Abstract
The cell-autonomous role of synaptic transmission in the regulation of neuronal structural and electrical properties is unclear. We have now employed a genetic approach to eliminate glutamatergic synaptic transmission onto individual CA1 pyramidal neurons in a mosaic fashion in vivo. Surprisingly, while electrical properties are profoundly affected in these neurons, as well as inhibitory synaptic transmission, we found little perturbation of neuronal morphology, demonstrating a functional segregation of excitatory synaptic transmission from neuronal morphological development.
Introduction
In the brain, the majority of excitatory synaptic transmission is mediated by glutamate acting on two types of ionotropic glutamate receptors: AMPA receptors (AMPARs) and NMDA receptors (NMDARs). The role of excitatory synaptic transmission in sculpting neuronal structure and function has been extensively studied by utilizing pharmacological blockade both in vitro and in vivo (Collin et al., 1997; Craig et al., 1994; Desai et al., 1999; Galante et al., 2000; Gomperts et al., 2000; Harms and Craig, 2005; Hartman et al., 2006; Kilman et al., 2002; Kim and Tsien, 2008; Lindskog et al., 2010; McAllister et al., 1996; McKinney; McKinney et al., 1999; Mitra et al., 2012; Murphy and Segal, 1996; Thiagarajan et al., 2005; Turrigiano, 2008; Turrigiano and Nelson, 2004). While pharmacological reagents globally block AMPAR- and NMDAR- mediated synaptic transmission, they do not separate the cell-autonomous function of excitatory synaptic transmission from the indirect effects on network activity associated with global blockade or indirect effects through glia. They might also eliminate competition among neurons (Buffelli et al., 2003), and thus mask potential roles that synaptic transmission might play in neuronal function. Furthermore, as integral transmembrane proteins, neurotransmitter receptors may play a structural role in neuronal morphogenesis, which cannot be addressed by pharmacological inhibition. To address these issues, we made a quadruple conditional knockout mouse line in which three genes encoding AMPAR subunits (GluA1, A2 and A3) (Lu et al., 2009) plus the gene encoding GluN1 (Adesnik et al., 2008; Lu et al., 2011; Tsien et al., 1996), the obligatory NMDAR subunit, are all conditional alleles (GRIA1-3fl/flGRIN1fl/fl). Through the injection of adeno-associated virus (AAV) expressing Cre recombinase fusion to GFP (GFP-Cre) into the hippocampus of GRIA1-3fl/flGRIN1fl/fl, both AMPARs and NMDARs of individual CA1 pyramidal neurons will be eliminated from within. With this approach the impact of silencing of excitatory synaptic transmission in single neurons in vivo can be directly compared with the vast majority of neighboring intact control neurons with all cells existing in an otherwise unperturbed microenvironment.
Result
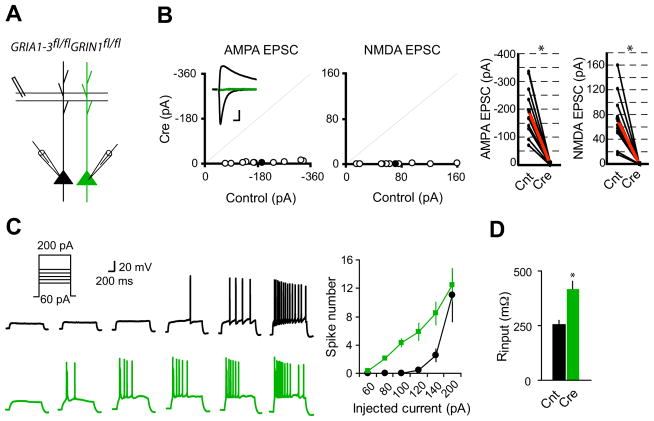
To express Cre recombinase in CA1 pyramidal neurons, we delivered CreGFP AAV virus to newborn GRIA1-3fl/flGRIN1fl/fl pups at p0. By controlling the volume and titer of the virus, we could genetically alter a very small percentage of hippocampal neurons in a mosaic fashion, thus enabling us to study the cell-autonomous effect of excitatory synaptic transmission on neuronal excitability. Dual whole-cell voltage-clamp recording revealed that AMPAR and NMDAR mediated synaptic transmission were reduced by about 70% by 12 days (Figure S1) and eliminated three weeks following virus injection (Figure 1A and B). In addition to elimination of synaptic NMDAR mediated currents (Figure 1B), deletion of GluN1 prevents GluN2 subunits from trafficking to synapses (Fukaya et al., 2003). To study the effect of complete elimination of glutamatergic synaptic transmission in vivo on neuronal electrical properties, we recorded neurons at 3–5 weeks of age. We delivered a series of current injections (60–140 pA, 1 s) to neurons expressing GFP-Cre under whole-cell current-clamp configurations. Loss of excitatory transmission in individual neurons dramatically increased the evoked action potential firing and significantly reduced the minimal current needed to fire a spike (Figure 1C). The increased excitability is likely due to the strong increase of input resistance (Figure 1D). In contrast, resting membrane potential, threshold for firing action potential, and action potential waveform were not altered (Figure S2). Furthermore, a saturating current injection (200 pA, 1 s) elicited similar numbers of spikes in controls and neurons expressing Cre (Figure 1C), indicating that mechanisms for generating action potentials were not affected in neurons lacking excitatory input. Together, these data demonstrate that loss of excitatory input onto individual neurons in vivo causes strong cell-autonomous adaptation by increasing neuronal excitability.
Figure 1. Single-cell synaptic silencing in vivo induced enhanced neuronal excitability.
(A) Schematic of dual whole-cell voltage-clamp recording in acute hippocampal slices from GRIA1-3fl/flGRIN1fl/fl p20-p28 mice infected with virus expressing CreGFP at p0.
(B) Scatter plots showed amplitudes of EPSCs for single pairs (open circles) and mean ± SEM (filled circles). (Inset) Representative superimposed traces (Black, Control (Cnt); Green, Cre). Scale bar: 0.02 s, 50 pA. Graphs in the right show that synaptic transmission mediated by AMPARs or NMDARs is essentially eliminated in CreGFP-expressing neurons (each black line represents a single pair and the red line represents the average; AMPA EPSCs, Cnt: −194.2 ± 29.8 pA; Cre: −4.92 ± 1.39 pA; n = 10; NMDA EPSCS, Cnt: 71.5 ± 14.03 pA; Cre: 0.56 ± 0.08 pA; n = 10). *P < 0.001.
(C) Sample action potential responses to step current injections (60, 80, 100, 120, 140 and 200 pA; 1000 ms) in control neurons (black) or CreGFP-expressing neurons (Green). Right is the summary graph showing that deletion of both AMPARs and NMDARs increased the excitability of CA1 pyramidal neurons. *P < 0.001.
(D) Bar graph shows that input resistance is significantly enhanced in neurons lacking both AMPARs and NMDARs. *P < 0.001.
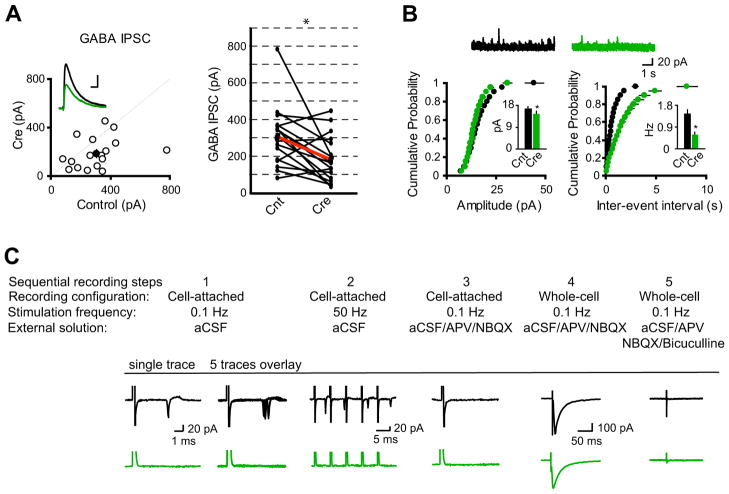
Functional balance between excitatory and inhibitory synaptic transmission is critical for pyramidal neuron firing output (Hartman et al., 2006; Kilman et al., 2002; Liu, 2004; Tao and Poo, 2005; Turrigiano et al., 1998; Turrigiano and Nelson, 2004; Vitureira et al., 2012). We thus examined how GABAergic synaptic transmission adapts in response to single-cell silencing of excitatory glutamatergic synaptic transmission in vivo. Dual-whole cell recording revealed that neurons lacking synaptic excitation had a substantial reduction of GABA receptor-mediated inhibitory postsynaptic currents (IPSCs) (Figure 2A). Indeed, evoked IPSCs from CA1 pyramidal neurons expressing Cre were about 60% of controls (Figure 2A). Supporting this finding, mini IPSC recording showed that there was a minor but significant reduction of amplitude and a large reduction of frequency of mini IPSCs in neurons expressing Cre (Figure 2B). These data demonstrated that single-cell elimination of glutamatergic synaptic transmission in vivo induced homeostatic reduction of GABAergic synaptic strength, suggesting a role for glutamatergic synaptic transmission in functional maturation of GABAergic synapses.
Figure 2. Single-cell elimination of AMPARs and NMDARs induced the reduction of GABAergic synaptic strength.
(A) Scatter plots showed amplitudes of IPSCs for single pairs (open circles) and mean ± SEM (filled circles). (Inset) Representative superimposed traces (Black, Cnt; Green, Cre). Scale bar: 0.05 s, 100 pA. Graph in the right shows that synaptic transmission mediated by GABAA receptors is significantly reduced in CreGFP-expressing neurons (each black line represents a single pair and the red line represents the average; Cnt: 309.00 ± 39.32 pA; Cre: 183.53 ± 32.13 pA; n = 17). *P < 0.001.
(B) Cumulative distributions of mIPSC amplitudes and inter-event intervals from control and CreGFP-expressing neurons. Above are sample traces from a control neuron (Black) and a CreGFP-expressing neuron (Green). Bar graphs show that both mIPSC amplitudes and inter-event intervals are reduced in neurons expressing CreGFP. *P < 0.05.
(C) Synaptic activation-evoked action potential firing is lost in CA1 pyramidal neurons expressing CreGFP. Synaptic activation-evoked action potentials were simultaneously recorded in one CreGFP expressing neuron and one neighboring control neuron under cell-attached mode without breaking-in. Synaptic activity was induced by stimulation of Schaffer Collateral pathway at either 0.1 Hz (step 1) or 50 Hz (step 2). After washing-in AP-V (100 μM) and NBQX (10 μM), synaptic activation-evoked action potentials were blocked (step 3). Neurons were then held in whole-cell mode under voltage-clamp configuration to record evoked IPSCs (step 4), which were completely blocked by 20 μM bicuculline (step 5).
During early development, due to higher intracellular Cl- concentration, GABAA receptor activation can generate membrane depolarization, contributing to neuronal firing (Ben-Ari, 2002; Ben-Ari et al., 2007; Owens et al., 1996). We wondered if glutamatergic synaptic transmission controls the switch of GABAergic synaptic transmission from excitation to inhibition during development. Under cell-attached mode, activation of axonal fibers in stratum radiatum of hippocampal CA1 region reliably led to neuronal firing in control neurons, but not in Cre-expressing neurons, during both low- and high-frequency stimulations (Figure 2C). While evoked IPSCs could be recorded from both control and Cre-expressing neurons, the firing was due to synaptic activation of glutamate receptors, as pharmacological blockade of AMPARs and NMDARs eliminated firing (Figure 2C). These data indicate that GABA receptor activation does not depolarize these neurons, as would occur if the chloride reversal potential remained depolarizing. Taken together, the data from Figures 1 and 2 demonstrate that loss of excitatory input onto individual neurons in vivo causes strong cell-autonomous adaptation by increasing neuronal excitability and decreasing inhibitory input.
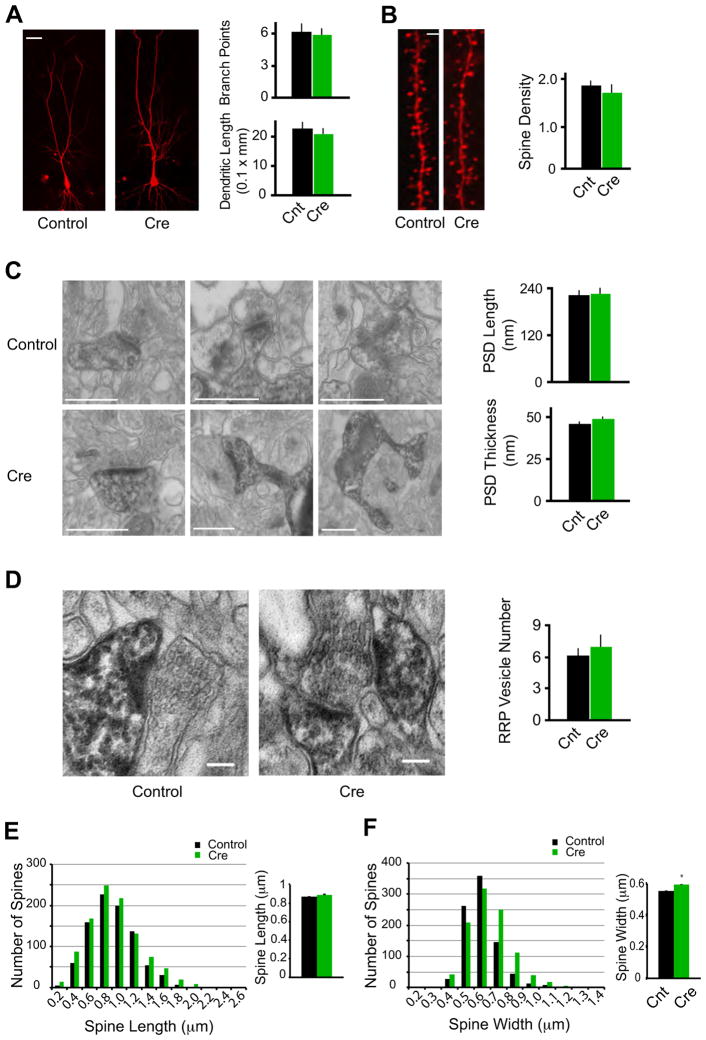
The adaptation of neuronal electrical properties induced by loss of excitatory input contrasted strikingly to the preservation of neuronal morphology (Figure 3). Infected neurons expressing Cre were fixed at about four weeks after p0 virus injection when glutamatergic synaptic transmission was completely lost in these neurons (Figure 3A). Fixed neurons were filled with fluorescent dyes and examined with confocal microscopy. In these neurons, we could detect no change in the average number of branch points of dendrites and dendritic length (Figure 3A), suggesting that dendritic morphogenesis does not require ongoing synaptic activity. Dendritic spines are the loci of the majority of excitatory synapses in pyramidal neurons. Surprisingly, the spine density is indistinguishable between control neurons and neurons expressing Cre (Figure 3B).
Figure 3.
Morphological conservation of CA1 pyramidal neurons devoid of synaptic excitation in vivo.
(A) Representative confocal stacks from Cnt and Cre cells. Bar graph in right shows average number dendritic branch points and dendritic length (Cnt: n = 10; Cre: n = 8; P > 0.05). Scale bar, 20 μm.
(B) Maximum intensity projections of representative dendrites from Cnt and Cre cells. Bar graph in right shows average spine density (the number of spines per micron) for Cnt and Cre neurons (Cnt: n = 7 neurons; Cre: n = 6; P > 0.05). Scale bar, 1 μm.
(C) Electron micrographs consistently reveal PSDs in spines from Cre-expressing cell, similar to Cnt cells. Bar graphs show no significant difference in PSD length or thickness between Cnt and Cre spines (Cnt: n = 33 spines; Cre: n = 40; P > 0.05). Scale bars, 500 nm.
(D) Pre-synaptic vesicle pools in boutons opposed to Cnt and Cre spines. Bar graph shows no significant difference in RRP vesicle numbers (Cnt: n = 15 spines; Cre: n = 15; P > 0.05). Scale bars, 100 nm.
(E–F) Histograms showing spine lengths (E) and widths (F) for all spines measured in confocal volumes of control (Black) and Cre-expressing (Green) neurons. In the bar graphs in the right, comparing the two conditions revealed no significant difference in mean spine length (E, Cnt: 0.87 ± 0.01 μm, n = 880; Cre: 0.88 ± 0.01 μm, n = 1021; P = 0.23), and a small but statistically significant increase in spine width in Cre-expressing neurons (F, Cnt: 0.55 ± 0.004 μm, n = 861; Cre: 0.59 ± 0.004 μm, n = 998; P < 0.01).
To study in more detail the morphological features of neurons lacking glutamatergic synaptic transmission, we performed single-cell electron microscopic analysis. CA1 pyramidal neurons from six-week old mice injected with rAAV-CreGFP virus at p0 were filled with Lucifer yellow, photoconverted using 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a substrate, and then serial section electron microscopy (ssEM) was performed. Under ssEM, we identified mature asymmetric synapses defined by the presence of a postsynaptic density (PSD), presynaptic vesicles apposed to the PSD, and a clearly defined synaptic cleft (Figure 3C). Quantitative analysis showed that the length and thickness of the PSDs and the number of presynaptic vesicles within 20 nm of the presynaptic membrane were no different from control neurons (Figure 3C and D). In addition, there is no difference of spine length and a slight but significant increase of spine width in Cre-expressing neurons (Figure 3E and F). The mechanism underlying the small increase of spine width in Cre-expressing neurons remains unclear at the current stage. Taken together, these data suggest that synaptic AMPAR and NMDAR complexes are not essential in establishing or maintaining pre- or post-synaptic structures in CA1 pyramidal neurons in vivo.
Discussion
Through a genetic approach, we discovered a dichotomy in the regulation of electrical properties and neuronal morphology by excitatory synaptic input. While electrical properties, as well as synaptic inhibition, are profoundly regulated by the presence of excitatory input (Hartman et al., 2006; Turrigiano and Nelson, 2004), the development and maintenance of key neuronal morphological features are largely independent of excitatory synaptic input onto the neuron. Our data clearly show that excitatory synaptic input is not necessary for development and maintenance of neuronal morphology, even if suppressed in vivo, in a cell-autonomous manner. In addition, the maintenance of the PSD is normal in neurons lacking both AMPARs and NMDARs, suggesting that the presence of neurotransmitter receptor protein at synapses is not required for the maintenance of the PSD. In contrast, the profound adaptation in electrical properties in neurons lacking both AMPARs and NMDARs suggests a tight cell-autonomous relationship between intrinsic membrane properties and ongoing excitatory synaptic inputs. In addition, the down-regulation of synaptic inhibition indicates that the presence of synaptic excitation is important for development and maintenance of inhibitory synaptic transmission at the level of individual neurons.
Neuronal activity has long been shown to play an important role in the development and maintenance of the nervous system (Feller, 2009; Hanson and Landmesser, 2004; Hubel and Wiesel, 1970; Hubel et al., 1977; Huberman et al., 2006; Katz and Crowley, 2002; Katz and Shatz, 1996; McLaughlin et al., 2003; Mizuno et al., 2007; Shatz and Stryker, 1988; Sretavan et al., 1988; Wang et al., 2007; Xu et al., 2011; Zhang et al., 2012), likely through activity-dependent regulation of gene expression (Flavell and Greenberg, 2008). During early development of the neuromuscular junction (NMJ) in vivo, synaptic activity regulates synaptogenesis and competition among different synaptic inputs plays a crucial role in synapse elimination (Buffelli et al., 2003; Misgeld et al., 2002). In the visual cortex, convincing evidence demonstrates the importance of neuronal activity in determining the organization of ocular dominance column (Huberman et al., 2008; Katz and Crowley, 2002). These data suggest an instructive role for neuronal activity in fine sculpting the structure and function of the nervous system. However many developmental events of the nervous system appear not to require neuronal activity. Indeed, synaptic transmission has been shown to be dispensable for the maintenance of mature NMJs (Young et al., 2008). More significantly, the initial assembly of the nervous system including de novo synapse formation also does not depend on synaptic transmission (Varoqueaux et al., 2002; Verhage et al., 2000). In addition, early development of thalamocortical topographic arrangement does not require evoked neurotransmitter release (Molnar et al., 2002). Furthermore, neuronal activity is not essential for synapse formation in adult newborn neurons in olfactory bulb (Lin et al., 2010). These data highlight the imperative role for intrinsic determinants of neuronal form, instead of experience-dependent sensory inputs, in shaping the development, maintenance and function of the nervous system. We have now delineated, for the first time under the stringent condition of cell-autonomous manipulation, the impact of glutamatergic excitatory synaptic transmission in guiding neuronal structure and function from early development to maturity. Our data demonstrate that at the level of individual neurons in the hippocampus, the genetic program essentially dictates dendritic and synapse development and maintenance.
However it is clear that learning leads to new spine formation, presumably through patterned synaptic activity (Moser et al., 1994; Xu et al., 2009; Yang et al., 2009). One possible explanation for this discrepancy is synaptic competition within a neuron. In this case the activity of a subset of synapses on a neuron generated during the learning, in contrast to the global silencing of all excitatory synapses on a neuron, is key for the mechanisms underlying learning-induced spine changes. Supporting this notion, it has been reported that spatial learning-dependent new spine formation only affects basal but not apical dendrites in hippocampal CA1 pyramidal neurons (Moser et al., 1994; Moser et al., 1997), suggesting a compartmentalized effect of experience-induced synaptic activity on neuronal morphological remodeling. In addition, recent studies have shown that new spines induced by learning accounts for a very small percentage of total spines (< 0.1%) (Yang et al., 2009), and thus may not have a detectable effect on total spines. It is also plausible that turnover rates of spines, but not the steady-state spine density are regulated by neuronal activity as revealed in cortical pyramidal neurons from in vivo studies (Bhatt et al., 2009; Holtmaat and Svoboda, 2009; Majewska and Sur, 2003; Oray et al., 2004; Tropea et al.; Zuo et al., 2005). Indeed, in vivo imaging in mouse primary visual cortex demonstrated that binocular deprivation from before the time of eye-opening for more than two weeks up-regulated spine motility without changing the spine density in layer 5 neurons (Majewska and Sur, 2003). It is also worth noting that Environmental Enrichment (EE) leads to neuronal morphological changes in animals (Comery et al., 1995; Johansson and Belichenko, 2002; Rampon et al., 2000; Restivo et al., 2005; Turner and Greenough, 1985; Turner et al., 2003; van Praag et al., 2000) (but also see (Bindu et al., 2007; Connor and Diamond, 1982), although it remains unclear how EE induces morphological reorganizations in the nervous system. It has been shown that EE-induced structural changes in hippocampal CA1 pyramidal neurons is independent of the presence of synaptic NMDARs (Rampon et al., 2000), suggesting that other factors, such as metabolic or hormonal pathways (Lewis, 2004; Turner et al., 2002), other than activation of ionotropic glutamate receptors may play a critical role in EE-induced morphological plasticity. Lastly, it is important to emphasize that although this loss-of-function study demonstrates that ionotropic glutamate receptors are not essential for spine development and maintenance in hippocampal CA1 pyramidal neurons, our data do not exclude the possibility that persistent activation of ionotropic glutamate receptors can induce morphological plasticity. Indeed, it has been shown that LTP-like stimuli or direct activation of ionotropic glutamate receptors causes the outgrowth of new spines (Engert and Bonhoeffer, 1999; Kwon and Sabatini, 2011; Maletic-Savatic et al., 1999; Toni et al., 1999). Nevertheless, our single-cell genetic study demonstrates the activity-independence of steady state spine density in hippocampal CA1 pyramidal neurons, which is largely determined by intrinsic genetic programs. This differential role of excitatory synaptic transmission in neuronal form and function as revealed by our results may permit a dynamic regulation of electrical function of the neuron by ongoing synaptic activity while preserving the relative stability of neuronal morphology.
Experimental Procedures
Electrophysiology
Acute transverse hippocampal slices (300 μm) were prepared as described in the supplemental data. All paired voltage clamp recordings involved simultaneous dual whole-cell recordings from one GFP-positive neuron and one neighboring control neuron, as described in the Supplemental Data. Action potentials were recorded by injection of step-depolarizing current pulses under current clamp mode with potassium based internal solution.
Fluorescence Imaging
Mice were perfused with 4% paraformaldehyde (PFA) in PBS and 100 μm thick slices were cut on vibrating microtome as described in the supplemental data. GFP positive cells were identified by epifluorescence and were iontophoretically filled with Alexa Fluor 568 dyes using a sharp micropipette. Slices were then post-fixed, mounted, and scanned with confocal microscopy as described in the Supplemental Data.
Electron Microscopy
Mice were perfused with 4% paraformaldehyde (PFA) in PBS and GFP positive cells were filled with 5% Lucifer Yellow using a sharp micropipette. Dye-filled cells were photo-converted and processed for electron microscopy imaging as described in the Supplemental Data.
Supplementary Material
Acknowledgments
We thank K. Bjorgan and M. Cerpas for technical assistance, and all members from the Nicoll lab and Ellisman lab for helpful discussions. We are also grateful to the generosity of Professors Peter Seeburg and Rolf Sprengel at Max Planck Institute for Medical Research, Heidelberg, Germany, for sharing individual gene targeted conditional mice for GluA1, GluA2 or GluA3, from which we were able to make the triple floxed Gria1-3fl/fl mice. WL was funded by a NIH Pathway to Independence Award from NIMH and is supported by the NINDS Intramural Research Program. RAN is funded by grants from NIMH. The microscopy studies were carried out at the National Center for Microscopy and Imaging Research at UCSD supported by an award from NIH NCRR (P41 GM103412) and the National Institute of General Medical Sciences (8 P41 GM103412-24) from the National Institutes of Health to MHE. The image data obtained for this project are available in the Cell Image Library/Cell Centered Database supported by grants from the NIH NIGMS (R01 GM082949) to MHE and Maryann Martone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature reviews Neuroscience. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiological reviews. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Bindu B, Alladi PA, Mansooralikhan BM, Srikumar BN, Raju TR, Kutty BM. Short-term exposure to an enriched environment enhances dendritic branching but not brain-derived neurotrophic factor expression in the hippocampus of rats with ventral subicular lesions. Neuroscience. 2007;144:412–423. doi: 10.1016/j.neuroscience.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Collin C, Miyaguchi K, Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. Journal of neurophysiology. 1997;77:1614–1623. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- Comery TA, Shah R, Greenough WT. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiol Learn Mem. 1995;63:217–219. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- Connor JR, Diamond MC. A comparison of dendritic spine number and type on pyramidal neurons of the visual cortex of old adult rats from social or isolated environments. The Journal of comparative neurology. 1982;210:99–106. doi: 10.1002/cne.902100111. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nature neuroscience. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural development. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annual review of neuroscience. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4855–4860. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante M, Nistri A, Ballerini L. Opposite changes in synaptic activity of organotypic rat spinal cord cultures after chronic block of AMPA/kainate or glycine and GABAA receptors. The Journal of physiology. 2000;523(Pt 3):639–651. doi: 10.1111/j.1469-7793.2000.t01-1-00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. The Journal of comparative neurology. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nature neuroscience. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature reviews Neuroscience. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annual review of neuroscience. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52:247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nature reviews Neuroscience. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Tsien RW. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron. 2008;58:925–937. doi: 10.1016/j.neuron.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH. Environmental complexity and central nervous system development and function. Ment Retard Dev Disabil Res Rev. 2004;10:91–95. doi: 10.1002/mrdd.20017. [DOI] [PubMed] [Google Scholar]
- Lin CW, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron. 2010;65:32–39. doi: 10.1016/j.neuron.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog M, Li L, Groth RD, Poburko D, Thiagarajan TC, Han X, Tsien RW. Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21806–21811. doi: 10.1073/pnas.1016399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nature neuroscience. 2004;7:373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- Lu W, Gray JA, Granger AJ, During MJ, Nicoll RA. Potentiation of synaptic AMPA receptors induced by the deletion of NMDA receptors requires the GluA2 subunit. Journal of neurophysiology. 2011;105:923–928. doi: 10.1152/jn.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:16024–16029. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McKinney RA. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. The Journal of physiology. 588:107–116. doi: 10.1113/jphysiol.2009.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nature neuroscience. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- Mitra A, Mitra SS, Tsien RW. Heterogeneous reallocation of presynaptic efficacy in recurrent excitatory circuits adapting to inactivity. Nature neuroscience. 2012;15:250–257. doi: 10.1038/nn.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, Tagawa Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6760–6770. doi: 10.1523/JNEUROSCI.1215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Lopez-Bendito G, Small J, Partridge LD, Blakemore C, Wilson MC. Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:10313–10323. doi: 10.1523/JNEUROSCI.22-23-10313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Egeland T, Andersen P. Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. The Journal of comparative neurology. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature neuroscience. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988;336:468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Tropea D, Sur M, Majewska AK. Experience-dependent plasticity in visual cortex: Dendritic spines and visual responsiveness. Commun Integr Biol. 2011;4:216–219. doi: 10.4161/cib.4.2.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Turner CA, Lewis MH, King MA. Environmental enrichment: effects on stereotyped behavior and dendritic morphology. Dev Psychobiol. 2003;43:20–27. doi: 10.1002/dev.10116. [DOI] [PubMed] [Google Scholar]
- Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature reviews Neuroscience. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature reviews Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Vitureira N, Letellier M, Goda Y. Homeostatic synaptic plasticity: from single synapses to neural circuits. Current opinion in neurobiology. 2012;22:516–521. doi: 10.1016/j.conb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan SM, Xiong ZQ, Ding YQ. Activity-dependent development of callosal projections in the somatosensory cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, Zhou ZJ, Butts DA, Tian N, Picciotto MR, Crair MC. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron. 2011;70:1115–1127. doi: 10.1016/j.neuron.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G. Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nature neuroscience. 2008;11:721–728. doi: 10.1038/nn.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ackman JB, Xu HP, Crair MC. Visual map development depends on the temporal pattern of binocular activity in mice. Nature neuroscience. 2012;15:298–307. doi: 10.1038/nn.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.