.ed. [DOI] [PubMed] [Google Scholar]
- 6.Buchsbaum GM, Duecy EE. Incontinence and pelvic organ prolapse in parous/nulliparous pairs of identical twins. Neurourol Urodyn. 2008;27:496–498. doi: 10.1002/nau.20555. [DOI] [PubMed] [Google Scholar]
- 7.Epstein LB, Graham CA, Heit MH. Systemic and vaginal biomechanical properties of women with normal vaginal support and pelvic organ prolapse. Am J Obstet Gynecol. 2007;197:165, e1–6. doi: 10.1016/j.ajog.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Jessee EF, Owen DS, Jr, Sagar KB. The benign hypermobile joint syndrome. Arthritis Rheum. 1980;23:1053–1056. doi: 10.1002/art.1780230914. [DOI] [PubMed] [Google Scholar]
- 9.AL-Rawi ZS, Al-Rawi ZT. Joint hypermobility in women with genital prolapse. Lancet. 1982;1(8287):1439–1441. doi: 10.1016/s0140-6736(82)92453-9. [DOI] [PubMed] [Google Scholar]
- 10.Norton PA, Baker JE, Sharp HC, Warenski JC. Genitourinary prolapse and joint hypermobility in women. Obstet Gynecol. 1995;85:225–228. doi: 10.1016/0029-7844(94)00386-R. [DOI] [PubMed] [Google Scholar]
- 11.Aydeniz A, Dikensoy E, Cebesoy B, Altindağ O, Gürsoy S, Balat O. The relation between genitourinary prolapse and joint hypermobility in Turkish women. Arch Gynecol Obstet. 2010;281:301–304. doi: 10.1007/s00404-009-1103-3. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh LJ, Stanitski DF, Mallett VT, Frahm JD, Richardson DA, Evans MI. Ehlers-Danlos syndrome: relationship between joint hypermobility, urinary incontinence, and pelvic floor prolapse. Gynecol Obstet Invest. 1996;41:135–139. doi: 10.1159/000292060. [DOI] [PubMed] [Google Scholar]
- 13.Calguneri M, Bird HA, Wright V. Changes in joint laxity occurring during pregnancy. Ann Rheum Dis. 1982;41(2):126–128. doi: 10.1136/ard.41.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marnach ML, Ramin KD, Ramsey PS, Song SW, Stensland JJ, An KN. Characterization of the relationship between joint laxity and maternal hormones in pregnancy. Obstet Gynecol. 2003;10:331–335. doi: 10.1016/s0029-7844(02)02447-x. [DOI] [PubMed] [Google Scholar]
- 15.Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27:1777–1779. [PubMed] [Google Scholar]
- 16.Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:272–284. doi: 10.1007/s00192-005-1314-5. [DOI] [PubMed] [Google Scholar]
- 17.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 18.Dolan AL, Hart DJ, Doyle DV, Grahame R, Spector TD. The relationship of joint hypermobility, bone mineral density, and osteoarthritis in the general population: the Chingford Study. J Rheumatol. 2003;30:799–803. [PubMed] [Google Scholar]
- 19.Klemp P, Williams SM, Stansfield SA. Articular mobility in Maori and European New Zealanders. Rheumatology (Oxford) 2002;41:554–557. doi: 10.1093/rheumatology/41.5.554. [DOI] [PubMed] [Google Scholar]
- 20.Gedalia A, Press J, Klein M, Buskila D. Joint hypermobility and fibromyalgia in schoolchildren. Ann Rheum Dis. 1993;52:494–496. doi: 10.1136/ard.52.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]