Summary
Articular cartilage injuries of the knee are difficult to treat due to the poor healing ability of cartilage and conventional treatment methods often give unsatisfactory results. Mesenchymal Stem Cells (MSCs) have generated interest as an alternative source of cells for cartilage tissue engineering due to their chondrogenic potential and their easy isolation from bone marrow. It has been reported that the use of scaffold in cartilage engineering acts as a support for cell adhesion, keeping the cells in the cartilage defects and therefore facilitating tissue formation, and that Hyaluronic acid (HA) is a molecule of particular interest for producing scaffold for tissue engineering.
In this study we evaluated the in vitro selection and expansion of Bone Marrow MSCs (BM-MSCs) and by residual BM+HA membrane (BM-HA-MSCs) used as scaffold. Sixty mL of BM have been aspirated by the posterior iliac crest and HA membrane (Hyalograft-C, Fidia Advanced Biopolimers) was used as scaffold. BM-MSCs were cultured with D-MEM supplemented with Desamethasone, Ascorbic Acid, β-Transforming Growth Factor and Insulin. When cultured in chondrogenic selective medium MSCs from both BM and HA membrane were able to differentiate into chondrogenesis, but BM-HA-MSCs showed a higher staining intensity than BM-MSCs when they were stained with Toluidine blue. The interaction of MSCs with the HA-scaffold seems to promote by itself chondrogenesis.
Keywords: bone marrow, cartilage injuries, mesenchymal stem cells, tissue engineering
Introduction
Autologous chondrocyte implantation was the first-generation cell therapy for cartilage repair started more than 20 years ago and the treatment of articular cartilage lesions of the knee, using chondrocytes seeded into a scaffold, has been investigated in several clinical trials1–3. However the application of autologous chondrocytes in cartilage repair procedure is associated with several disadvantages including injury of healthy cartilage and the need of a two step procedure surgeries. Mesenchymal stem cells (MSC) have generated interest as an alternative source for cartilage tissue engineering since they have the ability to self replicate and to differentiate down multiple cell lineages, including chondrocytes4–6. The use of MSC in tissue repair is evolving in different approaches which include either the use of entire bone marrow (BM) or in vitro expanded MSC re-administered either as cell suspension or by using scaffolds or hydrogel. MSC are typically obtained by BM, but they can be isolated from several other tissues among which umbilical cord blood, placenta, adipose tissue etc.7
It has been reported that BM is the MSC source an appropriate in the setting of cartilage regeneration to treat focal lesions8,9.
The use of scaffold in cartilage engineering acts as a support for cell adhesion, keeping the cells in the cartilage defects and therefore facilitating tissue formation. In recent years both natural and synthetic biomaterials have been used to create 3D scaffold capable of inducing and enhancing tissue generation through cell organization, mechanical forces and bioactive molecule delivery. A molecule of particular interest for producing scaffold for tissue engineering is hyaluronic acid (HA) since it is found natively in cartilage tissue10,11.
In this study we evaluated the in vitro selection and expansion of MSC obtained respectively by samples of BM after concentration and samples of HA membrane additioned with BM and platelet enriched plasma (PRP) and used as scaffold in the treatment of patients with severe chondral lesions.
Materials and methods
Samples
Five patients (3 males and 2 females age 19–42 years) underwent knee arthroscopy. Intra-operatively 60 mL of BM were aspirated by the posterior iliac crest using heparin-coated syringes and concentrated by the Smart-Prep2 Centrifuge System (Harvest Technologies GmbH) to a final volume of 7 mL. BM concentrated cells were used to prepare a scaffold of HA membrane (Hyalograft-C, Fidia Advanced Biopolimers). The membrane was embebbed with 5 mL BM concentrated cell suspension and additioned with autologous PRP plus thrombin (1+1 mL) and 1 mL of Ca-Gluconate, before beeing applied locally to completely cover the area of cartilage lesion.
Samples of BM concentrated cells and of HA membrane additioned with BM+PRP+Thrombin+Ca-gluconate from the five patients were sent to Stem Cell Manipulation Laboratory for in vitro selection, expansion and characterization of MSC.
MSC isolation
BM concentrated samples and HA membrane samples additioned with BM+PRP+Thrombin+Ca-gluconate were cultured in parallel following two different procedures.
BM samples – Thanks to their density MSC are usually isolated from entire BM in the mononuclear cell ring of a density gradient centrifugation. Therefore concentrated BM samples were diluted in Phosphate Buffered Saline (PBS) and sedimented on Fycoll-Hypaque gradient to isolate mononuclear cells (MNC). Five or ten x 106 isolated MNC were then cultured in T25 sterile flasks at the concentration of 1 x 106 cells/mL in D-MEM medium (LiStarFish) additioned with 10% platelet lysate (complete medium, CM) to allow selection of BM derived MSC adherent cells. These cells were identified as BM-MSC. After 48 h the non-adherent cells were removed and fresh medium added to the cultures. The medium was then changed twice a week until the BM-MSC reached the confluence ≥ 80% (passage 1).
BM-HA-membrane samples – To investigate if MSC can be released in vitro from the HA membrane scaffold additioned with BM+PRP+Thrombin+Ca-gluconate samples of the scaffold (1 cm2 more or less) were left in a Petri dish with 2 ml of CM. At intervals of 4 days the HA membrane was transferred in new Petri for four times generating 4 “daughter” Petri dishes and after the last transfer it was discarded. The cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2 and checked by inverted microscopy to detect the release and the growth of adherent cells until confluence (passage 1). These cells were identified as BM-HA derived MSC adherent cells (BM-HA-MSC).
MSC Characterization and expansion
When BM-MSC and BM-HA-MSC reached confluence 3 80% (passage 1) the cells were detached by trypsin (Li StarFish) treatment, harvested and washed before counting. BM-HA-MSC s derived from the 4 Petri dishes were mixed and counted all together.
In order to avoid the intrinsic variance of the visual counting method which is quite operator dependent, MSC counts were performed by flow cytometry as absolute cell count using fluorescent beads in conjuction with monoclonal antibody (Mo-Ab) specific for a receptor of MSC, such as CD105 or CD90, and a Mo-Ab against CD44, which is expressed on surface of different cell populations, including MSC. The cells were gated by their physical features (forward and side scatter) and fluorescent signals. Two counts were performed for each sample and the average value was accepted as the absolute cell number.
BM-MSC and BM-HA-MSC immunophenotypic characterization included a combination of markers required to identify MSC population as CD105, CD73, CD90, CD166 (all expressed by MSC) and CD34, CD45 (not expressed by MSC). Flow-cytometry analysis was performed by using FacsCalibur (Becton-Dickinson San Jose’, California, USA) and the following Mo-Ab anti-CD71 (allophycocyanin, APC-conjugated, BD), anti-CD73 (phycoerytrhin, PE-conjugated, BD), anti-CD90 (allophycocyanin, APC-conjugated, BD), anti-CD105 (peridinin chlorophyll protein complex, PerCP-conjugated, BD), anti-CD166 (PE conjugated, BD), anti-CD45 (FITC-conjugated, BD) e anti-CD34 (FITC-conjugated, BD).
If BM-MSC and BM-HA-MSC were found > 95% CD10+, CD73+, CD90+, CD166+ and < 2% CD34+ and CD45+ they were identified as a homogeneous MSC population and therefore underwent next cell expansion passages.
For expansion cultures, BM-MSC and BM-HA-MSC were diluted to 4000 cells/cm2 in fresh CM and following the P1, the cells undewent three further passages. The expansion rates were calculated by dividing the total number of cells obtained at each confluence step by the number of cells seeded at each passage.
MSC Chondrogenesis Differentiation
The potential of BM-MSC and BM-HA-MSC to differentiate into chondrogenesis was assessed at passage 4.
Chondrogenesis is usually performed in micromass pellets. To this aim, approximately 200.000 cells obtained from both BM-MSC and BM-HA-MSC were cultured in 15 ml conical tube in a-MEM (Gibco Invitrogen, San Giuliano Milanese, Italia) supplemented with 5% PL Desamethasone 10−7M (Sigma-Aldrich), Ascorbic Acid 10−4M (Sigma-Aldrich), b-Transforming Growth Factor 10ng/mL (Sigma-Aldrich) and Insulin 10mg/mL (Sigma-Aldrich). The medium was changed every 3–4 days. After 21 days of culture the cell pellets were harvested, formalin fixed and paraffin embebbed. Histochemical analyses sections were stained with both Toluidine blue and Hematoxylin/Eosin dyes.
In addition to chondrogenesis induced in micromass pellets, 200.000 BM-MSC and BM-HA-MSC were also seeded in six-well dish plates, left to adhere to the plastic in CM before it was re-placed by the selective medium (above described) to induce chondrogenesis. The medium was changed every 3–4 days and after 21 culture days the cells in the adherent phase were stained ony with Toluidine Blue (Bio-Optica, Milano, Italy).
Results
BM-MSC isolate by culturing BM derived MNC reached >80% confluence (P1) within 13–20 days as already observed by culturing MSC derived from BM donors (data not shown).
On the other hand BM-HA-MSC released by HA membrane in the 4 Petri dishes required a longer time to reach confluence which was observed within 22–36 days. Actually, during the culture period the visual observation of the BM-HA-MSC showed a slow release, from the HA membrane, of cells with fibroblastoid morphology which adhere to the Petri plastic surface and the contemporary disruption of the scaffold matrix with release of single fibers.
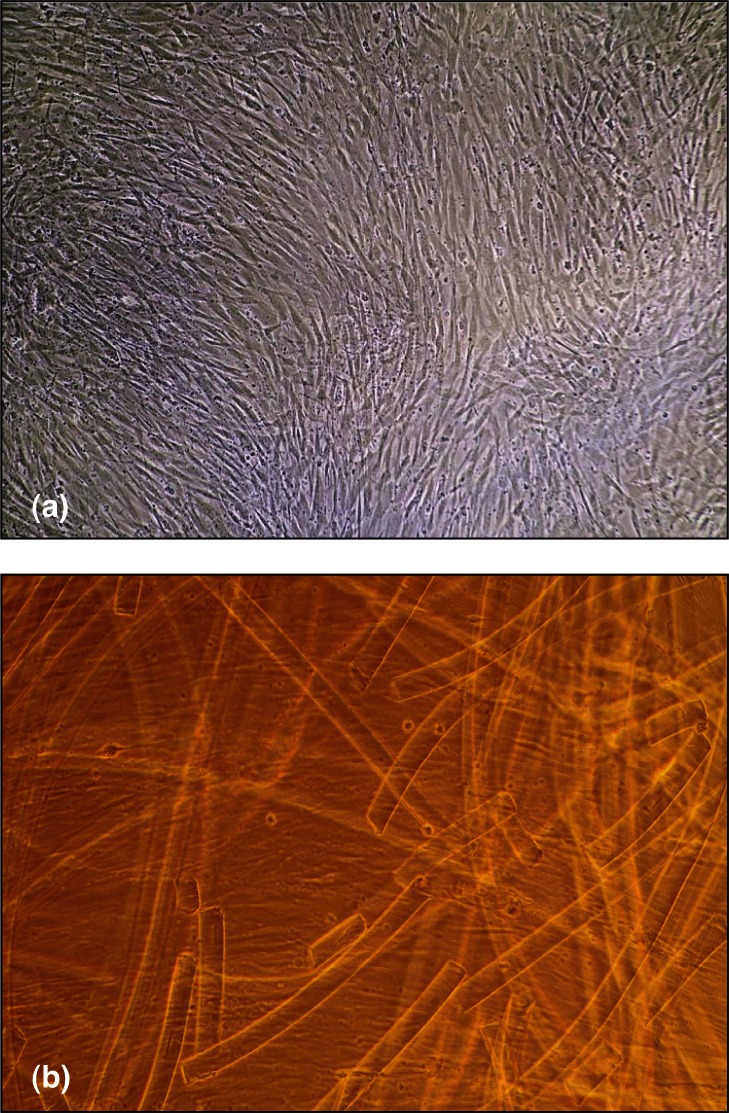
Figure 1 shows the images of BM-MSC (a) and BM-HAMSC (b) monolayers where HA membrane degraded into small fragment is clearly recognizable.
Figure 1.
Images of BM-MSC (a) and BM-HA-MSC (b) at confluence phase. In the BM-HA-MSC picture, besides the cells, small fragments of HA membrane, broken down during the culture period, are clearly recognizable.
All the samples of BM-MSC and BM-HA-MSC underwent 4 passages along the expansion and the corresponding time schedule is summarized in Table 1.
Table 1.
BM-MSC and BM-HA-MSC time schedule through the four passages to reach confluence during the whole expansion time of cell culture.
| BM MSC | Days to reach | BM-HA MSC | Days to reach | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P1 | P2 | P3 | P4 | ||
| Pt 1 | 20 | 29 | 37 | 47 | Pt 1 | 26 | 37 | 47 | 60 |
|
| |||||||||
| Pt 2 | 14 | 22 | 26 | 34 | Pt 2 | 22 | 26 | 34 | 41 |
|
| |||||||||
| Pt 3 | 18 | 25 | 32 | 39 | Pt 3 | 25 | 39 | 46 | 55 |
|
| |||||||||
| Pt 4 | 18 | 27 | 36 | 42 | Pt 4 | 36 | 49 | 59 | 62 |
|
| |||||||||
| Pt 5 | 13 | 20 | 30 | 43 | Pt 5 | 35 | 43 | 53 | 61 |
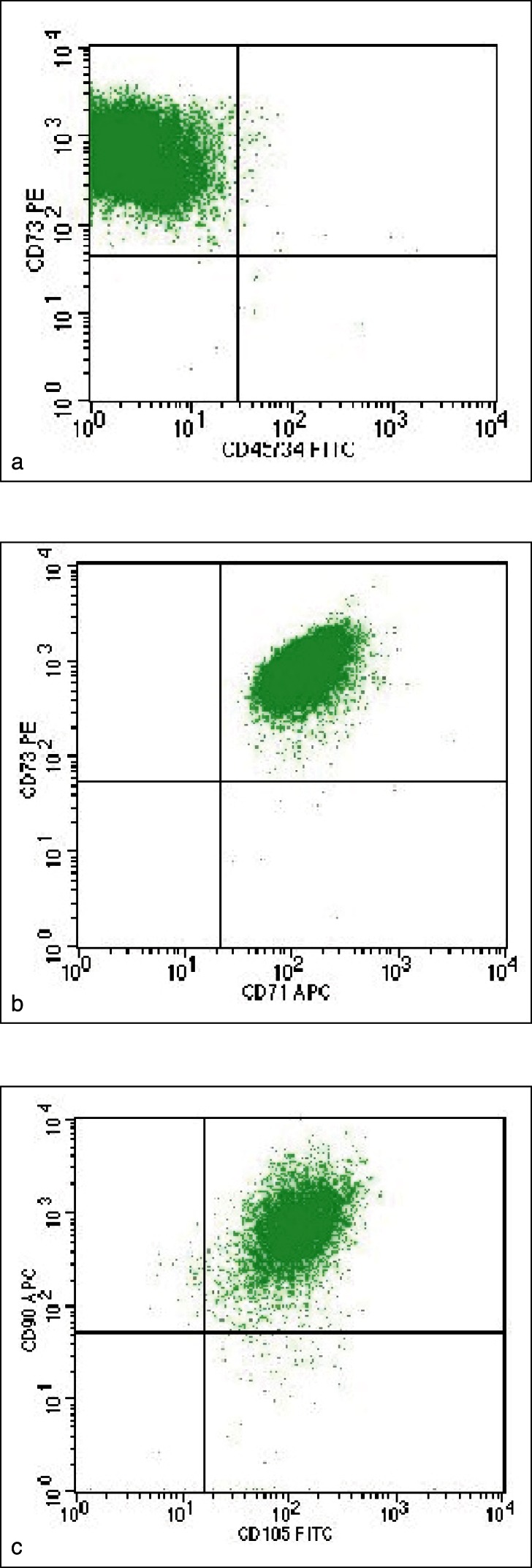
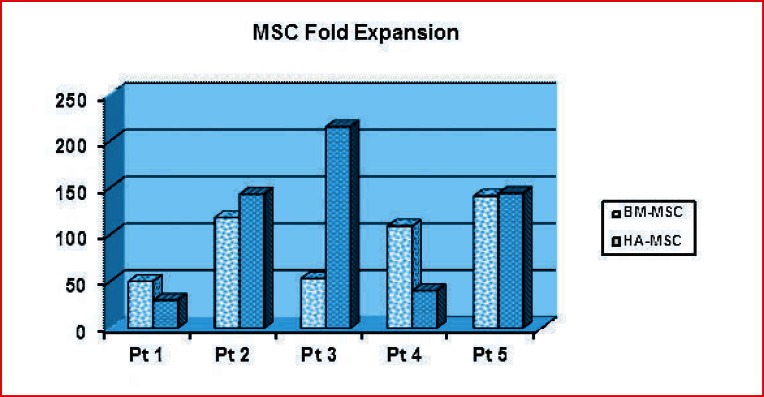
At P1 flow-cytometry analysis showed that > 95% were identified as MSC showing the expression of the following surface receptors CD45− CD34− CD71+ CD73+ CD90+ CD105+ (Fig. 2) as required by the minimal criteria for defining multipotent mesenchymal stromal cells stated by the International Society of Cell Therapy (ISCT). The yield of MSC obtained at the end of the culture (passage 4) for both BM-MSC and BM-HA-MSC are summarized respectively in Table 2 and 3 while the fold expansion is shown in Figure 3. BM-MSC from patient 1 and 3 expanded at a lower rate in comparison with the remaining three patients. By comparing the expansion of BMMSC and HA-BM-MSC the expansion rates are comparable in patients 1, 2 and 5 whereas relevant differences have been observed in patients 3 and 4.
Figure 2.
Dot plots of flow-cytometry analysis to identify MSC on the base of surface receptor expression: a) CD73+/CD45-CD34-b) CD73+/CD71+ c) CD90+/CD105+.
Table 2.
BM-MSC yield obtained at the end of the culture (passage 4).
| Patients | MNC x 106 Starting culture | BM-MSC x 106 P1 | BM-MSC x 106 P4 |
|---|---|---|---|
| Pt 1 | 10 | 0.25 | 12.5 |
| Pt 2 | 10 | 1.9 | 224 |
| Pt 3 | 5 | 4.9 | 260 |
| Pt 4 | 5 | 1.5 | 164 |
| Pt 5 | 10 | 6.4 | 902 |
Table 3.
BM-HA-MSC yield obtained at the end of the culture (passage 4).
| Patients | BM-HA-MSC x 106 P1 | BM-HA-MSC x 106 P4 |
|---|---|---|
| Pt 1 | 0.14 | 4.2 |
| Pt 2 | 0.79 | 113 |
| Pt 3 | 0.02 | 4.3 |
| Pt 4 | 0.56 | 22.5 |
| Pt 5 | 0.73 | 105 |
Figure 3.
BM-MSC and BM-HA-MSC fold expansion at the end of the culture time (passage 4). The total fold expansion was calculated by multiplying individual rate evaluated at each passage as the ratio between the number of cells seeded and those found at next confluence step.
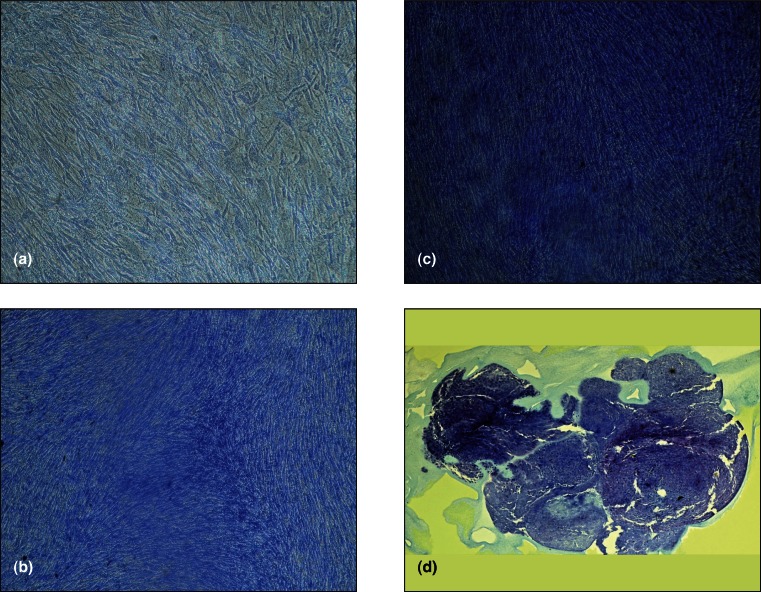
When cultured in micromass pellets and chondrogenic selective medium both BM-MSC and BM-HA-MSC were able to show differentiation into chondrogenesis as assessed by histochemical analyses stained with Toluidine blue and Hematoxylin/eosin dyes (Fig. 4).
Figure 4.
Chondrogenesis differentiation of BM-MSC and BM-HA-MSc cultured in micromass pellets and chondrogenic selective medium. The histochemical analyses was performed by staining the sections with Hematoxylin/Eosin (a) and Blue Toluidine (b).
When BM-MSC and BM-HA-MSC where cultured in adherent phase by using chondrogenesis selective medium and after 21 days stained with toluidine-blue BM-MSC were less stained than BM-HA-MSC.
Discussion and conclusion
Articular cartilage injuries have poor repair capabilities and conventional treatment methods often give unsatisfactory results. Development of innovative solutions are under study and recently MSC have been explored as an alternative cell source for cartilage regeneration and repair due to their chondrogenic potential and their easy isolation from BM. The great challenge when considering MSC for articular cartilage repair is to generate cells with features of stable chondrocytes avoiding the risk of hypertrophy. Therefore biological studies which evaluate in vitro the isolation, expansion and chondrogenic differentiation of BM derived MSC could be helpful when correlated with long-term in vivo results. Here, we focused on the ex vivo selection, expansion and characterization of MSC obtained by patients treated for severe chondral lesions. To select MSC we cultured in vitro either BM samples or samples of HA membrane seeded with BM being this biomaterial most frequently used in vivo to regenerate cartilage tissue.
The results of in vitro MSC selection and growth showed that BM-MSC from two patients expanded at a lower rate in comparison with the remaining ones and these findings could be likely due to the individual variability12. By comparing the growth of BM-MSC and BM- HA-MSC the expansion rates were comparable in three patients, whereas differences have been observed in the other two patients. In this regard it has to be considered that the size of HA membrane left in culture was different among the patients. Actually the residual of the scaffold sent to laboratory was depending on the extension of the lesion to be treated and this starting variability could have influenced the yield of MSC obtained in ex vivo cell cultures. By daily optical examination under microscopy of the BM-HA-MSC cultures it was possible to reveal that MSC were slowly released from the scaffold in the growth culture medium and therefore they reached the confluence later than MSC derived from BM samples directly cultivated. The slow release of MSC from the HA membrane could be explained by the interaction via CD44 receptor expressed on MSC surface. Via this receptor MSC links to HA, and this mechanism likely is the mean by which the HA scaffold retains the cells in the lesion site. It has been reported that the use of scaffold, as a support for for cell adhesion, may also provide a favourable microenvironment for MSC in vivo chondrogenesis11. Hyaluronic acid is a natural glycosaminoglycan present in soft tissues and it is in particularly high concentrated in the extracellular matrix of articular cartilage where it contributes to maintain the cartilage matrix homeostasis.
In our hands both BM-MSC and BM-HA-MSC were able to differentiate towards chondrogenesis when cultured in micro-mass according to the commonly used protocols. In addition to culture in micro-mass, we tried to induce chondrogenesis by using appropriate selective medium but leaving BM-MSC and BM-HA-MSC adherent to the plastic. When these cells were stained with toluidine blue BM-HA-MSC showed a higher staining intensity than BMMSC which may suggest a higher detection of proteoglycans in BM-HA-MSC in respect to BM-MSC. This in vitro observation seems to sustain the hypothesis that the interaction of MSC with the scaffold such as HA membrane may contribute to positively influence the differentiation process towards chondrogenesis.
Many other factors may help MSC in vitro differentiation into chondrocytes as the spatial conformation of the culture system, additional reagents to the medium as PRP, PL or TGF-b1 (valutare referenze), but the influence of the structure and composition of the scaffold is particularly relevant since it could provide favourable microenviroment for MSC chondrogenesis not only in vitro but also in vivo when employed in regenerative tissue engineering procedure. More extensive data are required to deeply investigate the role of scaffold matrix in inducing MSC differentiation both in vitro and in vivo in order to allow the choice of the most appropriate one for the treatment of different tissue injury.
References
- 1.Komarek J, Valis P, Repko M, Chaloupka R, Krbec M. Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation: long term results. Acta Chir Orthop Traumatol Ceck. 2010;77(4):291–295. [PubMed] [Google Scholar]
- 2.De Girolamo L, Bertolini G, Cervellin M, Sozzi G, Volpi P. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro chracterization of mesenchymal stem cells from iliac crest and sub-chondral bone. Injury. 2010;41(11):1172–1177. doi: 10.1016/j.injury.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 4.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Delaine-Smith RM, Reilly GC. Mesenchymal stem cell responses to mechanical stimuli. MLTJ. 2012;2(3):169–180. [PMC free article] [PubMed] [Google Scholar]
- 6.Bouffi C, Thomas O, Bony C, Giteau A, Venier-Julienne MC, Jorgensen C, Montero-Menei C, Noel D. The role of pharmacologically active micro-carriers releasing TGF-beta 3 in cartilage formation in vivo by mesenchymal stem cells. Biomaterials. 2010;31(25):6485–6493. doi: 10.1016/j.biomaterials.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Bernardo ME, Cometa AM, Pagliara D, Vinti L, Rossi F, Cristantielli R, Palumbo G, Locatelli F. Ex-vivo Expansion of mesenchymal stromal cells. Best Practice and research clinical haematology. 2011;24:73–81. doi: 10.1016/j.beha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Giai Via A, Frizziero A, Oliva F. Biological properties of Mesenchymal Stem Cells from different sources. MLTJ. 2012;2(3):154–162. [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobsen RB, Shahdadfar A, Reinholt FP, Brinchmann JE. Chondrogenesis in a hyaluronic acid scaf-fold: comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthosc. 2010;18(10):1407–1416. doi: 10.1007/s00167-009-1017-4. [DOI] [PubMed] [Google Scholar]
- 10.Tognana E, Borrione A, De Luca C, Pavesio A. Hyalograft C: Hyaluronan based scaffolds in tissue engineered-cartilage. Cells Tissues Organs. 2007;186:97–103. doi: 10.1159/000102539. [DOI] [PubMed] [Google Scholar]
- 11.Chung C, Burdick JA. Influence of 3D Hyaluronic acid microenviroments on mesenchyma stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raggi C, Berardi AC. Mesenchymal stem cells, aging and regenerative medicine. MLTJ. 2012;2(3):239–242. [PMC free article] [PubMed] [Google Scholar]