Abstract
Recent advances in metabolomics and computational analysis have deepened our appreciation for the role of specific metabolic pathways in dictating cell fate. Once thought to be a mere consequence of the state of a cell, metabolism is now known to play a pivotal role in dictating whether a cell proliferates, differentiates or remains quiescent. Here, we review recent studies of metabolism in stem cells that have revealed a shift in the balance between glycolysis, mitochondrial oxidative phosphorylation and oxidative stress during the maturation of adult stem cells, and during the reprogramming of somatic cells to pluripotency. These insights promise to inform strategies for the directed differentiation of stem cells and to offer the potential for novel metabolic or pharmacological therapies to enhance regeneration and the treatment of degenerative disease.
Keywords: Blastocyst metabolism, Pluripotent stem cells, Hematopoietic progenitors, Reprogramming, Metabolomics
Introduction
As the zygote develops into a multicellular organism, the evolving demands of rapid cell growth, tissue formation and organogenesis place drastically different metabolic requirements on embryos, early pluripotent stem cells, tissue-resident adult stem cells, transient amplifying tissue progenitors and, ultimately, mature adult tissues. During the 1960s and 1970s, advances in our understanding of the metabolism of embryos led to techniques for in vitro culture of blastocysts and the development of in vitro fertilization (IVF). More recently, advances in cancer cell metabolism have led to an explosion in our understanding of how aerobic glycolysis (the ‘Warburg effect’, see Glossary, Box 1), rather than normal glycolysis (see Glossary, Box 1) linked to the Krebs cycle (see Glossary, Box 1) and to oxidative phosphorylation (OxPhos, see Glossary, Box 1), supports rapid cellular growth. This understanding has led to new drug targets for cancer therapy. Within the past decade, studies of metabolism in various stem cell populations have also highlighted a role for cell-specific metabolic pathways that are modulated during differentiation or during reprogramming to pluripotency. These novel insights have challenged the long-held assumption that all metabolic enzymes perform the same housekeeping functions in all cells, and have provoked a resurgence of interest in metabolism. Overall, these results paint a picture of tissue- and cell-specific metabolic pathways and isoenzymes that are tightly regulated during development and perform unique functions in specific contexts. Such cell- and tissue-specific isoenzymes provide a therapeutic window for pharmacological manipulation. Hence, the application of metabolomics to the study of stem cells during development could lead to new breakthroughs in our understanding of embryogenesis and adult tissue homeostasis, with implications for ongoing efforts in stem cell biology, tissue regeneration and therapies for degenerative diseases.
Box 1. Glossary
- Anaplerosis.
Reactions replenishing Krebs cycle intermediates that are shunted into other biosynthetic pathways.
- Eicosanoids.
Oxidized lipids derived from the oxidation of 20-carbon omega 3 or omega 6 essential fatty acids. There are four families of eicosanoids – prostaglandins, prostacyclins, thromboxanes and leukotrienes.
- Fatty acid β-oxidation.
A series of dehydrogenation, hydration, oxidation and thiolysis reactions that repeatedly cleave off the 2-carbon acetyl-CoA from fatty acids, which then feeds into the Krebs cycle.
- Gluconeogenesis.
Metabolic pathway that generates glucose from other carbon sources, such as pyruvate, lactate or glucogenic amino acids. It involves many glycolytic enzymes running in reverse.
- Glycolysis.
A metabolic pathway that breaks down one molecule of glucose into two molecules of pyruvate or lactate, generating ATP in the process. Glycolytic intermediates can also be shunted into anabolic pathways for biosynthesis, e.g. pentose phosphate, glycerol-3-phosphate, serine or glycine.
- Krebs cycle.
Cyclic series of condensation, isomerization, oxidation, decarboxylation and hydration reactions involving carboxylic acids in the mitochondria to generate energy through the oxidization of acetyl-CoA into CO2. In addition, the cycle provides precursors to generate certain amino acids, as well as NADH. Each complete turn of the cycle generates one GTP, two CO2, one FADH2 and three NADH molecules.
- Mitochondrial membrane potential (ΔΨm).
The voltage difference across the inner mitochondrial membrane that results from the proton gradient generated by proton pumps in the electron transport chain.
- Oxidative phosphorylation (OxPhos).
The mitochondrial reactions that generate and harness energy released from the oxidation of nutrients such as pyruvate, via a proton gradient, to synthesize ATP.
- Pentose phosphate pathway (PPP).
The metabolic pathway that converts glucose-6-phosphate into ribose-5-phosphate for nucleotide synthesis, generating NADPH in the process.
- Pluripotent.
Having the developmental potential to differentiate into any of the three germ layers – endoderm, mesoderm or ectoderm – that form the embryo proper.
- Reactive oxygen species (ROS).
Chemically reactive molecules containing oxygen, including superoxide and peroxide. ROS form as a by-product of OxPhos and irradiation, potentially causing damage to cell structures. Cumulatively, this is known as oxidative stress.
- Totipotent.
Having the developmental potential to differentiate into all the cells in an organism, including any of the three germ layers – endoderm, mesoderm or ectoderm – as well as the extra-embryonic trophoblast, which forms the placenta in mammals.
- Warburg effect.
The observation that proliferative cancer or embryonic cells exhibit a high rate of glycolysis followed by lactate production, instead of a low rate of glycolysis followed by mitochondrial oxidation of pyruvate, despite high levels of O2. Also known as aerobic glycolysis.
In this Review, we outline the specific metabolic pathways that are active in mammalian totipotent stem cells (TSCs), in pluripotent stem cells before and after differentiation, in quiescent adult stem cells, and in proliferative stem/progenitor cells during mammalian tissue development. We also discuss the role of stemness factors in governing stem cell metabolism, and examine the role of stem cell metabolism during aging using insights gleaned from invertebrate models.
Metabolism in TSCs and the early (pre-blastocyst) embryo
Before the morula stage of development in preimplantation embryos, each individual early blastomere is totipotent (see Glossary, Box 1) and retains the ability to generate an entire organism and its placenta. Despite differences between mammalian species in the time spent in gestation, the amount of time required to develop to the blastocyst stage is remarkably conserved (Brinster, 1974). In the first week, blastomeres, which are considered here as TSCs in vitro, undergo a few self-renewing cell divisions and rounds of DNA replication, but the embryo as a whole experiences no net growth (Leese, 1995). TSCs likely autophagocytose their protein reserves, which drop by 26% by the eight-cell stage (Brinster, 1967).
Studies indicate that metabolism in TSCs is tightly controlled by the rate-limiting glycolysis enzymes hexokinase (HK) and phosphofructokinase 1 (PFK1), thus glycolysis rates are initially low (Fig. 1A) (Barbehenn et al., 1978). Instead, the only energy and carbon sources that TSCs can use are pyruvate analogs, a fact that is also true for primordial germ cells, oocytes and spermatocytes, which use pyruvate secreted by ovarian follicle cells and lactate secreted by testicular Sertoli cells. The advantages of this preference for pyruvate in TSCs remain unknown. Early embryo development is actually inhibited by high concentrations of glucose (Brinster and Troike, 1979). Only at the morula stage does the relative rate of glucose oxidation rival that of pyruvate (Leese and Barton, 1984). The oxidation of pyruvate in mitochondria then fuels the Krebs cycle, which in turn generates carbon intermediates and fuels OxPhos.
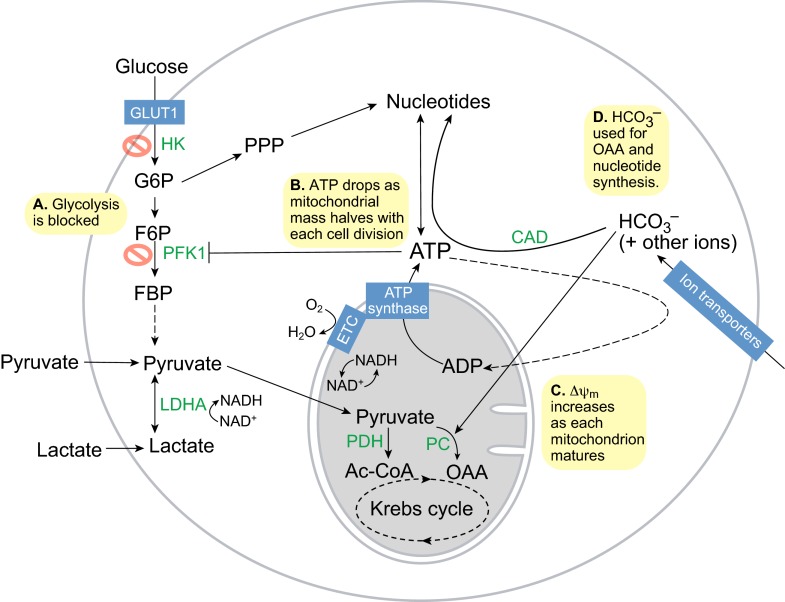
Fig. 1.
Metabolism in totipotent stem cells. (A) Glycolysis is impaired due to the low activities of the rate-limiting enzymes hexokinase (HK) and phosphofructokinase 1 (PFK1). Totipotent stem cells use pyruvate as their major energy and carbon source instead, via pyruvate dehydrogenase (PDH) to generate acetyl-CoA (Ac-CoA) and via pyruvate carboxylase (PC) to generate oxaloacetate (OAA) for anaplerosis or gluconeogenesis. (B) ATP synthesis is dependent on mitochondrial oxidative phosphorylation driven by the electron transport chain (ETC) and ATP synthase. However, as mitochondrial replication has not yet initiated, the halving of mitochondrial mass with each round of mitosis leads to a drop in ATP levels during embryo cleavage. (C) Simultaneously, each mitochondrion matures and the inner mitochondrial membrane potential (ΔΨm) increases steadily, thus turning the exponential drop in ATP into a linear drop. (D) Bicarbonate (HCO3−) is needed to buffer the pH and also provides a carbon source to OAA in the Krebs cycle for anaplerosis via PC or to nucleotide synthesis for DNA and RNA via carbamoyl phosphate synthetase (CAD). F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; G6P, glucose-6-phosphate; LDHA, lactate dehydrogenase A; PPP, pentose phosphate pathway.
Despite the preference for pyruvate oxidation, the O2 consumption rate of TSCs is relatively low, comparable with that of adult skin or bone (Brinster and Troike, 1979). Consistent with the low O2 consumption rate, mitochondria are structurally immature in TSCs. Oocyte and TSC mitochondria are typically spherical elements at least 1 μm in diameter with few truncated cristae, which contain a matrix of high-electron density. By the end of early embryogenesis, mitochondria elongate and develop networks of cristae containing a matrix of low-electron density (Van Blerkom, 2009). The inner mitochondrial membrane potential (ΔΨm, see Glossary, Box 1) per mitochondrion also increases with embryo cleavage. However, prior to blastocyst implantation and mitochondrial replication, mitochondrial numbers are halved with each cell division. Accordingly, total ATP levels and the ATP/ADP ratio are high initially in TSCs but decrease linearly with time during development (Fig. 1B,C), whereas the NADH/NAD+ ratio remains relatively high throughout (Quinn and Wales, 1971; Wales, 1974). Considering that ATP is a potent allosteric inhibitor of PFK1, this decrease in ATP may play a role in activating glycolysis at the blastocyst stage (Johnson et al., 2003).
The ATP generated by mitochondrial OxPhos is also used to actively transport ions into the early embryo. For example, the pH inside the early embryo is kept alkaline by importing high levels of bicarbonate from the oviduct and uterine fluid (Vishwakarma, 1962). Bicarbonate is important not just for pH buffering and membrane potential – it also contributes significantly to the carbon pool within TSCs (Graves and Biggers, 1970; Brinster, 1973). TSCs might use bicarbonate for carbon fixation via mitochondrial pyruvate carboxylase to generate oxaloacetate for anaplerosis (see Glossary, Box 1) or gluconeogenesis (see Glossary, Box 1), or via carbamoyl phosphate synthetase to generate nucleotides for DNA and RNA synthesis (Fig. 1D).
Metabolism in pluripotent stem cells and the blastocyst
During morula compaction, blastomeres undergo the first round of differentiation to segregate into trophectoderm (which becomes the placenta) and the pluripotent inner cell mass (ICM, which becomes the embryo proper). Accompanying this transformation is a sharp increase in net growth and metabolic activity (Fig. 2). First, glucose uptake rises sharply as the embryo upregulates the expression of the glucose transporters GLUT1 and GLUT3 (SLC2A1 and SLC2A3) (Pantaleon and Kaye, 1998). Glycolytic flux hence rises sharply, leading to increased lactate synthesis (Fig. 2A) (Leese and Barton, 1984). The importance of glycolysis to blastocysts is evident in vivo, as mutations in four glycolytic enzymes – glucose-6-phosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, triosephosphate isomerase and lactate dehydrogenase A (LDHA) – result in early postimplantation lethality (West et al., 1990; Pretsch, 2000; Merkle and Pretsch, 1989; Merkle et al., 1992). OxPhos rates also increase and O2 consumption in blastocysts rises to a high rate, comparable with that observed in the adult brain (Brinster, 1974). However, this increase is largely due to mitochondria of the trophectoderm, which have a high ΔΨm. By contrast, mitochondria in the ICM, which is not involved in organizing the blastocyst cavity, have a lower ΔΨm (Van Blerkom, 2009).
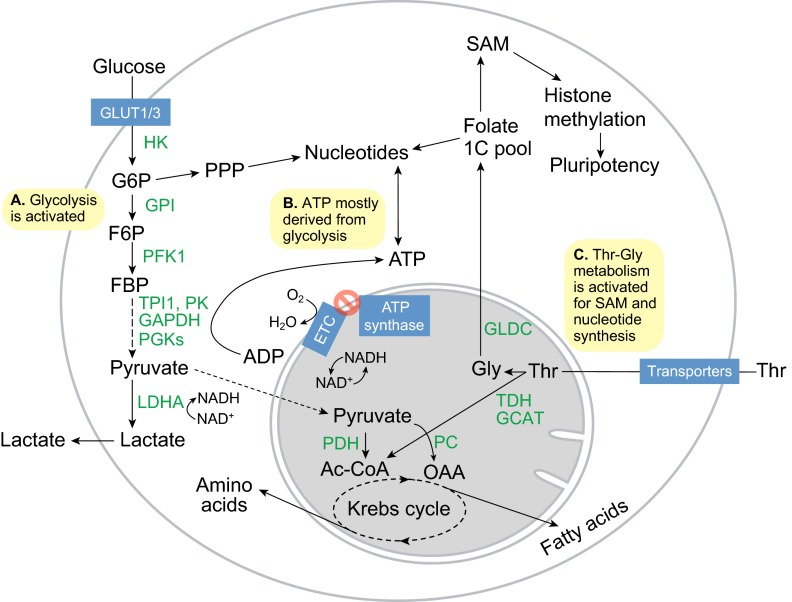
Fig. 2.
Metabolism in pluripotent stem cells. (A) Glucose flux increases with the increase in GLUT1/3 expression, and the hexokinase (HK) and phosphofructokinase 1 (PFK1) enzymes become activated to sharply increase glycolytic flux. As a result, flux into the pentose phosphate pathway (PPP) for nucleotide synthesis increases. (B) ATP synthesis is more dependent on the reactions carried out by glycolytic phosphoglycerate kinases (PGKs) and pyruvate kinases (PKs), and is decoupled from O2 consumption by the mitochondrial electron transport chain (ETC). (C) Activation of threonine dehydrogenase (TDH), glycine C-acetyltransferase (GCAT) and glycine decarboxylase (GLDC) promotes Thr-Gly catabolism to feed the folate one-carbon (1C) pool, which in turn fuels S-adenosylmethionine (SAM) and nucleotide synthesis to maintain pluripotency and proliferation. Ac-CoA, acetyl coenzyme A; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; G6P, glucose-6-phosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPI, glucose-6-phosphate isomerase; LDHA, lactate dehydrogenase A; OAA, oxaloacetate; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase complex; PK, pyruvate kinase; TPI1, triosephosphate isomerase.
Consistent with the lower ΔΨm in ICM mitochondria, pluripotent embryonic stem cells (ESCs) derived from in vitro culture of the ICM show high rates of glycolysis (Chung et al., 2007; Kondoh et al., 2007; Prigione et al., 2010; Varum et al., 2011; Zhang et al., 2011a; Panopoulos et al., 2012; Shyh-Chang et al., 2013). A switch from OxPhos back to glycolysis is also seen during the conversion of differentiated cells into induced pluripotent stem cells (iPSCs) and reflects metabolic reprogramming (Folmes et al., 2011; Shyh-Chang et al., 2013). However, the upregulation of glycolysis precedes the reactivation of pluripotency markers (Hansson et al., 2012; Shyh-Chang et al., 2013). This indicates that the activation of glycolysis is not necessarily specific to a pluripotent (see Glossary, Box 1) state but, rather, represents the preferred metabolic state of rapidly proliferating cells. Furthermore, cell proliferation enhances iPSC reprogramming stochastically (Hanna et al., 2009), further confounding the relationship between glycolysis, proliferation and pluripotency. That said, promotion of glycolysis enhances iPSC reprogramming, whereas pharmacological inhibition of glycolysis or stimulation of OxPhos impairs iPSC reprogramming (Yoshida et al., 2009; Zhu et al., 2010; Folmes et al., 2011). In the Warburg effect, cancer cells are thought to shunt glycolytic intermediates into amino acid, lipid and nucleotide synthesis for cell proliferation (Vander Heiden et al., 2009). Similarly, mouse ESCs show increased activity in the pentose phosphate pathway (PPP, see Glossary, Box 1), which allows rapid nucleotide synthesis (Filosa et al., 2003; Manganelli et al., 2012; Varum et al., 2011). Thus, anabolic glycolysis is a common feature of metabolism in both ESCs and cancer cells.
Despite the low levels of OxPhos occurring in undifferentiated ESCs, O2 consumption is still important. However, ATP synthesis appears to be decoupled from O2 consumption, and depends on glycolysis instead (Fig. 2B) (Zhang et al., 2011a). Although the mechanism and reasons for this decoupling remain unclear, it is possible that ESCs consume O2 through the electron transport chain (ETC) to oxidize NADH into NAD+ and maintain the Krebs cycle flux. This might also allow ESCs to maintain an optimal redox potential for lipid synthesis from citrate and for amino acid synthesis from OAA or α-ketoglutarate (Shyh-Chang et al., 2011). In line with this, ESCs with high ΔΨm form teratomas more efficiently than ESCs with low ΔΨm (Schieke et al., 2008), and blastocysts deficient in mitochondrial oxidation enzymes, including the pyruvate dehydrogenase (PDH) complex, show developmental defects (Johnson et al., 1997; Johnson et al., 2001).
Mouse ESCs also use a unique mode of amino acid metabolism to maintain their pluripotent epigenetic state (Fig. 2C). In a screen for dependence on each of the 20 amino acids, restriction of Thr alone (and to a lesser extent, Met or Cys) uniquely abolished mouse ESC growth. By contrast, other proliferative cell lines such as HeLa, 3T3 or mouse embryonic fibroblasts were sensitive to restriction of other amino acids but not to Thr restriction (Wang et al., 2009). Consistent with these findings, expression of the Thr-catabolizing enzyme threonine dehydrogenase (TDH) is dramatically upregulated in early blastocysts and ESCs (and after iPSC reprogramming), relative to differentiated cells (Wang et al., 2009; Shyh-Chang et al., 2013). TDH is a mitochondrial enzyme that controls the the rate-limiting step in the metabolism of Thr into Gly and acetyl-CoA. Gly can be subsequently used by the mitochondrial enzyme glycine decarboxylase (GLDC) to generate 1-carbon equivalents for the folate pool. Like TDH, GLDC is also upregulated in pluripotent cells relative to differentiated cells (Shyh-Chang et al., 2013). Furthermore, pharmacological inhibition or knockdown of TDH disrupts mouse ESC colony growth (Alexander et al., 2011; Shyh-Chang et al., 2013). Collectively, these findings suggest that the metabolites generated by Thr degradation may be used specifically for the self-renewal of pluripotent ESCs. Indeed, it has recently been demonstrated that TDH and GLDC regulate the synthesis of 5-methyl-tetrahydrofolate, thereby modulating S-adenosylmethionine (SAM) metabolism and histone H3K4 tri-methylation (Shyh-Chang et al., 2013) (Box 2). H3K4 tri-methylation is associated with open euchromatin, which is crucial for the epigenetic plasticity of pluripotent ESCs and their self-renewal (Azuara et al., 2006; Gaspar-Maia et al., 2011; Ang et al., 2011). TDH regulation of the folate pool was also found to promote rapid proliferation in mouse ESCs (Wang et al., 2009), consistent with findings that GLDC regulates the folate pool to promote nucleotide synthesis and rapid proliferation in cancer stem cells (Zhang et al., 2012).
Box 2. Metabolic regulation of epigenetics during stem/progenitor cell differentiation
Acetyl-CoA, besides being a Krebs cycle substrate, is important for protein acetylation (Choudhary et al., 2009; Kaelin and McKnight, 2013), which is known to impact protein function and gene expression. In particular, histone H3 acetylation leads to an open euchromatin conformation that regulates gene expression in stem cells. Reduction of acetyl-CoA by knockdown of ATP citrate lyase (ACL, the enzyme responsible for cytosolic acetyl-CoA synthesis) induces myoblast differentiation (Bracha et al., 2010), whereas inhibition of histone deacetylases promotes iPSC reprogramming (Huangfu et al., 2008). In addition, glycolysis fuels the rise in acetyl-CoA and histone acetylation required for pre-adipocyte differentiation (Wellen et al., 2009).
Akin to the role of acetyl-CoA in histone acetylation, S-adenosylmethionine (SAM) is crucial for histone methylation (Shyh-Chang et al., 2013). Like histone acetylation, histone H3K4 di- or tri-methylation leads to euchromatin formation. Reduction in the ratio of SAM to S-adenosylhomocysteine (SAH), induced by the SAH hydrolase inhibitor 3-deaza-adenosine (DZA), leads to rapid loss of H3K4 tri-methylation in ESCs, and causes ESCs to differentiate and die (Shyh-Chang et al., 2013). Thr-Gly metabolism was shown to fuel SAM synthesis in mouse ESCs, thus maintaining the high levels of H3K4 tri-methylation that are crucial to mouse ESC pluripotency and reprogramming. This demonstrates that changes in amino acid metabolism can regulate histone methylation via SAM, thereby influencing epigenetic control of stem cell fate.
The Krebs cycle intermediate α-ketoglutarate (αKG) is also an important co-factor for the Fe2+-dependent Jumonji family of dioxygenases, which mediate histone demethylation, and the TET family of dioxygenases, which mediate DNA demethylation. Somatic mutations in isocitrate dehydrogenases IDH1/2 lead to reduced αKG levels, thus disrupting normal histone demethylation and consequently hematopoetic stem cell differentiation (Figueroa et al., 2010; Sasaki et al., 2012), as well as pre-adipocyte and neural progenitor differentiation (Lu et al., 2012). This leads to cellular transformation and cancer. Interestingly, another co-factor for Fe2+-dependent dioxygenases, ascorbate or vitamin C, has also been shown to improve iPSC reprogramming, thereby promoting DNA and H3K36 demethylation (Chung et al., 2010; Stadtfeld et al., 2012; Wang et al., 2011).
Metabolism in differentiating ESCs
When ESCs differentiate, glycolytic flux decreases dramatically (Fig. 3A,B), Thr-Gly metabolism is extinguished and mitochondrial OxPhos fueled by glucose and fatty acids increases (Fig. 3C,D) (Cho et al., 2006; Chung et al., 2007; Facucho-Oliveira et al., 2007; Wang et al., 2009). Thus, cells acquire an even more oxidized state. Furthermore, pluripotent ESCs are enriched with unsaturated lipids such as omega 3 and omega 6 fatty acids that contain readily oxidizable carbon-carbon double bonds (Yanes et al., 2010). These unsaturated lipids prime ESCs to differentiate after oxidation by reactive oxygen species (ROS, see Glossary, Box 1) to form eicosanoids (Fig. 3E). In support of this concept, pharmacological inhibition of enzymes in the eicosanoid synthesis pathway (see Glossary, Box 1), which oxidizes these unsaturated lipids, preserves ESC pluripotency (Yanes et al., 2010).
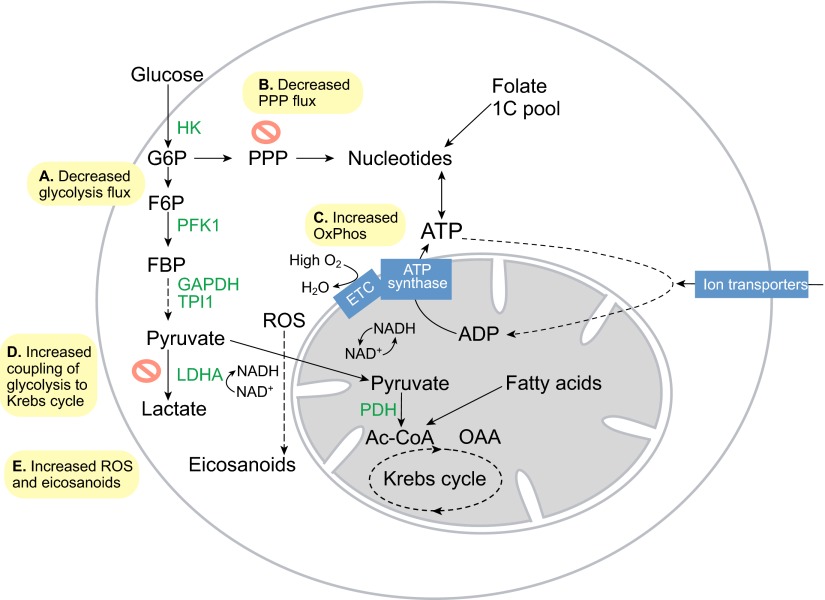
Fig. 3.
Metabolism in differentiating embryonic stem cells. (A) Glycolytic flux and lactate production drop rapidly upon embryonic stem cell differentiation. (B) Flux into the pentose phosphate pathway (PPP) decreases as a result. (C) O2 consumption increases sharply as the electron transport chain (ETC) again becomes coupled to ATP synthase to fulfill the needs of cell differentiation. (D) Glycolysis also becomes more coupled to the Krebs cycle, as pyruvate is transported more efficiently into mitochondria. (E) Increased ETC activity leads to increased reactive oxygen species (ROS) and eicosanoid signaling, which promote cell differentiation. Ac-CoA, acetyl coenzyme A; HK, hexokinase; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; G6P, glucose-6-phosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LDHA, lactate dehydrogenase A; OAA, oxaloacetate; Ox Phos, oxidative phosphorylation; PDH, pyruvate dehydrogenase complex; PFK1, phosphofructokinase 1; TPI1, triosephosphate isomerase.
Metabolism in quiescent adult stem cells
Unlike proliferative ESCs, most adult stem cells are quiescent. Such quiescent adult stem cells reside in various tissues and include long-term hematopoietic stem cells (LT-HSCs) and mesenchymal stem cells (MSCs) in the bone marrow, neural stem cells (NSCs) in the brain, epidermal stem cells in the hair follicle bulge, and satellite cells in skeletal muscles. It is thought that quiescent adult stem cells generally maintain a slow-cycling state to avoid cellular damage from ROS and to ensure life-long tissue renewal capacity (Rossi et al., 2008; Suda et al., 2011). But is the metabolic program in adult stem cells intrinsically required for stem cell self-renewal or is it an adaptive response to the often hypoxic environment?
It is known, for instance, that adult LT-HSCs prefer to use glycolysis (Fig. 4A), a phenotype that some have argued to be an adaptation to the hypoxic environment of the bone marrow niche (Suda et al., 2011). One potential advantage of hypoxia-induced glycolysis in LT-HSCs is the associated reduction in ROS production from mitochondria. LT-HSCs are sensitive to ROS, and they either undergo differentiation or apoptosis in the presence of excessive ROS when the stress response is defective (Tothova et al., 2007). NSCs in the hypoxic brain exhibit similar responses to ROS (Renault et al., 2009). This suggests, however, that glycolysis is not simply an environmental adaptation but an intrinsic necessity for quiescent adult stem cells. In line with this, the pro-glycolytic phenotype of LT-HSCs appears to be programmed by the HSC transcription factor MEIS1 via its target hypoxia-inducible factor 1α (HIF1α) (Simsek et al., 2010), which upregulates many glycolytic enzymes. Similar to embryonic TSCs and ESCs, adult HSCs possess low mitochondrial mass and immature mitochondrial morphology (Chung et al., 2007). In fact, the majority of LT-HSCs can be metabolically sorted by gating for low ΔΨm and low endogenous NADH, and the resultant cells have greater hematopoietic capacity in vivo (Simsek et al., 2010). The low ΔΨm could be partly because LT-HSCs express higher levels of the pyruvate dehydrogenase kinases PDK1 and PDK3, which inhibit PDH and mitochondrial pyruvate oxidation (Fig. 4B) (Klimmeck et al., 2012). Interestingly, another pair of PDKs regulated by HIF1α, PDK2 and PDK4, are also required by LT-HSCs, demonstrating that PDK-regulated OxPhos generally acts as a switch for LT-HSC function (Takubo et al., 2013). Overall, it appears that OxPhos capacity is reduced in LT-HSCs. However, peroxisome proliferator activator receptor δ-driven fatty acid β-oxidation (see Glossary, Box 1) has been shown to promote LT-HSC self-renewal (Fig. 4C) (Ito et al., 2012), suggesting that it is not the absolute quantity per se, but the efficiency of OxPhos that might also be important.
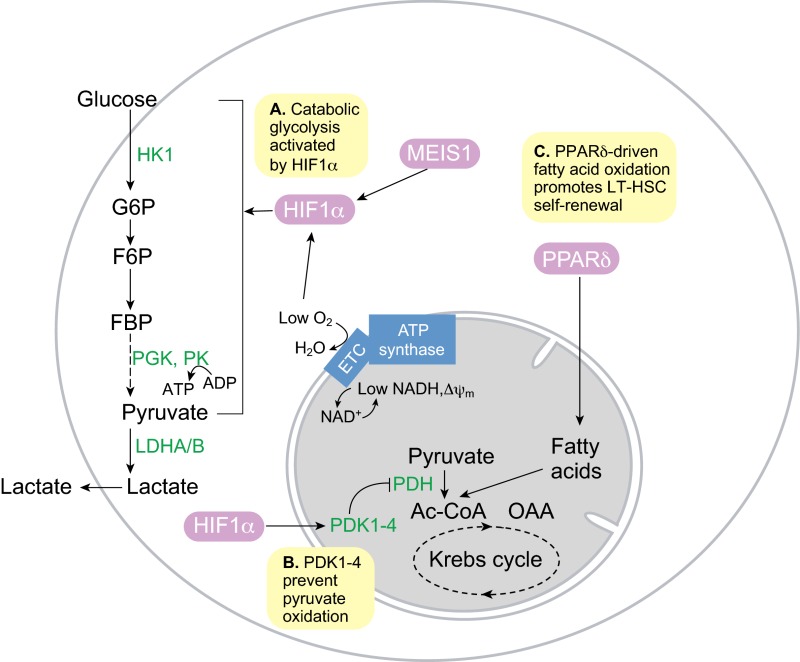
Fig. 4.
Metabolism in quiescent long-term hematopoietic stem cells. (A) The hematopoietic stem cell (HSC) transcription factor MEIS1 and low O2 levels combine to activate hypoxia-inducible factor 1α (HIF1α) activity, which in turn promotes glycolysis in quiescent long-term HSCs (LT-HSCs). (B) HIF1α-dependent pyruvate dehydrogenase kinases (PDK1-4) prevent pyruvate oxidation by suppressing pyruvate dehydrogenase complex (PDH). (C) Peroxisome proliferator activator receptor δ (PPARδ)-driven fatty acid oxidation in the mitochondria is required for LT-HSC self-renewal and quiescence. Inhibition of fatty acid oxidation leads to LT-HSC proliferation and differentiation. Ac-CoA, acetyl coenzyme A; ETC, electron transport chain; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; G6P, glucose-6-phosphate; HK1, hexokinase 1; LDHA, lactate dehydrogenase A/B; PGK, phosphoglycerate kinase; PK, pyruvate kinase.
MSCs also reside in hypoxic environments in vivo. Relative to the more differentiated osteoblasts within the bone marrow, MSCs express higher levels of glycolytic enzymes and lower levels of OxPhos proteins, suggesting that MSCs rely more on glycolysis than do osteoblasts (Chen et al., 2008a). Nevertheless, MSCs expanded under normoxia can still use OxPhos with a high O2 consumption rate, suggesting that glycolysis may be an environmental adaptation for MSCs (Pattappa et al., 2011). In fact, MSC proliferative and colony formation capacity is significantly increased in normoxia (Pattappa et al., 2013). The switch to OxPhos comes at a cost, however, as MSCs expanded under normoxia show a three- to fourfold increase in senescence, suggesting that hypoxia-induced glycolysis limits MSC proliferation to prevent oxidative stress-induced senescence and preserve MSC long-term self-renewal (Pattappa et al., 2013). Thus, in at least three types of adult stem cells, namely LT-HSCs (Tothova et al., 2007), MSCs (Chen et al., 2008a) and NSCs (Renault et al., 2009), the induction of ROS forces adult stem cells out of quiescence in hypoxia, and into a more proliferative state in normoxia. This may be a common mechanism for priming stem cell differentiation.
Metabolism in proliferative adult stem/progenitor cells
In addition to long-lived quiescent stem cells, a number of adult tissues contain proliferative stem and progenitor cells that contribute to tissue homeostasis and renewal. These include hematopoietic progenitors, neural progenitors, mesenchymal progenitors, skeletal myoblasts, cardiomyocyte progenitors, intestinal stem cells (ISCs) and germline stem cells (GSCs). As we discuss below, these are all highly proliferative undifferentiated cells that rely on different combinations of glycolysis and OxPhos for proliferation (summarized in Table 1).
Table 1.
Summary of metabolism in respective stem and progenitor cells
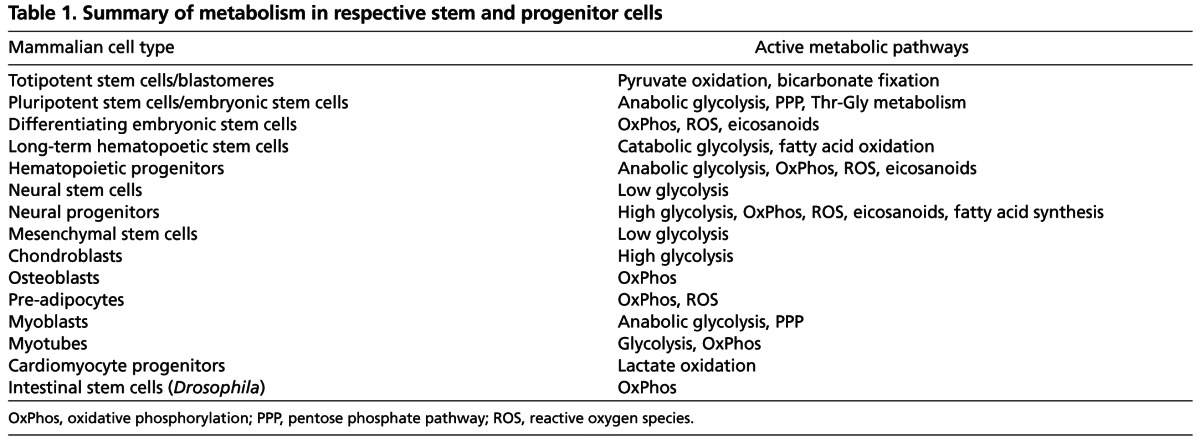
Hematopoietic progenitors
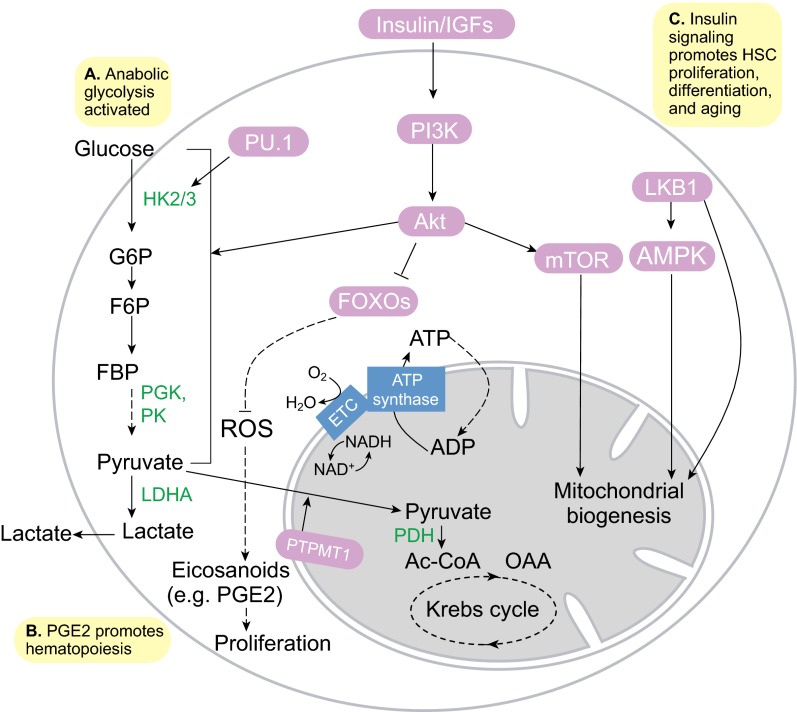
The metabolic switch from anaerobic glycolysis under hypoxia for quiescent adult stem cells, to a mixture of aerobic glycolysis and OxPhos for proliferative adult stem/progenitor cells is well exemplified in hematopoiesis. A proteomics study of the transition from HSCs to myeloid progenitors showed that among the hexokinase isoforms, HK1 expression is higher in HSCs, whereas HK2 and HK3 expression levels are higher in myeloid progenitors (Klimmeck et al., 2012). HK1 is primarily associated with catabolism, whereas HK2 and HK3 have anabolic roles (Wilson, 2003), suggesting that HSCs rely on glycolysis for energy, whereas myeloid progenitors use glycolysis for anabolic growth (Fig. 5A). In fact, myeloid cells express high levels of the transcription factor PU.1, which transactivates HK3 to drive myeloid differentiation and maintain myeloid identity (Federzoni et al., 2012). Myeloid progenitors also express lower levels of PDKs, suggesting that PDK-mediated suppression of OxPhos in HSCs is relieved upon myeloid commitment (Klimmeck et al., 2012; Takubo et al., 2013). In addition, the mitochondrial phosphatase PTPMT1, which dephosphorylates phosphatidylglycerol phosphate during cardiolipin synthesis (Zhang et al., 2011b), appears to be another master regulator of the OxPhos switch (Fig. 5B) (Yu et al., 2013).
Fig. 5.
Metabolism in proliferative hematopoietic stem and progenitor cells. (A) Anabolic glycolysis is driven in part by the myeloid transcription factor PU.1 and the Akt kinase. (B) Increased reactive oxygen species (ROS) production during oxidative phosphorylation (OxPhos), fueled by PTPMT1-driven pyruvate oxidation, might lead to increased synthesis of eicosanoids, e.g. prostaglandin E2 (PGE2), which promote hematopoiesis. (C) Insulin-PI3K-Akt signaling activates glycolysis, promotes ROS production by repressing the FOXO-mediated stress response, and promotes mitochondrial biogenesis by activating mTOR signaling. This leads to hematopoietic stem cell (HSC) proliferation, differentiation and aging. AMPK, AMP-activated protein kinase; Ac-CoA, acetyl coenzyme A; ETC, electron transport chain; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; G6P, glucose-6-phosphate; HK2/3, hexokinase 2/3; LDHA, lactate dehydrogenase A; LKB1, serine/threonine protein kinase 11; OAA, oxaloacetate; PDH, pyruvate dehydrogenase complex; PGK, phosphoglycerate kinase; PK, pyruvate kinase.
Concomitant with the increase in OxPhos activity as HSCs differentiate, ROS levels also increase (Fig. 5B) (Tothova et al., 2007). In the Drosophila larval lymph gland, hematopoietic cells resembling mammalian myeloid progenitors require ROS to differentiate into mature blood cells (Owusu-Ansah and Banerjee, 2009). The targets of ROS that prime HSC differentiation still remain unknown, but it is possible that components of the eicosanoid biosynthesis pathway, which oxidizes lipids in ESCs (Yanes et al., 2010), may be involved. In fact, the eicosanoid product prostaglandin E2 has been shown to significantly enhance HSC proliferation, hematopoietic colony formation and hematopoiesis in vivo by activating Wnt signaling (Goessling et al., 2009), suggesting that eicosanoids might be important for HSC proliferation and differentiation.
Neural progenitors
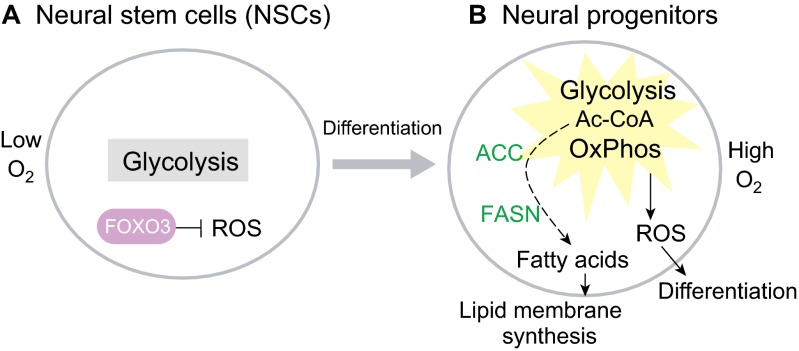
Like hematopoietic progenitors, proliferative neural progenitors show high levels of glycolysis mediated by HK2 (Gershon et al., 2013); and like hematopoietic progenitors, the directed differentiation of ESCs into neural progenitors is stimulated by the eicosanoid pathway and also by fatty acid metabolism (Yanes et al., 2010). However, it is unclear whether these effects of fatty acids are due to mitochondrial fatty acid β-oxidation or fatty acid use in lipogenesis, or both. The oxidative stress response mediated by FOXO3 becomes rapidly deactivated upon NSC differentiation, suggesting that mitochondrial oxidation-induced ROS is required in neural progenitors (Fig. 6A) (Renault et al., 2009). In fact, deficiency in FOXO3 causes depletion of adult brain NSCs and an expansion of oligodendrocytes in the corpus callosum (Renault et al., 2009). However, neural progenitors require lipogenesis, which is mediated by fatty acid synthase and acetyl-CoA carboxylase (ACC), for lipid membrane synthesis and for neural progenitor proliferation (Knobloch et al., 2013). These results suggest that both fatty acid synthesis and oxidation-induced ROS might be crucial for neural progenitors (Fig. 6B). Alternatively, it is possible that fatty acid β-oxidation is only required in quiescent NSCs, resembling the situation observed in LT-HSCs (Ito et al., 2012). Upon differentiation into neural progenitors, the ACC-mediated increase in malonyl-CoA could then allosterically inhibit fatty acid β-oxidation and promote fatty acid synthesis. Yet another possibility is that fatty acid-derived lipids are oxidized via the eicosanoid pathway to promote neurogenesis. More work is required to resolve these issues.
Fig. 6.
Metabolism in neural stem cells and progenitors. (A) Neural stem cells (NSCs) remain quiescent in a hypoxic niche with low O2. NSCs require FOXO3 to suppress reactive oxygen specis (ROS). (B) Neural progenitors, which exist under normoxia, upregulate both glycolysis and oxidative phosphorylation (OxPhos). In normoxia, FOXO3 is repressed and leads to increased ROS, which prime NSCs for differentiation. Activation of acetyl-CoA (Ac-CoA) carboxylase (ACC) and fatty acid synthase (FASN) increase fatty acid synthesis from Ac-CoA to fuel phospholipid membrane synthesis.
Mesenchymal progenitors
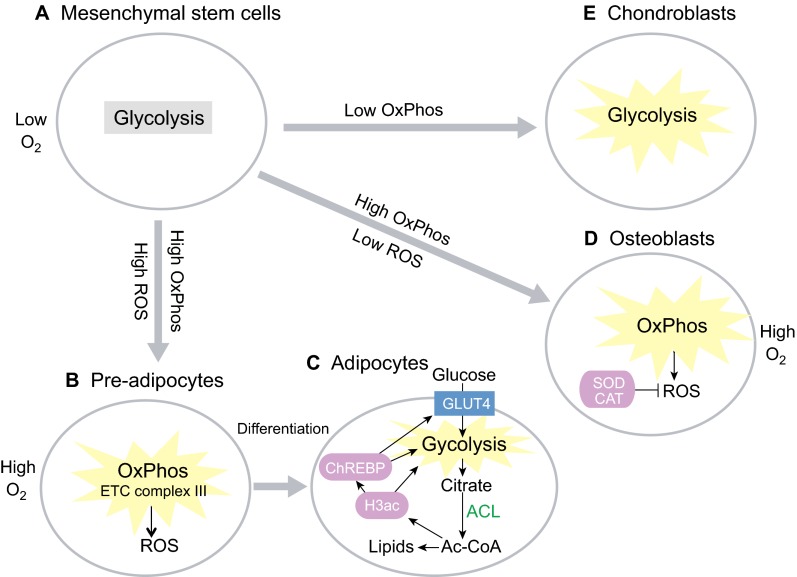
During adipogenesis, mesenchymal pre-adipocytes activate a lipogenic program involving ATP citrate lyase (ACL) to increase glucose metabolism and to synthesize lipids for fat storage (Fig. 7A,B). Adipocytes also rely upon ACL to increase their supply of acetyl-CoA for increased histone acetylation during adipogenesis (Fig. 7B,C). This is necessary for transactivation of key metabolic proteins such as the glucose transporter GLUT4, HK2, PFK1, LDHA and the carbohydrate-responsive element-binding protein (ChREBP) in the lipogenic program (Wellen et al., 2009). Directed adipogenesis of MSCs also increases mammalian target of rapamycin complex 1 (mTORC1)-dependent mitochondrial biogenesis, OxPhos and ROS (Fig. 7B). Endogenous ROS generated from the mitochondrial ETC complex III is required to initiate adipogenesis, suggesting that OxPhos and ROS are not simply a consequence of adipogenesis but are a causal factor in promoting it (Tormos et al., 2011). Similarly, directed osteogenesis of MSCs leads to an increase in mitochondrial biogenesis and OxPhos in osteoblasts (Chen et al., 2008a). Unlike adipogenesis, however, osteogenesis cannot tolerate ROS (Fig. 7D). In fact, antioxidant enzymes such as superoxide dismutase and catalase are simultaneously upregulated with OxPhos in osteoblasts to prevent ROS accumulation (Chen et al., 2008a). In contrast to adipogenesis and osteogenesis, MSCs undergoing chondrogenesis have significantly reduced O2 consumption and OxPhos, indicating a shift towards increased glycolysis (Fig. 7E) (Pattappa et al., 2011). Furthermore, hypoxia inhibits MSC osteogenesis, whereas chondrogenesis is unaffected (Pattappa et al., 2013). Taken together, these studies demonstrate that careful manipulation of oxidative metabolism can direct the differentiation of MSCs either into adipocytes, osteoblasts or chondroblasts.
Fig. 7.
Metabolism in mesenchymal stem cells and progenitors. (A) Bone marrow mesenchymal stem cells remain quiescent in a hypoxic niche and use glycolysis. (B) Pre-adipocytes upregulate oxidative phosphorylation (OxPhos), and reactive oxygen species (ROS) production from the electron transport chain (ETC) complex III is highly active, to prime adipocyte differentiation. (C) Adipocytes upregulate glycolysis and ATP citrate lyase (ACL), which leads to increased cytosolic acetyl-CoA (Ac-CoA) synthesis and, hence, an increase in histone H3 acetylation (H3ac) and in lipid synthesis. H3ac, in turn, leads to activation of the carbohydrate-responsive element-binding protein (ChREBP) transcription factor to promote GLUT4-mediated glucose uptake and glycolysis in order to generate the acetyl-CoA needed. (D) In osteoblasts, which give rise to bone, OxPhos and O2 consumption are upregulated, but ROS is suppressed via superoxide dismutase (SOD) and catalase (CAT). (E) Glycolysis is further upregulated during chondrogenesis in chondroblasts, which give rise to cartilage.
Skeletal myoblasts
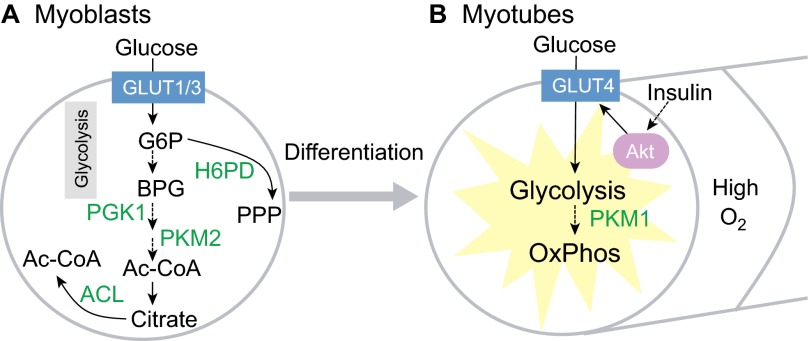
Myogenesis during muscle regeneration is mediated by the proliferation of myoblasts derived from satellite cells, which undergo fusion to form myotubes. The expression of glucose transporters is tightly regulated during this process. GLUT1, for example, is expressed specifically in satellite cells and myoblasts (Fig. 8A). Levels of the higher affinity transporter GLUT3 increase markedly in myoblasts during myogenesis, and decrease in myotubes. By contrast, the high-affinity and insulin-sensitive GLUT4 is expressed specifically in myotubes (Fig. 8B). Accordingly, the rate of glucose transport rises significantly during myogenesis, suggesting a switch in glucose metabolism during this process (Guillet-Deniau et al., 1994). This switch is supported by findings that myoblast differentiation leads to a dramatic switch in the expression of pyruvate kinase isoforms, mediated by RNA-binding proteins, from the pro-glycolytic PKM2 (pyruvate kinase, muscle isoform 2) to the pro-OxPhos PKM1 (Fig. 8A,B) (Clower et al., 2010; David et al., 2010). In fact, myoblasts require glycolytic phosphoglycerate kinase 1 (PGK1) for self-renewal (Bracha et al., 2010). Hexose-6-phosphate dehydrogenase (H6PD) in the pentose phosphate pathway (PPP), and ACL-driven acetyl-CoA synthesis also appear to be required for myoblast self-renewal in vitro (Bracha et al., 2010).
Fig. 8.
Metabolism in skeletal myoblasts and myotubes. (A) Myoblasts use GLUT1 and GLUT3 for glucose uptake. Glycolysis mediated by phosphoglycerate kinase 1 (PGK1) is necessary for myoblast self-renewal. The low activity pyruvate kinase isoform M2 (PKM2) then facilitates accumulation of glycolytic intermediates for anabolic metabolism in myoblasts. For example, the pentose phosphate pathway (PPP), which is mediated by hexose-6-phosphate dehydrogenase (H6PD), shunts glucose-6-phosphate (G6P) into ribose and NADPH synthesis. ATP citrate lyase (ACL), which generates acetyl-CoA (Ac-CoA) from citrate, is also necessary for myoblast self-renewal. (B) Upon cell fusion and differentiation into myotubes, glucose uptake and glycolysis are increased by GLUT4, which is sensitive to regulation by insulin-PI3K-Akt signaling. Mitochondrial oxidative phosphorylation (OxPhos) also increases, partly owing to the switch to the high activity pyruvate kinase isoform M1 (PKM1).
Cardiomyocyte progenitors
In the fetal heart, cardiomyocyte progenitors prefer to use lactate to fuel OxPhos (Werner and Sicard, 1987). Knowledge of these metabolic features has aided the efficient derivation of cardiomyocytes from ESCs and iPSCs during directed differentiation. Lactate media without glucose effectively eliminate human ESCs and iPSCs from differentiating embryoid bodies, while stimulating the rapid proliferation of cardiomyocyte progenitors. The resultant human cardiomyocytes showed physiologically relevant action potential configurations and drug responses, and can be transplanted without forming tumors (Tohyama et al., 2013). These results demonstrate how understanding stem cell metabolism can lead to effective strategies for directed differentiation and mass production of functional cells and tissues.
Metabolism and ‘stemness’ factors: establishing a link
After decades of research in stem cell biology, candidate factors that define ‘stemness’ across the myriad of tissue lineages and stem cells are emerging. One outstanding issue in stem cell biology is whether such a stemness factor, if it exists, drives specific cellular functions such as metabolism in a specific manner to maintain stemness. One such candidate factor is LIN28, an RNA-binding protein first identified in C. elegans through mutagenesis screens for regulators of developmental timing, or heterochrony (Ambros and Horvitz, 1984; Shyh-Chang and Daley, 2013). In mammals, LIN28A/B have been found to be regulators of pluripotent ESCs and iPSCs, fetal HSCs, neural crest progenitors, skeletal myoblasts during muscle regeneration and spermatogonia (Viswanathan et al., 2008; Yu et al., 2007; Yuan et al., 2012; Molenaar et al., 2012; Polesskaya et al., 2007; Zheng et al., 2009). Interestingly, LIN28A/B regulate glucose metabolism in vivo by modulating insulin-PI3K-mTOR signaling via the let-7 microRNA (Zhu et al., 2011). Furthermore, transcripts encoding glycolysis and mitochondrial enzymes are among the top mRNA targets directly bound by LIN28A in human ESCs (Peng et al., 2011). These findings suggest that the effects of LIN28 on stem/progenitor cell self-renewal across multiple lineages might be mediated in part by metabolic programming – a hypothesis that awaits testing.
Stem cell metabolism and aging
Several lines of evidence support the notion that enhanced tissue regeneration by adult stem cells can delay aging, whereas a decline in adult stem cell numbers and function drives aging-related syndromes (Sahin and Depinho, 2010). Accumulating damage from ROS, for example, can compromise stem cell self-renewal and promote aging (Rossi et al., 2008; Sahin and Depinho, 2010). Nutrient-sensitive signaling pathways that regulate organismal aging, such as the insulin-PI3K, Akt-FOXO, mTOR and AMPK pathways, also regulate the balance between quiescence and proliferation of stem cells during aging (Chen et al., 2009; Jasper and Jones, 2010; Kharas et al., 2010; Kalaitzidis et al., 2012; Magee et al., 2012). As one example of the crosstalk between nutrient-sensitive signaling and ROS in mammals, deficiency in the FOXO transcription factors dampens the oxidative stress response and depletes mouse LT-HSCs (Tothova et al., 2007). Likewise, deletion of the AMPK regulator LKB1 (serine/threonine protein kinase 11) leads to loss of mouse LT-HSC quiescence, depletion of LT-HSC pools and impaired hematopoiesis, probably owing to mitochondrial defects (Gan et al., 2010; Gurumurthy et al., 2010; Nakada et al., 2010). The mTOR signaling pathway also plays a major role in governing stem cell fate. For example, deletion of the metabolic sensor TSC1 (tuberous sclerosis 1), which upregulates mTORC1 signaling, drives mouse LT-HSCs into proliferation due to increased mitochondrial biogenesis, ultimately depleting the LT-HSCs and impairing hematopoiesis (Chen et al., 2008b). Consistently, mTORC1 inhibition with rapamycin delays mouse LT-HSC aging by preserving adult LT-HSC self-renewal and hematopoietic capacity (Chen et al., 2009). Excessive mTOR signaling also leads to adult epidermal stem cell exhaustion and progressive hair loss in mice – a phenomenon that rapamycin can delay (Castilho et al., 2009). Perhaps more strikingly, rapamycin treatment late in adulthood can extend organismal longevity in mice (Harrison et al., 2009). These mechanisms appear to be well-conserved in Drosophila too, as insulin-PI3K signaling also regulates the proliferative capacity of Drosophila hematopoietic progenitors (Shim et al., 2012), neuroblasts (Chell and Brand, 2010; Sousa-Nunes et al., 2011), intestinal stem cells (ISCs) (Biteau et al., 2010; Choi et al., 2011; O'Brien et al., 2011) and germline stem cells (GSCs) (McLeod et al., 2010). Thus, metabolic signaling pathways that regulate aging might do so via stem cell metabolism, by acting as nutrient sensors that modulate regenerative capacity during aging (Fig. 5C).
Drosophila models of ISCs provide strong evidence that efficient oxidative metabolism can enhance stem cell self-renewal during aging and contribute to organismal longevity. One example is the demonstration that intestine-specific upregulation of the Drosophila PGC1 homolog Spargel, a master regulator of mitochondrial biogenesis, can delay ISC aging and the consequent accumulation of mis-differentiated cells during aging by enhancing the efficiency of OxPhos. Moreover, Spargel upregulation in ISCs improves intestinal integrity and extends adult organismal longevity in old flies (Rera et al., 2011). In the same vein, it is well known that redox balance regulates ISC proliferation. In the Drosophila intestine, high levels of ROS are produced by enterocytes to control bacterial populations. ISCs respond to this oxidative stress by proliferating as part of a regenerative response. But over time this can lead to hyperproliferation, stem cell exhaustion and epithelial degeneration in the aging animal. In fact, ROS-induced hyperproliferation can be prevented with antioxidants (Hochmuth et al., 2011). Moreover, organismal longevity can be increased upon gain-of-function of nuclear factor erythroid 2-related factor 2 (NRF2) or its C. elegans homolog SKN1, master regulators of the oxidative stress response (Hochmuth et al., 2011). By contrast, tissue-specific loss of NRF2 in ISCs causes accumulation of ROS and accelerates aging-related degeneration of the intestine (Hochmuth et al., 2011). Interestingly, loss of the NRF2 regulator kelch-like ECH-associated protein 1 (KEAP1) leads to hyperkeratosis in the gastrointestinal tract in mice (Wakabayashi et al., 2003), suggesting that NRF2 might also control ISC proliferation and aging in mammals.
Although very little is known regarding GSC metabolism, GSC proliferation is known to be regulated by the availability of various nutrients. GSCs in the Drosophila ovary adjust their proliferation rates to nutritional conditions (Drummond-Barbosa and Spradling, 2001), and the nutrient-sensitive insulin/IGF1 and TOR signaling pathways are required for this response (LaFever and Drummond-Barbosa, 2005; Hsu et al., 2008; Ueishi et al., 2009; LaFever et al., 2010). Given that studies in C. elegans have shown that GSCs can regulate C. elegans longevity (Arantes-Oliveira et al., 2002; Wang et al., 2008), it will be interesting to see whether GSC metabolism plays a role in regulating C. elegans and mammalian longevity.
Calorie restriction might also promote longevity in part by promoting the self-renewal of stem cells. Calorie restriction not only extends longevity but also prolongs the health of organisms, ranging from worms and flies to rodents and primates, by improving metabolic homeostasis and decreasing the incidence of degenerative diseases and cancer (Barger et al., 2003). However, the detailed mechanisms underlying the benefits of calorie restriction in specific tissues had remained unclear until recently. In Drosophila, calorie restriction conditions that extend longevity also delay the loss of male GSCs over the course of aging (Mair et al., 2010). In mammalian muscles, calorie restriction boosts the number and myogenic function of skeletal satellite cells, by increasing mitochondrial biogenesis and enhancing OxPhos (Cerletti et al., 2012). In fact, the pro-myogenic effect of calorie restriction can be recapitulated in vitro by replacing glucose in satellite cell media with galactose to force the use of fatty acid oxidation and OxPhos. In the intestinal villus crypts, calorie restriction leads to upregulation of cyclic ADP-ribose (cADPR) signaling in the intestinal niche Paneth cells by inhibiting mTOR signaling. As a result, cADPR signaling induces the proliferation of leucine rich repeat-containing G protein-coupled receptor 5 (LRG5)-positive ISCs during calorie restriction (Yilmaz et al., 2012). Thus, calorie restriction can lead to complex effects in niche and stem cells to promote stem cell function and counteract aging-related degeneration.
Conclusions
By the end of the 20th century, early seminal discoveries of metabolism in early embryos led to the perfection of techniques in blastocyst culture and IVF. More recent insights into the metabolism in human ESCs have also led to rapid advances in chemically defined conditions for human ESC culture, iPSC reprogramming and their use in human disease models. As we move forwards, a deeper understanding of human stem and progenitor cell metabolism could lead to similar breakthroughs in our efforts to define conditions to culture functional human tissues in vitro via directed differentiation of human ESCs. Furthermore, it is clear that our understanding of stem cell metabolism could also be useful in fighting aging-related degeneration in patients. Studies of metabolism in stem cells thus harbor enormous potential for the field of regenerative medicine. One recent example is the mass production of functional human cardiomyocytes from human ESCs achieved by changing the primary carbon source in growth media from glucose to lactate (Tohoyama et al., 2013), thus yielding a source of cells that could help treat degenerative heart diseases in the future. But progress is slow elsewhere. Much remains unclear about metabolism in quiescent adult stem cells. This gap in our knowledge represents a crucial barrier in our ability to grow, study and use adult stem cells ex vivo, and to derive transplantable adult stem cells from human ESCs. A case in point is that highly desired methods for long-term culture and expansion of LT-HSCs do not yet exist. Similarly, much remains unknown about the metabolic requirements of various adult progenitor cells and their differentiated progeny, such that we are still unable to differentiate properly many adult stem and progenitor cells into fully functional, terminally differentiated cells that we can grow or maintain in vitro (Lyssiotis et al., 2011; Lairson et al., 2013). Although the traditional focus in this regard has been on growth factors and transcription factors, it would also be crucial to discover the necessary metabolic conditions. In fact, optimal metabolic conditions need not only be permissive for the directed differentiation of cells, but could also serve as instructive regulatory signals (see Box 2). Drugs could even be developed to manipulate pharmacologically the relevant enzymes, which have historically proven to be one of the most druggable classes of proteins in humans.
To achieve these goals, further advances in metabolomic technologies might be necessary. Much of the extant literature on stem cell metabolism is based on steady-state measurements of lyzed cells. Given the rapid kinetics of metabolic reactions, it will be crucial to measure metabolic fluxes inside cells. Isotope-tracing methods in gas or liquid chromatography mass spectrometry (GC-MS or LC-MS) are state-of-the-art techniques in this area with their high levels of sensitivity, but still suffer from the inability to track real-time changes in vivo. Nuclear magnetic resonance (NMR) imaging methods could potentially overcome this barrier, but they currently show poorer sensitivity compared with mass spectrometry. Another important barrier to advances in stem cell metabolism is the current lack of reliable methods for metabolomic profiling at the single-cell level. Current methods are limited to single fluorescent reporters of a few metabolic indicators, such as the ATP/AMP ratio, the NADH/NAD ratio or ROS abundance, instead of the hundreds of metabolites measurable by metabolomics. In addition, the intercellular metabolism and subcellular metabolism of stem cell niches in vivo also await further exploration. Therefore, we emphasize the need to explore beyond the current paradigm of in vitro stem cell metabolism to one that also encompasses the real-time changing metabolic needs of stem cells in vivo.
Another tradition among extant metabolic studies is the focus on the carbon and energy source of cells. This focus has led to numerous studies on key common pathways in glucose metabolism, i.e. glycolysis, OxPhos and ROS. However, insufficient attention has been dedicated to amino acid and lipid metabolism. Recent studies are just beginning to show that Thr-Gly metabolism is important to the folate pool and SAM/Met metabolism, with implications for nucleotide synthesis during proliferation and histone methylation for epigenetic regulation in stem cells. Acetyl-CoA metabolism is also beginning to emerge as a major regulator of protein (including histone) acetylation to regulate stem cells epigenetically. Furthermore, lipogenesis is emerging as a key pathway that sustains membrane synthesis and proliferation, whereas lipid oxidation via the eicosanoid pathway might generate a diversity of tissue-specific signaling molecules necessary to guide stem cell differentiation. An entire universe of cell- or tissue-specific metabolic pathways might be awaiting future discovery – with the potential to revolutionize regenerative medicine.
Acknowledgements
We apologize to those authors whose papers could not be cited owing to space constraints. We thank Costas Lyssiotis, Hao Zhu and the three anonymous reviewers for thoughtful and constructive feedback on our manuscript.
Footnotes
Funding
N.S.C. is supported by the NSS Scholarship from the Agency for Science, Technology and Research, Singapore. G.Q.D. is supported by the National Institutes of Health (NIH), the Ellison Medical Foundation and Alex's Lemonade Stand Foundation and is an investigator of the Howard Hughes Medical Institute. L.C.C. is supported by a grant from the NIH. Deposited in PMC for release after 12 months.
Competing interests statement
L.C.C. owns equity in and receives compensation from Agios Pharmaceuticals, and serves on the Board of Directors and Scientific Advisory Board of Agios Pharmaceuticals. Agios Pharmaceuticals is identifying metabolic pathways of cancer cells and developing drugs to inhibit such enzymes in order to disrupt tumor cell growth and survival. G.Q.D., as a co-founder and Scientific Advisory Board member, holds stock options and receives consulting fees from iPierian, a biopharmaceutical company that uses iPS cells in drug discovery against neurological disease.
References
- Alexander P. B., Wang J., McKnight S. L. (2011). Targeted killing of a mammalian cell based upon its specialized metabolic state. Proc. Natl. Acad. Sci. USA 108, 15828-15833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V., Horvitz H. R. (1984). Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409-416 [DOI] [PubMed] [Google Scholar]
- Ang Y.-S., Tsai S.-Y., Lee D.-F., Monk J., Su J., Ratnakumar K., Ding J., Ge Y., Darr H., Chang B., et al. (2011). Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145, 183-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Oliveira N., Apfeld J., Dillin A., Kenyon C. (2002). Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295, 502-505 [DOI] [PubMed] [Google Scholar]
- Azuara V., Perry P., Sauer S., Spivakov M., Jørgensen H. F., John R. M., Gouti M., Casanova M., Warnes G., Merkenschlager M., et al. (2006). Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 8, 532-538 [DOI] [PubMed] [Google Scholar]
- Barbehenn E. K., Wales R. G., Lowry O. H. (1978). Measurement of metabolites in single preimplantation embryos; a new means to study metabolic control in early embryos. J. Embryol. Exp. Morphol. 43, 29-46 [PubMed] [Google Scholar]
- Barger J. L., Walford R. L., Weindruch R. (2003). The retardation of aging by caloric restriction: its significance in the transgenic era. Exp. Gerontol. 38, 1343-1351 [DOI] [PubMed] [Google Scholar]
- Biteau B., Karpac J., Supoyo S., Degennaro M., Lehmann R., Jasper H. (2010). Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 6, e1001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha A. L., Ramanathan A., Huang S., Ingber D. E., Schreiber S. L. (2010). Carbon metabolism-mediated myogenic differentiation. Nat. Chem. Biol. 6, 202-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L. (1967). Protein content of the mouse embryo during the first five days of development. J. Reprod. Fertil. 13, 413-420 [DOI] [PubMed] [Google Scholar]
- Brinster R. L. (1973). Nutrition and metabolism of the ovum, zygote and blastocyst. In Handbook of Physiology (ed. Greep R. O., Astwood E. B.), pp. 165-185 Washington, DC: Americal Physiological Society; [Google Scholar]
- Brinster R. L. (1974). Embryo development. J. Anim. Sci. 38, 1003-1012 [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Troike D. E. (1979). Requirements for blastocyst development in vitro. J. Anim. Sci. 49 Suppl. 2, 26-34 [DOI] [PubMed] [Google Scholar]
- Castilho R. M., Squarize C. H., Chodosh L. A., Williams B. O., Gutkind J. S. (2009). mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5, 279-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M., Jang Y. C., Finley L. W. S., Haigis M. C., Wagers A. J. (2012). Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell 10, 515-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell J. M., Brand A. H. (2010). Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143, 1161-1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-T., Shih Y.-R. V., Kuo T. K., Lee O. K., Wei Y.-H. (2008a). Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26, 960-968 [DOI] [PubMed] [Google Scholar]
- Chen C., Liu Y., Liu R., Ikenoue T., Guan K. L., Liu Y., Zheng P. (2008b). TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205, 2397-2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Y., Liu Y., Zheng P. (2009). mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2, ra75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. M., Kwon S., Pak Y. K., Seol H. W., Choi Y. M., Park J., Park K. S., Lee H. K. (2006). Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 348, 1472-1478 [DOI] [PubMed] [Google Scholar]
- Choi N. H., Lucchetta E., Ohlstein B. (2011). Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA 108, 18702-18707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834-840 [DOI] [PubMed] [Google Scholar]
- Chung S., Dzeja P. P., Faustino R. S., Perez-Terzic C., Behfar A., Terzic A. (2007). Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 4 Suppl. 1, S60-S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T.-L., Brena R. M., Kolle G., Grimmond S. M., Berman B. P., Laird P. W., Pera M. F., Wolvetang E. J. (2010). Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 28, 1848-1855 [DOI] [PubMed] [Google Scholar]
- Clower C. V., Chatterjee D., Wang Z., Cantley L. C., Vander Heiden M. G., Krainer A. R. (2010). The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc. Natl. Acad. Sci. USA 107, 1894-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. J., Chen M., Assanah M., Canoll P., Manley J. L. (2010). HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463, 364-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D., Spradling A. C. (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231, 265-278 [DOI] [PubMed] [Google Scholar]
- Facucho-Oliveira J. M., Alderson J., Spikings E. C., Egginton S., St John J. C. (2007). Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J. Cell Sci. 120, 4025-4034 [DOI] [PubMed] [Google Scholar]
- Federzoni E. A., Valk P. J. M., Torbett B. E., Haferlach T., Löwenberg B., Fey M. F., Tschan M. P. (2012). PU.1 is linking the glycolytic enzyme HK3 in neutrophil differentiation and survival of APL cells. Blood 119, 4963-4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M. E., Abdel-Wahab O., Lu C., Ward P. S., Patel J., Shih A., Li Y., Bhagwat N., Vasanthakumar A., Fernandez H. F., et al. (2010). Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa S., Fico A., Paglialunga F., Balestrieri M., Crooke A., Verde P., Abrescia P., Bautista J. M., Martini G. (2003). Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 370, 935-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes C. D. L., Nelson T. J., Martinez-Fernandez A., Arrell D. K., Lindor J. Z., Dzeja P. P., Ikeda Y., Perez-Terzic C., Terzic A. (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 14, 264-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan B., Hu J., Jiang S., Liu Y., Sahin E., Zhuang L., Fletcher-Sananikone E., Colla S., Wang Y. A., Chin L., et al. (2010). Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Maia A., Alajem A., Meshorer E., Ramalho-Santos M. (2011). Open chromatin in pluripotency and reprogramming. Nat. Rev. Mol. Cell Biol. 12, 36-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon T. R., Crowther A. J., Tikunov A., Garcia I., Annis R., Yuan H., Miller C. R., MacDonald J., Olson J., Deshmukh M. (2013). Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer and Metabolism 1, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W., North T. E., Loewer S., Lord A. M., Lee S., Stoick-Cooper C. L., Weidinger G., Puder M., Daley G. Q., Moon R. T., et al. (2009). Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves C. N., Biggers J. D. (1970). Carbon dioxide fixation by mouse embryos prior to implantation. Science 167, 1506-1507 [DOI] [PubMed] [Google Scholar]
- Guillet-Deniau I., Leturque A., Girard J. (1994). Expression and cellular-localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat fetuses. J. Cell Sci. 107, 487-496 [PubMed] [Google Scholar]
- Gurumurthy S., Xie S. Z., Alagesan B., Kim J., Yusuf R. Z., Saez B., Tzatsos A., Ozsolak F., Milos P., Ferrari F., et al. (2010). The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468, 659-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J., Saha K., Pando B., van Zon J., Lengner C. J., Creyghton M. P., van Oudenaarden A., Jaenisch R. (2009). Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson J., Rafiee M. R., Reiland S., Polo J. M., Gehring J., Okawa S., Huber W., Hochedlinger K., Krijgsveld J. (2012). Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep. 2, 1579-1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth C. E., Biteau B., Bohmann D., Jasper H. (2011). Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8, 188-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-J., LaFever L., Drummond-Barbosa D. (2008). Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev. Biol. 313, 700-712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Maehr R., Guo W., Eijkelenboom A., Snitow M., Chen A. E., Melton D. A. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26, 795-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Carracedo A., Weiss D., Arai F., Ala U., Avigan D. E., Schafer Z. T., Evans R. M., Suda T., Lee C.-H., et al. (2012). A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 18, 1350-1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H., Jones D. L. (2010). Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 12, 561-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. T., Yang H. S., Magnuson T., Patel M. S. (1997). Targeted disruption of the murine dihydrolipoamide dehydrogenase gene (Dld) results in perigastrulation lethality. Proc. Natl. Acad. Sci. USA 94, 14512-14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. T., Mahmood S., Hyatt S. L., Yang H. S., Soloway P. D., Hanson R. W., Patel M. S. (2001). Inactivation of the murine pyruvate dehydrogenase (Pdha1) gene and its effect on early embryonic development. Mol. Genet. Metab. 74, 293-302 [DOI] [PubMed] [Google Scholar]
- Johnson M. T., Mahmood S., Patel M. S. (2003). Intermediary metabolism and energetics during murine early embryogenesis. J. Biol. Chem. 278, 31457-31460 [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, McKnight S. L. (2013). Influence of metabolism on epigenetics and disease. Cell 153, 56-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D., Sykes S. M., Wang Z., Punt N., Tang Y., Ragu C., Sinha A. U., Lane S. W., Souza A. L., Clish C. B., et al. (2012). mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell 11, 429-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharas M. G., Okabe R., Ganis J. J., Gozo M., Khandan T., Paktinat M., Gilliland D. G., Gritsman K. (2010). Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 115, 1406-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimmeck D., Hansson J., Raffel S., Vakhrushev S. Y., Trumpp A., Krijgsveld J. (2012). Proteomic cornerstones of hematopoietic stem cell differentiation: distinct signatures of multipotent progenitors and myeloid committed cells. Mol. Cell. Proteomics 11, 286-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M., Braun S. M. G., Zurkirchen L., von Schoultz C., Zamboni N., Araúzo-Bravo M. J., Kovacs W. J., Karalay O., Suter U., Machado R. A. C., et al. (2013). Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493, 226-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H., Lleonart M. E., Nakashima Y., Yokode M., Tanaka M., Bernard D., Gil J., Beach D. (2007). A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid. Redox Signal. 9, 293-299 [DOI] [PubMed] [Google Scholar]
- LaFever L., Drummond-Barbosa D. (2005). Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309, 1071-1073 [DOI] [PubMed] [Google Scholar]
- LaFever L., Feoktistov A., Hsu H.-J., Drummond-Barbosa D. (2010). Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development 137, 2117-2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairson L. L., Lyssiotis C. A., Zhu S., Schultz P. G. (2013). Small molecule-based approaches to adult stem cell therapies. Annu. Rev. Pharmacol. Toxicol. 53, 107-125 [DOI] [PubMed] [Google Scholar]
- Leese H. J. (1995). Metabolic control during preimplantation mammalian development. Hum. Reprod. Update 1, 63-72 [DOI] [PubMed] [Google Scholar]
- Leese H. J., Barton A. M. (1984). Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J. Reprod. Fertil. 72, 9-13 [DOI] [PubMed] [Google Scholar]
- Lu C., Ward P. S., Kapoor G. S., Rohle D., Turcan S., Abdel-Wahab O., Edwards C. R., Khanin R., Figueroa M. E., Melnick A., et al. (2012). IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis C. A., Lairson L. L., Boitano A. E., Wurdak H., Zhu S., Schultz P. G. (2011). Chemical control of stem cell fate and developmental potential. Angew. Chem. Int. Ed. Engl. 50, 200-242 [DOI] [PubMed] [Google Scholar]
- Magee J. A., Ikenoue T., Nakada D., Lee J. Y., Guan K.-L., Morrison S. J. (2012). Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 11, 415-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W., McLeod C. J., Wang L., Jones D. L. (2010). Dietary restriction enhances germline stem cell maintenance. Aging Cell 9, 916-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganelli G., Fico A., Masullo U., Pizzolongo F., Cimmino A., Filosa S. (2012). Modulation of the pentose phosphate pathway induces endodermal differentiation in embryonic stem cells. PLoS ONE 7, e29321 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McLeod C. J., Wang L., Wong C., Jones D. L. (2010). Stem cell dynamics in response to nutrient availability. Curr. Biol. 20, 2100-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle S., Pretsch W. (1989). Characterization of triosephosphate isomerase mutants with reduced enzyme activity in Mus musculus. Genetics 123, 837-844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle S., Favor J., Graw J., Hornhardt S., Pretsch W. (1992). Hereditary lactate dehydrogenase A-subunit deficiency as cause of early postimplantation death of homozygotes in Mus musculus. Genetics 131, 413-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar J. J., Domingo-Fernández R., Ebus M. E., Lindner S., Koster J., Drabek K., Mestdagh P., van Sluis P., Valentijn L. J., van Nes J., et al. (2012). LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat. Genet. 44, 1199-1206 [DOI] [PubMed] [Google Scholar]
- Nakada D., Saunders T. L., Morrison S. J. (2010). Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468, 653-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Soliman S. S., Li X., Bilder D. (2011). Altered modes of stem cell division drive adaptive intestinal growth. Cell 147, 603-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E., Banerjee U. (2009). Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos A. D., Yanes O., Ruiz S., Kida Y. S., Diep D., Tautenhahn R., Herrerías A., Batchelder E. M., Plongthongkum N., Lutz M., et al. (2012). The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 22, 168-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleon M., Kaye P. L. (1998). Glucose transporters in preimplantation development. Rev. Reprod. 3, 77-81 [DOI] [PubMed] [Google Scholar]
- Pattappa G., Heywood H. K., de Bruijn J. D., Lee D. A. (2011). The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 226, 2562-2570 [DOI] [PubMed] [Google Scholar]
- Pattappa G., Thorpe S. D., Jegard N. C., Heywood H. K., de Bruijn J. D., Lee D. A. (2013). Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Part C Methods 19, 68-79 [DOI] [PubMed] [Google Scholar]
- Peng S., Chen L.-L., Lei X.-X., Yang L., Lin H., Carmichael G. G., Huang Y. (2011). Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 29, 496-504 [DOI] [PubMed] [Google Scholar]
- Polesskaya A., Cuvellier S., Naguibneva I., Duquet A., Moss E. G., Harel-Bellan A. (2007). Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 21, 1125-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretsch W. (2000). Enzyme-activity mutants in Mus musculus. I. Phenotypic description and genetic characterization of ethylnitrosourea-induced mutations. Mamm. Genome 11, 537-542 [DOI] [PubMed] [Google Scholar]
- Prigione A., Fauler B., Lurz R., Lehrach H., Adjaye J. (2010). The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28, 721-733 [DOI] [PubMed] [Google Scholar]
- Quinn P., Wales R. G. (1971). Adenosine triphosphate content of preimplantation mouse embryos. J. Reprod. Fertil. 25, 133-135 [DOI] [PubMed] [Google Scholar]
- Renault V. M., Rafalski V. A., Morgan A. A., Salih D. A. M., Brett J. O., Webb A. E., Villeda S. A., Thekkat P. U., Guillerey C., Denko N. C., et al. (2009). FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5, 527-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M., Bahadorani S., Cho J., Koehler C. L., Ulgherait M., Hur J. H., Ansari W. S., Lo T., Jr, Jones D. L., Walker D. W. (2011). Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 14, 623-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D. J., Jamieson C. H. M., Weissman I. L. (2008). Stems cells and the pathways to aging and cancer. Cell 132, 681-696 [DOI] [PubMed] [Google Scholar]
- Sahin E., Depinho R. A. (2010). Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Knobbe C. B., Munger J. C., Lind E. F., Brenner D., Brüstle A., Harris I. S., Holmes R., Wakeham A., Haight J., et al. (2012). IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488, 656-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieke S. M., Ma M., Cao L., McCoy J. P., Jr, Liu C., Hensel N. F., Barrett A. J., Boehm M., Finkel T. (2008). Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J. Biol. Chem. 283, 28506-28512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J., Mukherjee T., Banerjee U. (2012). Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 14, 394-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N., Daley G. Q. (2013). Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12, 395-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N., Zheng Y., Locasale J. W., Cantley L. C. (2011). Human pluripotent stem cells decouple respiration from energy production. EMBO J. 30, 4851-4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N., Locasale J. W., Lyssiotis C. A., Zheng Y., Teo R. Y., Ratanasirintrawoot S., Zhang J., Onder T., Unternaehrer J. J., Zhu H., et al. (2013). Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek T., Kocabas F., Zheng J., Deberardinis R. J., Mahmoud A. I., Olson E. N., Schneider J. W., Zhang C. C., Sadek H. A. (2010). The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7, 380-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Nunes R., Yee L. L., Gould A. P. (2011). Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471, 508-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M., Apostolou E., Ferrari F., Choi J., Walsh R. M., Chen T., Ooi S. S. K., Kim S. Y., Bestor T. H., Shioda T., et al. (2012). Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat. Genet. 44, 398-405, S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takubo K., Semenza G. L. (2011). Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9, 298-310 [DOI] [PubMed] [Google Scholar]
- Takubo K., Nagamatsu G., Kobayashi C. I., Nakamura-Ishizu A., Kobayashi H., Ikeda E., Goda N., Rahimi Y., Johnson R. S., Soga T., et al. (2013). Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12, 49-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama S., Hattori F., Sano M., Hishiki T., Nagahata Y., Matsuura T., Hashimoto H., Suzuki T., Yamashita H., Satoh Y., et al. (2013). Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127-137 [DOI] [PubMed] [Google Scholar]
- Tormos K. V., Anso E., Hamanaka R. B., Eisenbart J., Joseph J., Kalyanaraman B., Chandel N. S. (2011). Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 14, 537-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z., Kollipara R., Huntly B. J., Lee B. H., Castrillon D. H., Cullen D. E., McDowell E. P., Lazo-Kallanian S., Williams I. R., Sears C., et al. (2007). FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325-339 [DOI] [PubMed] [Google Scholar]
- Ueishi S., Shimizu H., H Inoue Y. (2009). Male germline stem cell division and spermatocyte growth require insulin signaling in Drosophila. Cell Struct. Funct. 34, 61-69 [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. (2009). Mitochondria in early mammalian development. Semin. Cell Dev. Biol. 20, 354-364 [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varum S., Rodrigues A. S., Moura M. B., Momcilovic O., Easley C. A., 4th, Ramalho-Santos J., Van Houten B., Schatten G. (2011). Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 6, e20914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma P. (1962). The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil. Steril. 13, 481-485 [DOI] [PubMed] [Google Scholar]
- Viswanathan S. R., Daley G. Q., Gregory R. I. (2008). Selective blockade of microRNA processing by Lin28. Science 320, 97-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D. R., et al. (2003). Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 35, 238-245 [DOI] [PubMed] [Google Scholar]
- Wales R. G. (1974). The levels of NAD and NADH in mouse embryos during the preimplantation stages of development. Proc. Aust. Biochem. Soc. 7, 28 [Google Scholar]
- Wang M. C., O'Rourke E. J., Ruvkun G. (2008). Fat metabolism links germline stem cells and longevity in C. elegans. Science 322, 957-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Alexander P., Wu L., Hammer R., Cleaver O., McKnight S. L. (2009). Dependence of mouse embryonic stem cells on threonine catabolism. Science 325, 435-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chen K., Zeng X., Yang J., Wu Y., Shi X., Qin B., Zeng L., Esteban M. A., Pan G., et al. (2011). The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 9, 575-587 [DOI] [PubMed] [Google Scholar]
- Wellen K. E., Hatzivassiliou G., Sachdeva U. M., Bui T. V., Cross J. R., Thompson C. B. (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J. C., Sicard R. E. (1987). Lactate metabolism of isolated, perfused fetal, and newborn pig hearts. Pediatr. Res. 22, 552-556 [DOI] [PubMed] [Google Scholar]
- West J. D., Flockhart J. H., Peters J., Ball S. T. (1990). Death of mouse embryos that lack a functional gene for glucose phosphate isomerase. Genet. Res. 56, 223-236 [DOI] [PubMed] [Google Scholar]
- Wilson J. E. (2003). Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J. Exp. Biol. 206, 2049-2057 [DOI] [PubMed] [Google Scholar]
- Yanes O., Clark J., Wong D. M., Patti G. J., Sánchez-Ruiz A., Benton H. P., Trauger S. A., Desponts C., Ding S., Siuzdak G. (2010). Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 6, 411-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O. H., Katajisto P., Lamming D. W., Gültekin Y., Bauer-Rowe K. E., Sengupta S., Birsoy K., Dursun A., Yilmaz V. O., Selig M., et al. (2012). mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Takahashi K., Okita K., Ichisaka T., Yamanaka S. (2009). Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237-241 [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917-1920 [DOI] [PubMed] [Google Scholar]
- Yu W.-M., Liu X., Shen J., Jovanovic O., Pohl E. E., Gerson S. L., Finkel T., Broxmeyer H. E., Qu C.-K. (2013). Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 12, 62-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Nguyen C. K., Liu X., Kanellopoulou C., Muljo S. A. (2012). Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335, 1195-1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Khvorostov I., Hong J. S., Oktay Y., Vergnes L., Nuebel E., Wahjudi P. N., Setoguchi K., Wang G., Do A., et al. (2011a). UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30, 4860-4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Guan Z., Murphy A. N., Wiley S. E., Perkins G. A., Worby C. A., Engel J. L., Heacock P., Nguyen O. K., Wang J. H., et al. (2011b). Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab. 13, 690-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. C., Shyh-Chang N., Yang H., Rai A., Umashankar S., Ma S., Soh B. S., Sun L. L., Tai B. C., Nga M. E., et al. (2012). Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 148, 259-272 [DOI] [PubMed] [Google Scholar]
- Zheng K., Wu X., Kaestner K. H., Wang P. J. (2009). The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 9, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Li W., Zhou H., Wei W., Ambasudhan R., Lin T., Kim J., Zhang K., Ding S. (2010). Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7, 651-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Shyh-Chang N., Segrè A. V., Shinoda G., Shah S. P., Einhorn W. S., Takeuchi A., Engreitz J. M., Hagan J. P., Kharas M. G., et al. (2011). The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81-94 [DOI] [PMC free article] [PubMed] [Google Scholar]