Abstract
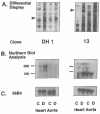
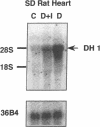
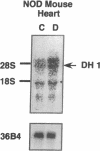
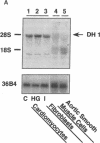
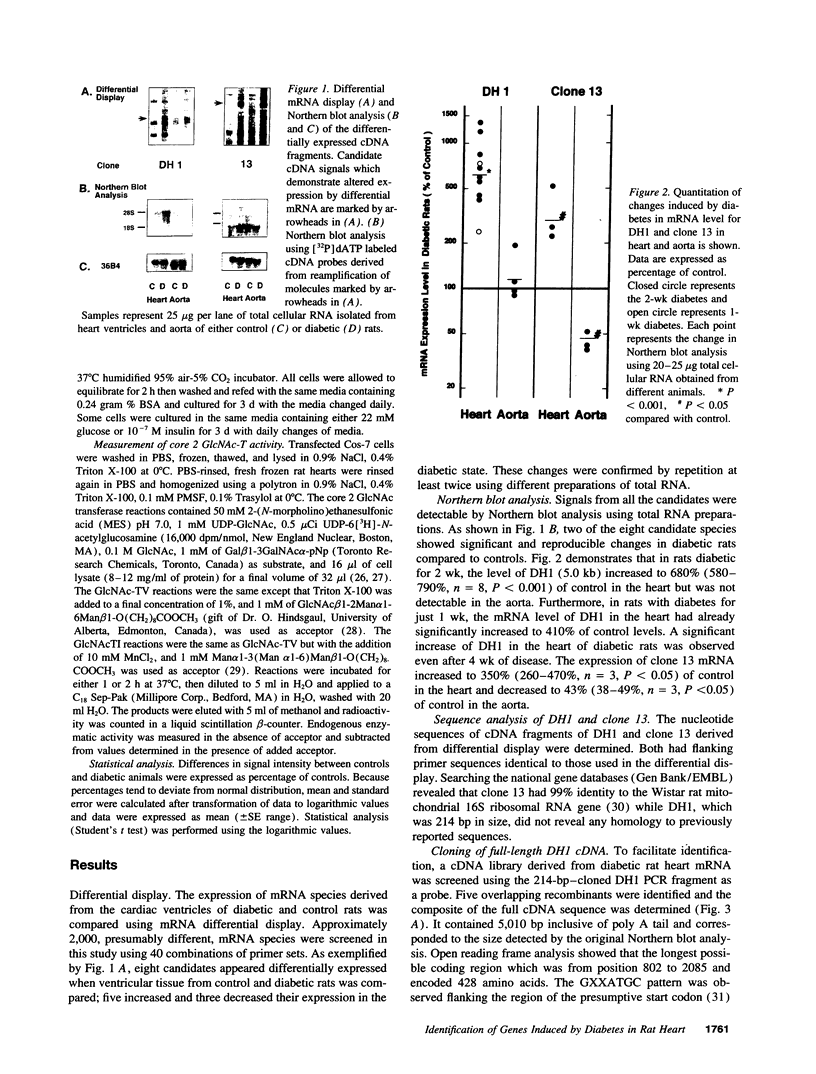
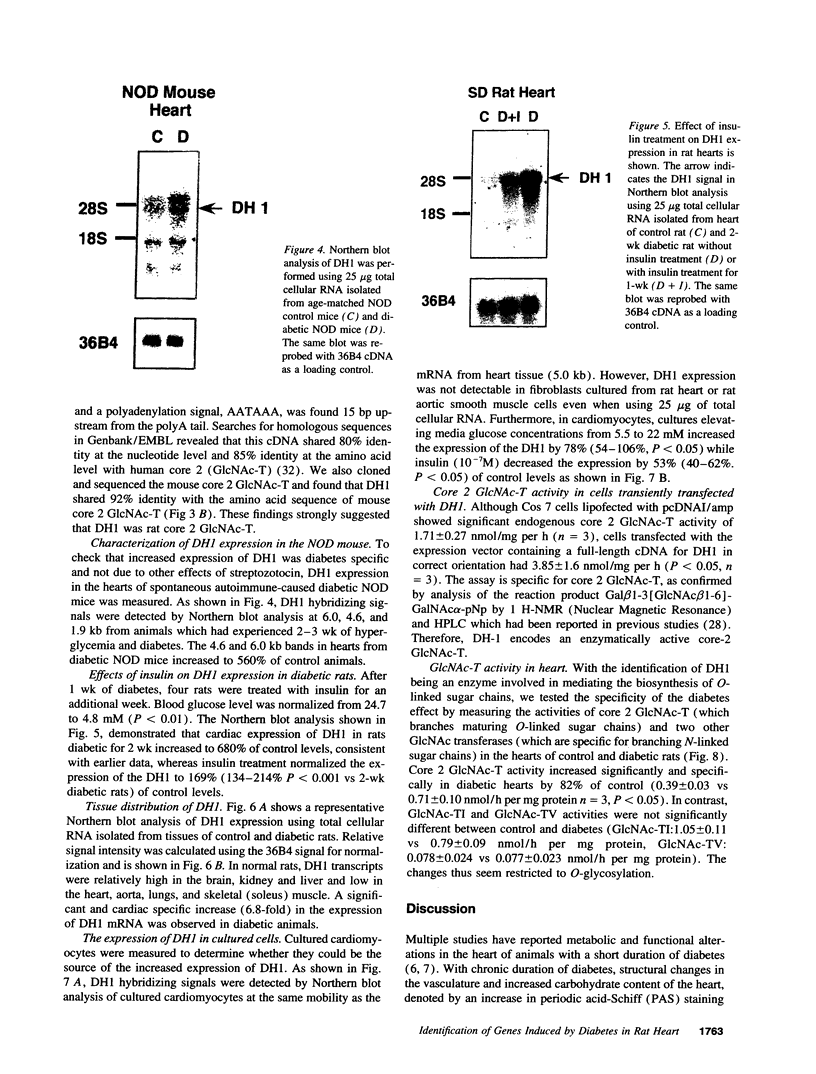
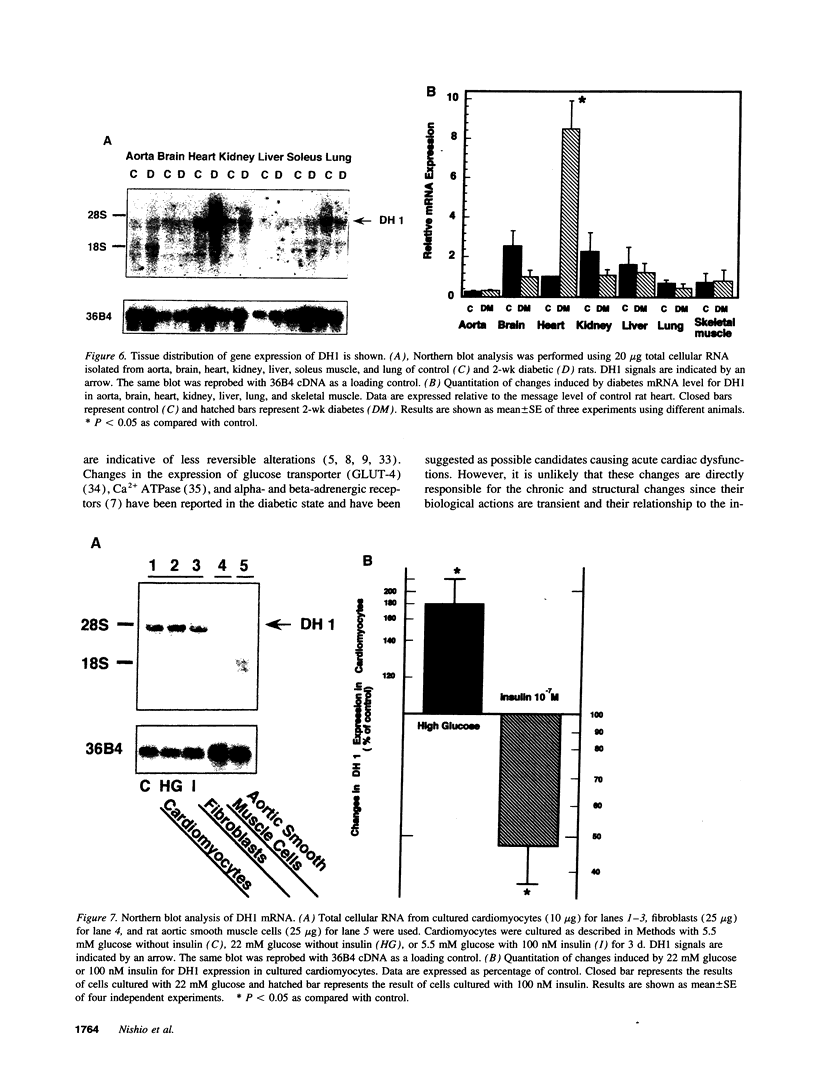
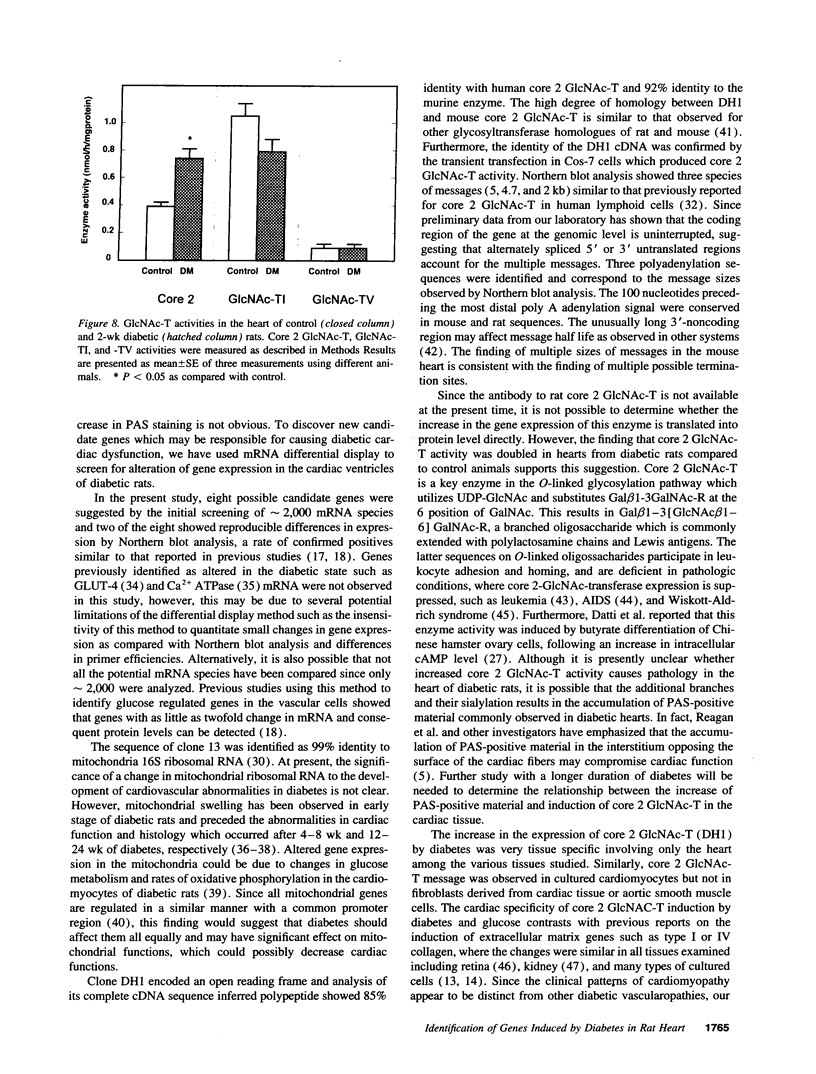
Primary cardiac abnormalities have been frequently reported in patients with diabetes probably due to metabolic consequences of the disease. Approximately 2,000 mRNA species from the heart of streptozotocin-induced diabetic and control rats were compared by the mRNA differential display method, two of eight candidate clones thus isolated (DH1 and 13) were confirmed by Northern blot analysis. The expression of clone 13 was increased in the heart by 3.5-fold (P < 0.05) and decreased in the aorta by twofold (P < 0.05) in diabetes as compared to control. Sequence analysis showed that clone 13 is a rat mitochondrial gene. DH1 was predominantly expressed in the heart with an expression level 6.8-fold higher in the diabetic rats than in control (P < 0.001). Insulin treatment significantly (P < 0.001) normalized the expression of DH1 in the hearts of diabetic rats. DH1 expression was observed in cultured rat cardiomyocytes, but not in aortic smooth muscle cells or in cardiac derived fibroblasts. The expression in cardiomyocytes was regulated by insulin and glucose concentration of culture media. The full length cDNA of DH1 had a single open-reading frame with 85 and 92% amino acid identity to human and mouse UDP-GlcNAc:Gal beta 1-3GalNAc alpha R beta 1-6 N-acetylglucosaminyltransferase (core 2 GlcNAc-T), respectively, a key enzyme determining the structure of O-linked glycosylation. Transient transfection of DH1 cDNA into Cos7 cells conferred core 2 GlcNAc-T enzyme activity. In vivo, core 2 GlcNAc-T activity was increased by 82% (P < 0.05) in diabetic hearts vs controls, while the enzymes GlcNAc-TI and GlcNAc-TV responsible for N-linked glycosylation were unchanged. These results suggest that core 2 GlcNAc-T is specifically induced in the heart by diabetes or hyperglycemia. The induction of this enzyme may be responsible for the increase in the deposition of glycoconjugates and the abnormal functions found in the hearts of diabetic rats.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello L. P., Robinson G. S., Lin Y. W., Nishio Y., King G. L. Identification of multiple genes in bovine retinal pericytes altered by exposure to elevated levels of glucose by using mRNA differential display. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6231–6235. doi: 10.1073/pnas.91.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhuizen M. F., Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Skottner A., Arnqvist H. J. In-vivo regulation of messenger RNA encoding insulin-like growth factor-I (IGF-I) and its receptor by diabetes, insulin and IGF-I in rat muscle. J Endocrinol. 1992 Nov;135(2):203–211. doi: 10.1677/joe.0.1350203. [DOI] [PubMed] [Google Scholar]
- Brockhausen I., Kuhns W., Schachter H., Matta K. L., Sutherland D. R., Baker M. A. Biosynthesis of O-glycans in leukocytes from normal donors and from patients with leukemia: increase in O-glycan core 2 UDP-GlcNAc:Gal beta 3 GalNAc alpha-R (GlcNAc to GalNAc) beta(1-6)-N-acetylglucosaminyltransferase in leukemic cells. Cancer Res. 1991 Feb 15;51(4):1257–1263. [PubMed] [Google Scholar]
- Buczek-Thomas J. A., Jaspers S. R., Miller T. B., Jr Adrenergic activation of glycogen phosphorylase in primary culture diabetic cardiomyocytes. Am J Physiol. 1992 Mar;262(3 Pt 2):H649–H653. doi: 10.1152/ajpheart.1992.262.3.H649. [DOI] [PubMed] [Google Scholar]
- Cagliero E., Maiello M., Boeri D., Roy S., Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988 Aug;82(2):735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M., Castelló A., Muñoz P., Monfar M., Testar X., Palacín M., Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J. 1992 Mar 15;282(Pt 3):765–772. doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Datti A., Dennis J. W. Regulation of UDP-GlcNAc:Gal beta 1-3GalNAc-R beta 1-6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc) in Chinese hamster ovary cells. J Biol Chem. 1993 Mar 15;268(8):5409–5416. [PubMed] [Google Scholar]
- Dennis J. W. Different metastatic phenotypes in two genetic classes of wheat germ agglutinin-resistant tumor cell mutants. Cancer Res. 1986 Sep;46(9):4594–4600. [PubMed] [Google Scholar]
- Dillmann W. H. Diabetes and thyroid-hormone-induced changes in cardiac function and their molecular basis. Annu Rev Med. 1989;40:373–394. doi: 10.1146/annurev.me.40.020189.002105. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987 Jul;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Sonnenblick E. H. Clinical and morphological features of human hypertensive-diabetic cardiomyopathy. Am Heart J. 1980 Apr;99(4):446–458. doi: 10.1016/0002-8703(80)90379-8. [DOI] [PubMed] [Google Scholar]
- Fein F. S., Strobeck J. E., Malhotra A., Scheuer J., Sonnenblick E. H. Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res. 1981 Dec;49(6):1251–1261. doi: 10.1161/01.res.49.6.1251. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M., Nakamura T., Ebihara I., Shirato I., Tomino Y., Koide H. ECM gene expression and its modulation by insulin in diabetic rats. Diabetes. 1992 Dec;41(12):1520–1527. doi: 10.2337/diab.41.12.1520. [DOI] [PubMed] [Google Scholar]
- Hsiao Y. C., Suzuki K., Abe H., Toyota T. Ultrastructural alterations in cardiac muscle of diabetic BB Wistar rats. Virchows Arch A Pathol Anat Histopathol. 1987;411(1):45–52. doi: 10.1007/BF00734513. [DOI] [PubMed] [Google Scholar]
- Ikegami H., Eisenbarth G. S., Hattori M. Major histocompatibility complex-linked diabetogenic gene of the nonobese diabetic mouse. Analysis of genomic DNA amplified by the polymerase chain reaction. J Clin Invest. 1990 Jan;85(1):18–24. doi: 10.1172/JCI114410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T., Battan R., Handler E., Sportsman J. R., Heath W., King G. L. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. V., McGrath G. M., Tahiliani A. G., Vadlamudi R. V., McNeill J. H. A functional and ultrastructural analysis of experimental diabetic rat myocardium. Manifestation of a cardiomyopathy. Diabetes. 1985 Sep;34(9):876–883. doi: 10.2337/diab.34.9.876. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Hjortland M., Castelli W. P. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974 Jul;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Kessler I. I. Mortality experience of diabetic patients. A twenty-six-year follow-up study. Am J Med. 1971 Dec;51(6):715–724. doi: 10.1016/0002-9343(71)90299-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Seki T., Yaginuma K., Koike K. Nucleotide sequences of small ribosomal RNA and adjacent transfer RNA genes in rat mitochondrial DNA. Gene. 1981 Dec;16(1-3):297–307. doi: 10.1016/0378-1119(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Kuo T. H., Moore K. H., Giacomelli F., Wiener J. Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes. 1983 Sep;32(9):781–787. doi: 10.2337/diab.32.9.781. [DOI] [PubMed] [Google Scholar]
- Lababidi Z. A., Goldstein D. E. High prevalence of echocardiographic abnormalities in diabetic youths. Diabetes Care. 1983 Jan-Feb;6(1):18–22. doi: 10.2337/diacare.6.1.18. [DOI] [PubMed] [Google Scholar]
- Ledet T. Histological and histochemical changes in the coronary arteries of old diabetic patients. Diabetologia. 1968 Nov;4(5):268–272. doi: 10.1007/BF01309899. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Kojima N., Wada E., Kurosawa N., Nakaoka T., Hamamoto T., Tsuji S. Cloning and expression of cDNA for a new type of Gal beta 1,3GalNAc alpha 2,3-sialyltransferase. J Biol Chem. 1994 Apr 1;269(13):10028–10033. [PubMed] [Google Scholar]
- Liang P., Averboukh L., Keyomarsi K., Sager R., Pardee A. B. Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 1992 Dec 15;52(24):6966–6968. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Möller G., Reck F., Paulsen H., Kaur K. J., Sarkar M., Schachter H., Brockhausen I. Control of glycoprotein synthesis: substrate specificity of rat liver UDP-GlcNAc:Man alpha 3R beta 2-N-acetylglucosaminyltransferase I using synthetic substrate analogues. Glycoconj J. 1992 Aug;9(4):180–190. doi: 10.1007/BF00731163. [DOI] [PubMed] [Google Scholar]
- Nishio Y., Aiello L. P., King G. L. Glucose induced genes in bovine aortic smooth muscle cells identified by mRNA differential display. FASEB J. 1994 Jan;8(1):103–106. doi: 10.1096/fasebj.8.1.8299882. [DOI] [PubMed] [Google Scholar]
- Nishio Y., Kashiwagi A., Kida Y., Kodama M., Abe N., Saeki Y., Shigeta Y. Deficiency of cardiac beta-adrenergic receptor in streptozocin-induced diabetic rats. Diabetes. 1988 Sep;37(9):1181–1187. doi: 10.2337/diab.37.9.1181. [DOI] [PubMed] [Google Scholar]
- Nishio Y., Kashiwagi A., Ogawa T., Asahina T., Ikebuchi M., Kodama M., Shigeta Y. Increase in [3H]PN 200-110 binding to cardiac muscle membrane in streptozocin-induced diabetic rats. Diabetes. 1990 Sep;39(9):1064–1069. doi: 10.2337/diab.39.9.1064. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Granner D. K. PEPCK gene as model of inhibitory effects of insulin on gene transcription. Diabetes Care. 1990 Mar;13(3):327–339. doi: 10.2337/diacare.13.3.327. [DOI] [PubMed] [Google Scholar]
- Page M. M., Smith R. B., Watkins P. J. Cardiovascular effects of insulin. Br Med J. 1976 Feb 21;1(6007):430–432. doi: 10.1136/bmj.1.6007.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller F., Le Deist F., Weinberg K. I., Parkman R., Fukuda M. Altered O-glycan synthesis in lymphocytes from patients with Wiskott-Aldrich syndrome. J Exp Med. 1991 Jun 1;173(6):1501–1510. doi: 10.1084/jem.173.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan T. J., Lyons M. M., Ahmed S. S., Levinson G. E., Oldewurtel H. A., Ahmad M. R., Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977 Oct;60(4):884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinilä A., Akerblom H. K. Ultrastructure of heart muscle in short-term diabetic rats: influence of insulin treatment. Diabetologia. 1984 Sep;27(3):397–402. doi: 10.1007/BF00304857. [DOI] [PubMed] [Google Scholar]
- Roy S., Maiello M., Lorenzi M. Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest. 1994 Jan;93(1):438–442. doi: 10.1172/JCI116979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubler S., Dlugash J., Yuceoglu Y. Z., Kumral T., Branwood A. W., Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972 Nov 8;30(6):595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Piller F., Fox R. I., Fukuda M. T-lymphocytic leukemia expresses complex, branched O-linked oligosaccharides on a major sialoglycoprotein, leukosialin. Blood. 1991 Apr 1;77(7):1491–1499. [PubMed] [Google Scholar]
- Shanker R., Neeley W. E., Dillmann W. H. Rapid effects of insulin on in vitro translational activity of specific mRNA in diabetic rat heart. Am J Physiol. 1986 May;250(5 Pt 1):E558–E563. doi: 10.1152/ajpendo.1986.250.5.E558. [DOI] [PubMed] [Google Scholar]
- Williams D., Schachter H. Mucin synthesis. I. Detection in canine submaxillary glands of an N-acetylglucosaminyltransferase which acts on mucin substrates. J Biol Chem. 1980 Dec 10;255(23):11247–11252. [PubMed] [Google Scholar]
- Yousefi S., Higgins E., Daoling Z., Pollex-Krüger A., Hindsgaul O., Dennis J. W. Increased UDP-GlcNAc:Gal beta 1-3GaLNAc-R (GlcNAc to GaLNAc) beta-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J Biol Chem. 1991 Jan 25;266(3):1772–1782. [PubMed] [Google Scholar]
- Zarain-Herzberg A., Yano K., Elimban V., Dhalla N. S. Cardiac sarcoplasmic reticulum Ca(2+)-ATPase expression in streptozotocin-induced diabetic rat heart. Biochem Biophys Res Commun. 1994 Aug 30;203(1):113–120. doi: 10.1006/bbrc.1994.2156. [DOI] [PubMed] [Google Scholar]