Abstract
Picornaviruses have been developed as potential therapies for gene delivery and vaccination. One drawback to their use is the potential for recombination and viral persistence. Therefore, the engineering strategies used must take into account the possibility for virus escape. We have developed Theiler's murine encephalomyelitis virus (TMEV) as a potential vaccine vector for use in immunotherapy. This study shows that insertion of a vaccine epitope at a unique site within the TMEV leader protein can dramatically increase the type I interferon (IFN) response to infection and promote rapid viral clearance. This live virus vaccine maintains its ability to drive antigen-specific CD8+ T-cell responses to a model antigen as well as to the weakly immunogenic tumor antigen Her2/neu. Furthermore, the epitope integration site does not affect the efficacy of this vaccine as cancer immunotherapy for treating models of melanoma and breast cancer as demonstrated by delayed tumor outgrowth and increased survival in animals implanted with these tumors. These findings show that an attenuated virus retaining limited ability to replicate nonetheless can effectively mobilize CD8+ cellular immunity and will be important for the design of picornavirus vectors used as immunotherapy in clinical settings.
Introduction
Engineered picornaviruses have been developed as vaccine vectors for immunotherapy because of their inherent immunogenicity and relatively small genomes that can be easily manipulated using standard techniques. The harnessing of picornaviruses for immunotherapeutic purposes has been attempted using several viruses within this family including enterovirus,1 coxsackievirus,2 rhinovirus,3 and cardiovirus.4,5,6 However, the enthusiasm for using these viruses as vaccine vectors has been tempered by concerns regarding their inherent genomic instability and the prospect of reversion to wild-type sequences. Consequently, several studies have focused on engineering new picornavirus vectors that retain the engineered virus identity.7,8
We have recently identified the potential of the cardiovirus Theiler's murine encephalomyelitis virus (TMEV) as a vaccine candidate for immunotherapy.6 We engineered vaccine antigens into the leader sequence of TMEV and demonstrated that a virus integrated epitope could drive strong CD8+ T-cell responses and inhibit tumor outgrowth after treatment with engineered TMEV virus. The leader protein is unique among picornavirus proteins in that it is translated before the capsid proteins by virtue of its placement with the genome. The leader protein has been linked to immunosuppression through its ability to inhibit type I interferon (IFN) signaling which is necessary to evade innate immune effectors.9,10 Mice with the appropriate major histocompatibility complex class I molecules subsequently clear the virus through adaptive immune mechanisms,11,12 notably the CD8+ T-cell response13,14,15 and remarkably, the activation of this T-cell response occurs in the absence of costimulation, CD4 help, tumor necrosis factor, or IFNγ.16
Furthermore, studies using cell lines deficient in type I IFN responsiveness have shown that TMEV absent the leader protein can replicate in vitro but fail to efficiently replicate in vivo.17 Our studies showed that insertion of the vaccine epitope within the leader protein at an XhoI site attenuated the virus to a degree, suggesting that insertion of vaccine epitopes within the leader may also serve as a mechanism for attenuating the virus.6
Although viral replication efficiency is often a concern in the development of attenuated live virus vaccines, little is known about how attenuation can influence the effective response to engineered virus vectors. In this study, we have engineered TMEV vaccines that use unique insertion sites within the leader protein and consequently have unique replication properties in vivo. We find that the insertion site can influence viral clearance by promoting RAG independent mechanisms of clearance without affecting vaccine responses. This strategy provides a safe vaccine vector that can be rapidly cleared before reversion to wild-type virus yet promotes effective CD8+ T-cell immunity to vaccine antigens.
Results
Derivation of wild-type and attenuated vaccine strains of TMEV from recombinant vectors
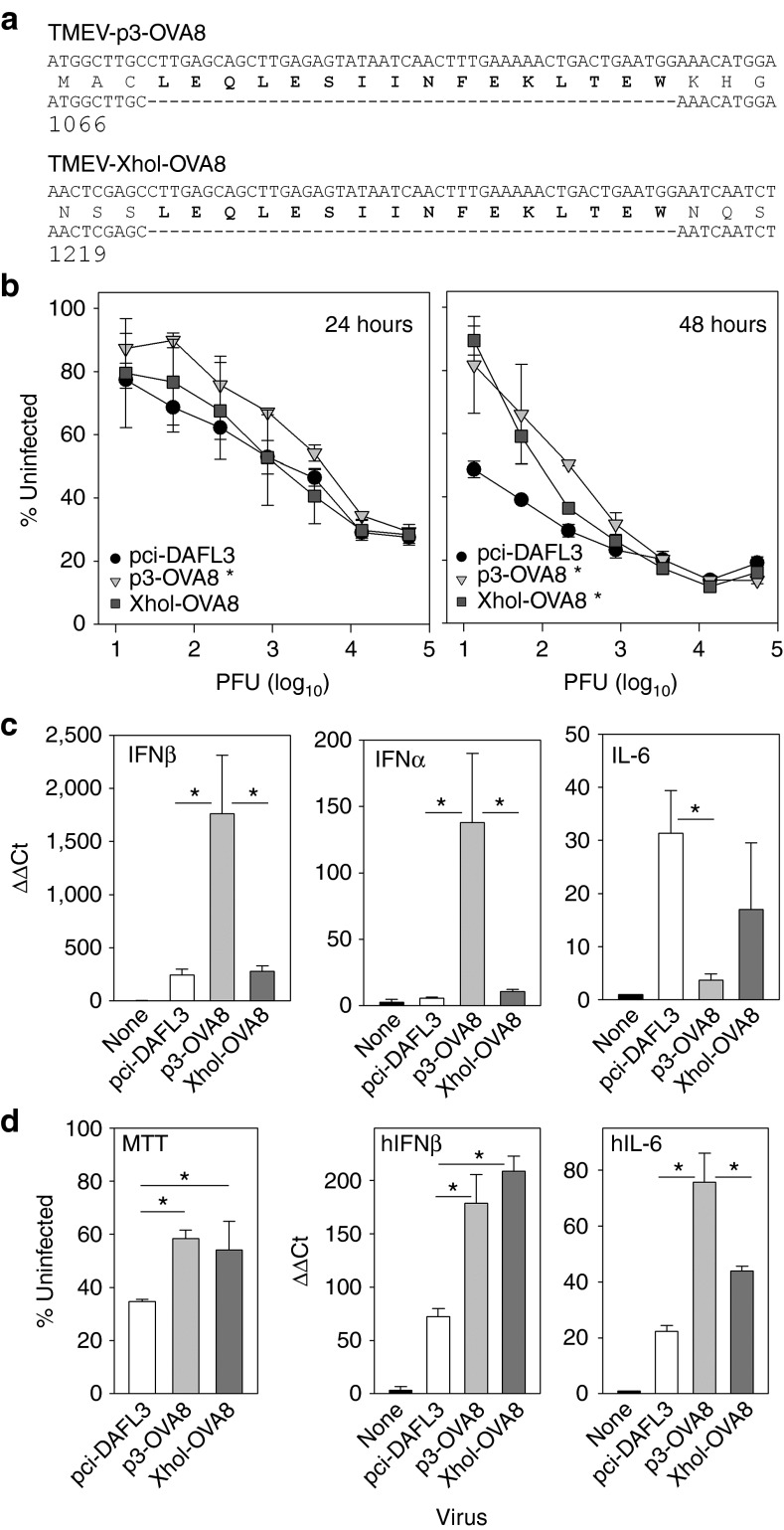
Previously, we developed a live TMEV vaccine through transfection of RNA transcripts generated from a modified TMEV cDNA clone.6 To bypass the need for generating RNA transcripts, we cloned the complete genome of the Daniel's strain of TMEV18 into a eukaryotic expression vector (pci-DA). The pci-DA clone was directly transfected into BHK cells and the resulting transfection resulted in the release of viral progeny. Using site-directed mutagenesis, we generated modified TMEV vectors that expressed a fragment of chicken ovalbumen (OVA244–260) that included the H-2Kb restricted CD8+ T-cell epitope SIINFEKL. This fragment was cloned into the pci-DA vector at positions 1074 and 1227 of the TMEV genome. These modified viruses encode small insertions after the third amino acid of the TMEV leader protein (p3-OVA8) and at the XhoI restriction site further downstream (XhoI-OVA8). Neither construct disrupted the coding frame after the epitope insertion site (Figure 1a).
Figure 1.
In vitro characterization of TMEV vaccines. (a) Amino-terminal sequence of TMEV L protein for the p3-OVA8 and XhoI-OVA8 viruses. OVA253–268 was engineered into the wild-type TMEV expression vector by site-directed mutagenesis. (b) Killing of BHK cells by engineered TMEV viruses at 24 and 48 hours after infection. Triplicate samples were assessed for MTT metabolism. Data are presented as the percentage of uninfected cell signal ±SD (*P < 0.05, versus pci-DA by Holm-Sidak method). (c) IFNβ, IFNα, and IL-6 gene regulation in response to infection with modified TMEV viruses for 24 hours (*P < 0.05 by Holm-Sidak method). (d) MTT metabolism, human IFNβ (hIFNβ), and human IL-6 (hIL-6) gene regulation after infection of human dermal fibroblasts (*P < 0.05 by Holm-Sidak method). PFU, plaque-forming units.
After transfection into cells, virus was recovered and quantified by plaque assay. The wild type, p3-OVA8, and XhoI-OVA8 demonstrated similar plaque size and infectivity compared with the wild-type virus (data not shown). To test the virulence of the engineered strains, we used an MTT assay to assess killing of BHK cells after infection with a titrating dose of each virus. After 24 hours of infection, the p3-OVA8 virus had a modest decrease in virulence compared with the other two viruses. However, the virulence of the wild-type virus was notably higher after 48 hours of infection compared with the p3-OVA8 and XhoI-OVA8 viruses, demonstrating attenuation of both modified strains (Figure 1b).
One unique aspect of viruses within the cardiovirus genus is the presence of a leader protein that modulates early cytokine release from infected cells as a mechanism of immune evasion. We infected B6 fibroblasts in vitro with modified viruses to determine whether the insertion sites we chose would modulate the cytokine response. We found that the p3-OVA8 virus elaborated more messenger RNA for IFNβ and IFNα after 24 hours of infection in vitro; in contrast, the wild-type pci-DA virus and XhoI-OVA8 virus elaborated an increased amount of IL-6 compared with the p3-OVA8 virus and uninfected control (Figure 1c). This suggests that regions within the leader can be disrupted by insertion of vaccine antigen with consequences toward host immune responses.
To verify the potential for using modified TMEV as therapy in humans, we infected human dermal fibroblasts and assessed their viability by MTT assay after 48 hours of infection. Similar to what we observed in mouse cells, we found that the p3-OVA8 and XhoI-OVA8 viruses were less virulent toward human cells when compared with the pci-DA wild-type virus (Figure 1d). Furthermore, we asked whether the innate immune response to infection of human cells could be modulated by using these viruses. We infected human fibroblasts for 24 hours and assessed the induction of human IFNβ and human IL-6 transcripts. We found that the p3-OVA8 and XhoI-OVA8 viruses elicited increased IFNβ in response to infection and that each of the three viruses responded with a unique IL-6 expression profile, supporting our hypothesis that the site of epitope integration can also modulate the innate immune response to viral infection in human cells.
Two distinct leader insertion sites modulate viral clearance
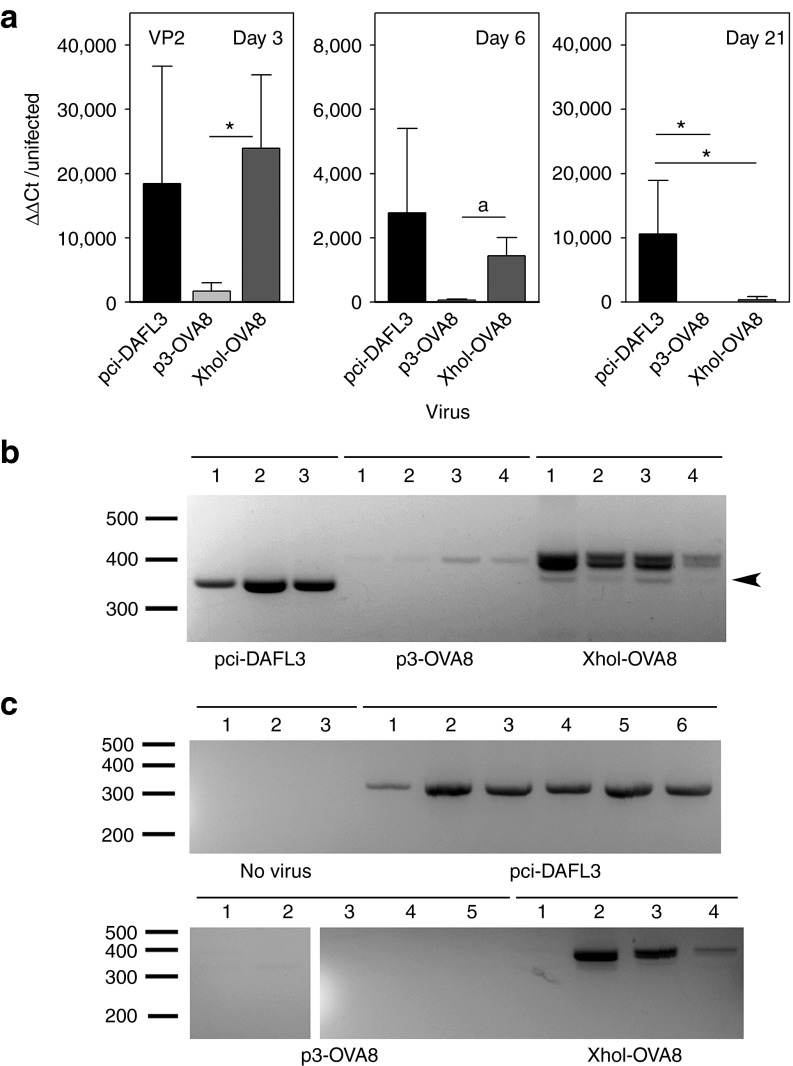
Clearance of TMEV from the CNS is determined by the H-2D region of major histocompatibility complex. B6 (H-2Db) mice are resistant to persistent infection and clear virus, where FVB (H-2Dq) mice fail to clear virus and therefore develop persistent infection. To determine how efficiently modified TMEV viruses can be cleared, we intracranially infected B6 mice for 3 and 6 days and FVB mice for 21 days to determine relative virus RNA levels. Three days after infection with pci-DA, p3-OVA8, or XhoI-OVA8 virus all mice had detectable levels of TMEV transcripts compared with uninfected mice. This level was reduced in all three groups at day 6, demonstrating that all three viruses replicated to some extent in the brain (Figure 2a). The p3-OVA8 virus never reached the RNA levels of the wild-type and XhoI-OVA8 viruses, demonstrating that this virus does not replicate as efficiently in vivo. Furthermore, infection of susceptible FVB mice reveals that the wild-type virus persists; however, the XhoI-OVA8 and p3-OVA8 RNA levels were reduced, with the p3-OVA8 being near the detection threshold (Figure 2a).
Figure 2.
Infection with engineered TMEV. (a) Relative virus levels as determined by semiquantitative RT-PCR for TMEV viral protein 2 (VP2) in mice intracerebrally infected with TMEV viruses. Day 3 and 6 infected data represent infection of C57BL/6 mice and day 21 data represent infection of FVB mice (fold increase from uninfected CNS ±SD, n = 5 per group at day 3, *P < 0.05 by Holm-Sidak method, aP < 0.05 by t-test). (b) RT-PCR amplification across antigen insertion site of RNA recovered from C57BL/6 mice infected for 6 days from a. Arrow indicates deletion mutant. (c) Analysis of RT-PCR products derived from FVB mice infected with pci-DA, p3-OVA8, and XhoI-OVA8 for 21 days. All bands were isolated and sequenced to confirm identity of wild-type virus, modified TMEV virus or to identify deletion mutants. RT-PCR, reverse transcriptase PCR.
To determine the virus replication fidelity, we amplified cDNA derived from infected animals in a manner that allowed us to discriminate the engineered viruses by amplicon size. Virus recovered from 6 day wild-type–infected B6 mice generated a single band that was consistent with the predicted size of 326 base pairs. In two out of four animals infected with p3-OVA8, viral RNA was detected and amplicons were consistent with the insert size of 377 bp and sequencing revealed identity to the engineered virus sequence (Figure 2b). All XhoI-OVA8 infected animals had amplified products consistent with the modified virus as well as two less prominent bands. To determine whether this represented virus that had reverted to wild type, we sequenced the most prominent product and the smaller of the two amplified products marked in Figure 2b. The major product was identical to the cDNA clone that was used to generate the modified virus. Sequencing of the smaller band revealed a deletion of the coding sequence that truncates the final six amino acids of the ovalbumen insert (Figure 1a); however, wild-type revertants were not detected.
This strategy was repeated using the 21-day–infected RNA from FVB animals. Size and sequence of amplicons verified the recovery of wild-type virus in all six animals infected with pci-DA. No virus was detected in mice infected with the p3-OVA8 virus and three out of four XhoI-OVA8 animals had detectable levels of virus. In the FVB strain, wild-type and XhoI-OVA8 virus PCR fragments matched the sequence of the infecting virus. All FVB animals had detectable titers of anti-TMEV antibody, verifying that all animals were infected with the three viruses (data not shown).
Clearance of the p3-OVA8 vaccine strain is mediated through type I IFNs
The clearance of p3-OVA8 virus and the generation of a strong type I IFN response suggest that innate immunity may be sufficient for eliminating this virus. We intracerebrally infected RAG−/− mice with wild-type TMEV or our two OVA8-expressing vaccine strains to determine whether adaptive immunity plays a role in viral clearance. Although these mice lack T and B cells, the innate immune response in these animals is still intact, providing an opportunity to determine whether innate immunity is sufficient for clearing virus. After infection, mice were monitored for 28 days for signs of infection, weight loss, and fecundity. The wild-type and XhoI-OVA8 viruses developed a severe disease with noticeable weight loss by day 14 in both groups. All RAG−/− animals infected with pci-DA or XhoI-OVA8 were killed by day 16 or 19 when mice became moribund (Figure 3a). The p3-OVA8 infected mice did not exhibit weight loss or morbidity and survived through the 28 day monitoring point.
Figure 3.
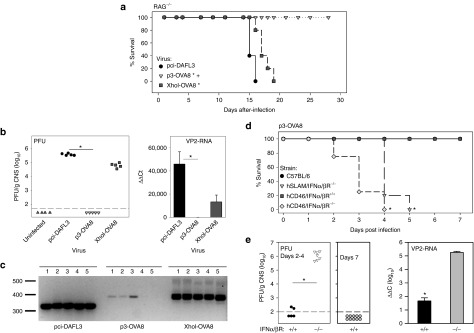
Adaptive immunity is not required for clearance of p3-OVA8. (a) Kaplan–Meier survival analysis in RAG−/− mice that were infected with pci-DA, p3-OVA8, and XhoI-OVA8 (P < 0.05 by Holm-Sidak method versus *pci-DA or +XhoI-OVA8). (b) Virus titers and viral RNA levels from CNS homogenates recovered from RAG−/− mice analyzed in a (*P < 0.05 Dunn's method). (c) Amplification of RNA recovered from the CNS of animals in a to determine identity of infectious virus. All bands were verified by sequencing to confirm virus identity. (d) Kaplan–Meier survival analysis of IFNα/β receptor knockout mice infected with p3-OVA8 (*P < 0.05 by Holm-Sidak method versus C57BL/6 or hCD46/IFNα/βR+/+). (e) Virus titers and viral RNA levels in CNS tissues recovered from animals in d (*P < 0.05 by t-test). PFU, plaque-forming units; VP2, viral protein 2.
As the p3-OVA8 infected mice survived, we asked whether infectious virus might still be present in the CNS. We performed plaque assays on CNS homogenates derived from each of the RAG−/− animals infected with pci-DA, XhoI-OVA8, and p3-OVA8 viruses. We found high titers of pci-DA or XhoI-OVA8 virus in the RAG−/− CNS infected with each of these viruses. No detectable virus plaques were observed in monolayers exposed to CNS homogenates from p3-OVA8 infected RAG−/− mice, similar to cells exposed to an uninfected CNS homogenate (Figure 3b). Finally, RNA was isolated from the CNS to determine whether RNA levels were consistent with infectious titers. pci-DA and XhoI-OVA8 RNA levels were consistent with data obtained from plaque assays. Low levels of p3-OVA8 RNA were detected in homogenates from infected animals, demonstrating that low levels of virus were present (Figure 3b). To verify the fidelity of each of these viruses after infection, cDNA derived from the CNS was sequenced, demonstrating that all the infected animals retained viruses with their respective engineered viral genome (Figure 3c).
As the p3-OVA8 virus could be cleared in the absence of T or B cells, we asked whether clearance was mediated through the type I IFN pathway. We infected IFNα/β receptor knockout mice with p3-OVA8 virus and monitored for weigh loss and morbidity. As observed previously, both C57BL/6 and littermate control animals displayed no signs of overt disease. In contrast, two different strains of IFNα/βR−/− mice quickly lost weight and succumbed to viral infection within 5 days after infection (Figure 3d), demonstrating the importance of this pathway for clearance of p3-OVA8. To verify the presence of live virus, we performed plaque assays on recovered CNS homogenates and found extremely high titers of p3-OVA8 virus in the IFNα/βR−/− animals that were infected for 2–4 days, in comparison with littermate controls or C57BL/6 animals infected for 2–4 or 7 days (Figure 3e). We verified the presence of viral RNA in all animals by semiquantitative reverse transcriptase PCR (Figure 3e).
p3-OVA8 and XhoI-OVA8 drive CD8 T-cell immunity that delays melanoma outgrowth
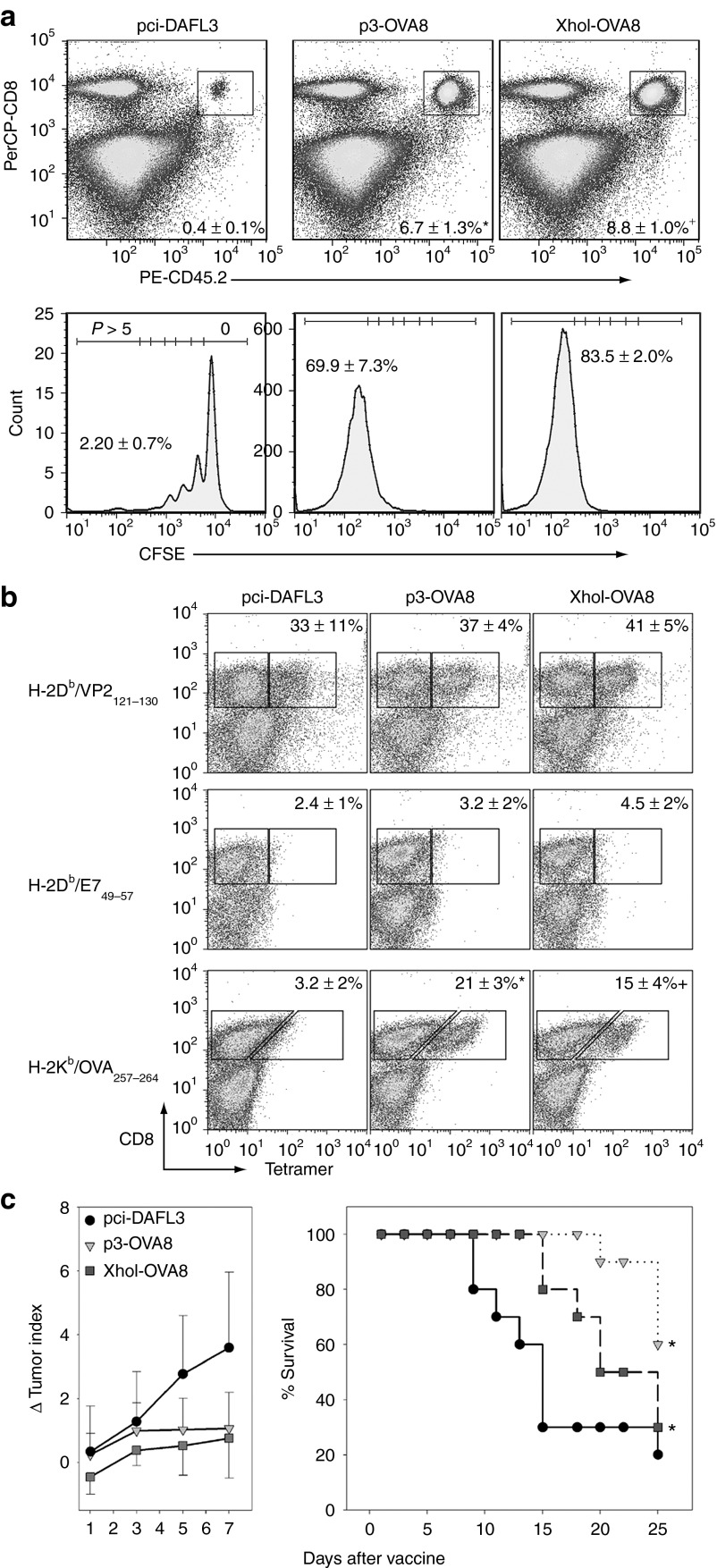
By engineering major histocompatibility complex class I epitopes into the leader sequence of TMEV, we have identified a strategy that mediates virus control and provides an added level of safety for our live virus vaccine. However, it is unclear whether attenuation of the virus in this manner will be detrimental to its use as a T-cell vaccine. To determine whether the p3-OVA8 and XhoI-OVA8 viruses can effectively drive proliferation of antigen-specific T cells, we determined how effectively these viruses drive proliferation of transgenic CD8+ T cells specific for OVA8. We found that infection with either the p3-OVA8 or the XhoI-OVA8 viruses drives proliferation of the T-cell population (Figure 4a). Furthermore, both viruses drove a significant dilution of Carboxyfluorescein diacetate succinimidyl ester (CFSE) indicating that these viruses were able to promote proliferation of T cells in response to infection with p3-OVA8 or XhoI-OVA8 when compared with pci-DA infection (Figure 4a).
Figure 4.
TMEV vaccines drive antigen-specific CD8+ T-cell responses and reveal their potential as immunotherapy. (a) Recovery and proliferation of CD45.2+ /CD8+ OT-1 T cells from splenocytes of vaccine infected mice (n = 5 for all groups, % recovered cells ± SD). Cells were binned according to rounds of proliferation as determined by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution. Data below are the percentage of cells that have gone through greater than five rounds of proliferation. *P < 0.05 pci-DA or XhoI-OVA8; +P < 0.05 versus pci-DA or p3-OVA8 by Holm-Sidak method. (b) Virus- and vaccine-specific CD8+ T-cell responses determined by MHC class I tetramers in naïve C57BL/6 mice infected with TMEV vaccines for 6 days (percentage of CD8+ T cells specific for VP2, E7 control or OVA ± SD). *P < 0.05 pci-DA or XhoI-OVA8; +P < 0.05 versus pci-DA or p3-OVA8 by Holm-Sidak method. (c) Delayed growth of established tumors and increased survival in mice challenged with the melanoma model tumor B16-OVA. Both p3-OVA8 and XhoI-OVA8 vaccines significantly delayed outgrowth as melanoma therapy by day 7 after treatment (*P < 0.05 versus pci-DA by Holm-Sidak) and increased survival during the 25 day observation period (*P < 0.05 versus pci-DA by Kaplan–Meier survival analysis with pairwise comparisons performed using Holm-Sidak method). MHC, major histocompatibility complex.
By demonstrating that antigen-specific T cells respond to our vaccines, we establish that these vectors can sufficiently present vaccine antigens and promote proliferation of a transgenic T-cell population. However, in the absence of a high-frequency T-cell receptor, we wanted to determine whether these vaccines drive de novo CD8 T-cell responses in naïve animals. To test this, we intracerebrally infected mice with pci-DA, p3-OVA8, and XhoI-OVA8 viruses and analyzed the CNS infiltrating lymphocytes after 6 days of infection. We compared the vaccine antigen-specific CD8+ T-cell responses to the endogenous VP2121–130 response typically observed in H-2Db mice. All viruses elicited a strong endogenous viral response after infection and no response was detected using the control tetramer E749 (Figure 4b). The p3-OVA8 and XhoI-OVA8 elicited OVA8 responses compared with the pci-DA wild-type virus, demonstrating their ability to drive a strong polyclonal response to this model antigen.
As these vaccines drive responses to the model antigen, we evaluated whether these vaccines would inhibit tumor outgrowth when used as immunotherapy. We established melanoma tumors in mice and allowed them to grow in the absence of intervention for 9 days. We treated tumor-bearing mice with p3-OVA8, XhoI-OVA8 or wild-type pci-DA virus and monitored changes in tumor size. Both viruses inhibited the outgrowth of the B16-OVA tumors and delayed outgrowth by day 7 after treatment with the virus vaccine compared with pci-DA virus (Figure 4c). Furthermore, these viruses significantly increased survival by delaying the growth of tumors through the 25 day monitoring window (Figure 4d).
Her2/neu vaccines drive antigen-specific CD8 T-cell responses and inhibit breast cancer growth
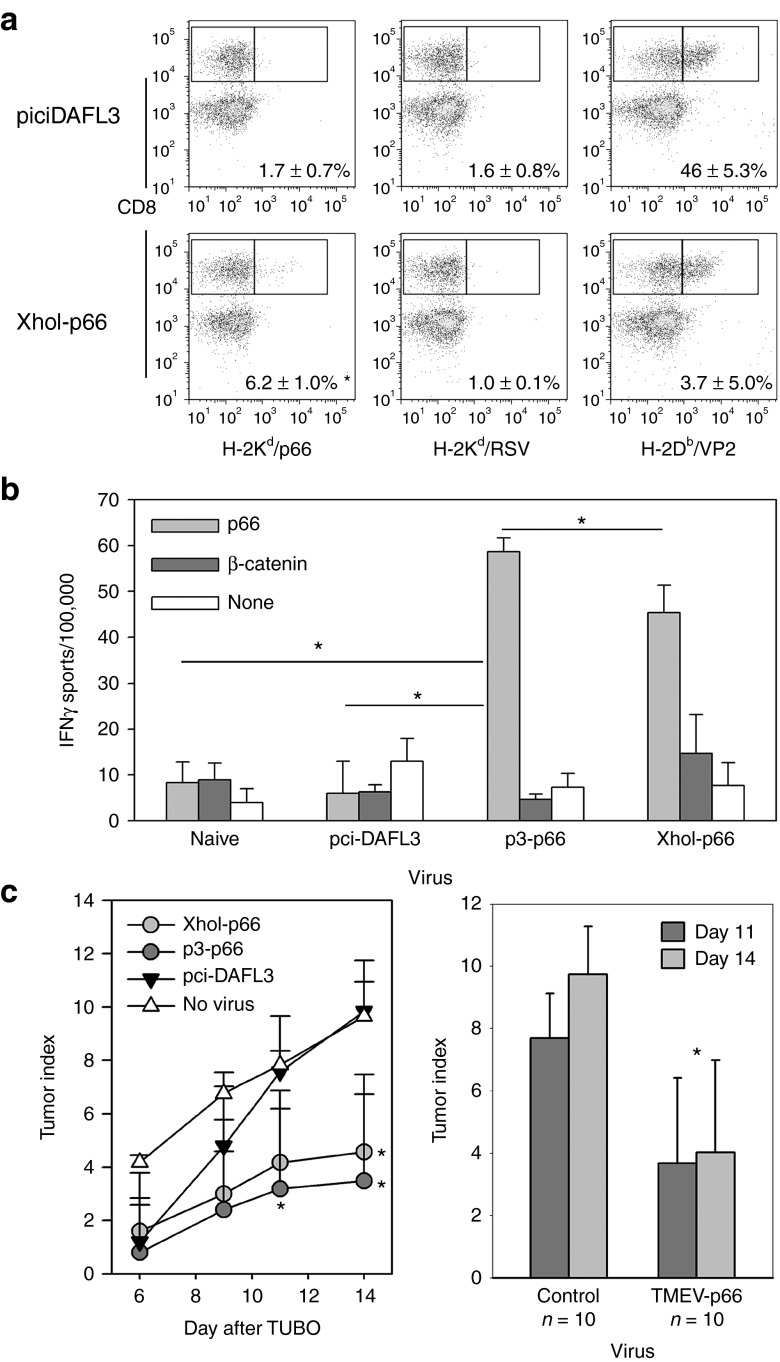
Although we demonstrate the ability to drive directed CD8 T-cell responses to the model antigen ovalbumen and show that this response inhibits tumor outgrowth, the potential of this vaccine approach may only be realized if responses to weakly immunogenic tumor antigens can be attained. To address this, we generated TMEV vaccines that encoded an H-2Kd peptide derived from Her2/neu, p66.19 We intracranially infected B6/Balb F1 mice with XhoI-p66 virus as well as the wild-type pci-DA. This allowed us to monitor the response to the H-2Db VP2121–130 peptide as well as the H-2Kd/p66 response by tetramer. CNS infiltrating lymphocytes were harvested after 6 days of infection. As expected, the wild-type TMEV infection yielded no response to H-2Kd/p66 or to the H-2Kd/RSV control peptide, but did respond to the endogenous VP2 antigen (Figure 5a). Infection with the XhoI-p66 virus yielded a weak, but significant CD8 response to the p66 peptide as measured by tetramer and no response to RSV demonstrating antigen specificity. Furthermore, the response to the endogenous VP2121–130 peptide was present, similar to wild-type virus (Figure 5a).
Figure 5.
TMEV-p66 vaccines promote Her2/neu immunity and inhibit outgrowth of a breast cancer tumor line. (a) Her2/neu-specific CD8+ T-cell responses in B6/Balb/c F1 mice (% of CD8+ T cells specific for the Her2/neu peptide p66, RSV, or VP2 ±SD, n = 3 per group, *P < 0.05 by Holm-Sidak method). (b) ELISPOT assay for p66-specific IFNγ response in Balb/c mice splenocytes after immunization with TMEV-p66 vaccines, control virus or no virus (*P < 0.05 by Holm-Sidak method). (c) Delayed outgrowth of TUBO tumors in p3-p66 and XhoI-p66 vaccinated mice (right) at 11 and 14 days after tumor in p3-OVA8 vaccinated animals and at day 14 in XhoI-OVA8 vaccinated (*P < 0.05 by Holm-Sidak method). Nonregressors were combined as control group compared with the p3-p66– and XhoI-p66–treated TMEV-p66 group at 11 and 14 days (*P < 0.05 by Holm-Sidak method).
Although the response to p66 could be detected in the F1 hybrids using the XhoI-p66 virus, we wanted to determine whether a p3-p66 virus and the XhoI-p66 viruses could induce antigen-specific CD8+ T-cell responses in Balb/c mice by assessing their induction of IFNγ by ELISPOT. To address this, we infected Balb/c mice intraperitoneally with pci-DA, p3-p66, and XhoI-p66 viruses and assessed IFNγ responses 7 days after, using purified CD8+ splenocytes. We find that both the p3-p66 and XhoI-p66 elicited a significant response to the relatively weak Her2/neu peptide, where naïve CD8+ splenocytes and wild-type infected failed to respond (Figure 5b), demonstrating that both vaccines are able to elicit a targeted and specific CD8+ T-cell response to this weak antigen.
As we demonstrate that this vaccine can elicit Her2/neu-specific CD8+ T-cell immunity, we assessed its potential as a breast cancer vaccine by treating mice with our TMEV-p66 viruses before the transfer of Her2/neu+ tumor cells onto the flank of Balb/c mice. After placement of the tumor, we monitored growth in the treated mice for 14 days after engraftment. We found that the p3-p66 treatment demonstrated a significant delay in outgrowth at day 11 and 14, where the XhoI-p66 significantly delayed outgrowth at day 14 (Figure 5c). The p3-p66 and XhoI-p66 demonstrated durable responses to the tumor with 3 out of 10 mice completely eradicating tumor, whereas all tumors grew in the control pci-DA and no treatment groups. As combined control or p66 vaccine groups, we find that there was a significant difference at both day 11 and day 14, with a decrease in tumor index in the p66 virus–treated groups (Figure 5c).
Discussion
Our objective is to engineer a live virus vaccine for use as immunotherapy. Previous studies demonstrate the potential use of TMEV as a vector that induces CD8+ T-cell responses against a model antigen and can suppress tumor growth in vivo.6 In this study, we identify strategies for manipulating the TMEV genome in a manner that can have consequences on the cytokine response elaborated after live virus infection, the development of protective CD8+ T-cell responses, and on live virus vaccine persistence. In addition, we demonstrate for the first time that TMEV can be used as a vector for driving CD8+ T-cell responses toward a weakly antigenic tumor antigen after a single treatment without additional immune modulation. These findings will be important for the further development of this virus as a vector for immunotherapy.
In an effort to identify insertion sites into the TMEV genome that might render the vaccine more immunogenic, we used the XhoI restriction site of the TMEV leader protein as well as a unique site after the third amino acid of the leader. As the TMEV genome is translated as one large polyprotein that is subsequently cleaved, we preferably chose sites at the amino terminus to promote more efficient major histocompatibility complex class I presentation of the virus encoded antigens. Both of these virus vaccines drive proliferation and activation of CD8+ T cells in a manner that demonstrates their potential for immunotherapy. However, we also identified some potential advantages for using the p3 insertion site when developing TMEV as a vaccine. One advantage of using the p3 vaccine is that infectious virus can be cleared in animals with severe immunodeficiency using protective mechanisms mediated through IFNα/β receptor signaling. However, attenuation of virulence does not inhibit the ability to drive CD8+ T-cell responses using this approach. Although the increased IFNβ response may be directly responsible for viral clearance after infection, we speculate that modification of the cytokine milieu may also directly or indirectly affect the quality of the CD8+ T-cell response. Studies have shown that type I IFN responsiveness by CD8+ T cells can affect the quality of their effector function, responsiveness to recall antigens and antitumor activity.20,21 Further studies will address how increases in the secretion of IFNβ in response to infection can alter the CD8 T-cell response.
Although we were able to identify two insertion sites that were amenable to the production of attenuated live virus, each virus elaborated a unique cytokine response after infection. This suggests that a critical immune modulating function of the virus may be disrupted by insertion of CD8+ T-cell epitopes into the leader protein and suggests a potential mechanism for differential cytokine production. Previously, it had been shown that the leader sequence of TMEV was important for inhibiting immediate/early IFNα/β production after infection.9 This line of investigation went on to further determine that this activity was associated with a zinc finger motif at the amino terminus of the protein.10 Our modification sites are immediately after the cysteine at amino acid position 3, an amino acid that is necessary for the formation of the zinc binding motif22 and after amino acid position 54 of the TMEV genome. Although our virus constructs do not remove the necessary amino acid residues needed for binding zinc, the introduction of new sequences may interfere with its function as a modifier of IFNβ production, thus inhibiting the immune modulating capability of the leader protein. Of interest was the ability of the XhoI-OVA8 virus to induce expression of IFNβ after infection of human cells, suggesting that the ability of the virus to modulate human innate immunity may be unique compared with infection in mice. Consequently, future studies with aims toward clinical development may be more predictive if innate immune responses are assessed in human cell lines.
In addition to modulation of IFNα/β production after virus infection, we found that by engineering vaccine epitopes into the leader protein of TMEV, we could modulate IL-6 mRNA production after infection of primary fibroblasts. Previous studies have demonstrated that TLR3 responsiveness is necessary for driving proinflammatory cytokine responses after TMEV infection.23 This was primarily mediated through interaction with viral RNA and subsequent activation of NFκB, a transcription factor necessary for the production of IL-6 and other proinflammatory cytokines. Although all of our viruses elicited a detectable amount of IL-6 after infection, the induction of this cytokine by the p3-OVA8 virus varied by species, with an increase in IL-6 induction observed in human fibroblasts compared with mouse. Whether this is related to how the leader protein functions in human cells is not clear at this time. Previous studies using TMEV have identified an interdependence between NFκB activation and viral replication as well as proinflammatory induction,23–25 which would explain the IL-6 response to the wild-type virus infection. Although the precise mechanism for the lack of IL-6 production after infection with the p3-OVA8 virus in mouse cells is not known, one possibility is that increased type I IFN production inhibits viral replication and spread thereby decreasing the overall potential for activating NFκB and downstream proinflammatory cytokines. Although the precise mechanisms for modulation of cytokine responses to the engineered TMEV vaccines is not known, it is clear that these modifications can be used to modulate the immune response to infection in both human and mouse cells.
We found that the insertion of epitopes into the XhoI restriction site of TMEV renders infectious particles that are capable of driving antigen-specific T-cell responses; however, infection of C57BL/6 mice with the XhoI-OVA8 virus resulted in partial deletion of the vaccine epitope. Although this was a less prominent fraction of the amplified TMEV, it reveals the potential for virus instability and emergence of deletion mutants that may have a growth advantage in vivo. Several mechanisms have been identified as contributors to genomic variability in picornaviruses.26 However, the ability to delete short genomic segments seems to be predominantly through the use of short direct repeats that act as parting and anchoring sites and allow the deletion of intervening genomic segments.27 Although it is not known precisely how the deletion of TMEV genomic RNA occurs in our studies, the mechanism of deletion proposed by Pilepenko and colleagues is likely. Consequently, genomic virus constructs could be rationally designed to minimize these repeats thereby inhibiting the development of deletion mutants and enhancing stability and safety for use as immunotherapy.28
Several picornaviruses have been identified as potential treatments for human disease.1,6,29,30 These have reached clinical trials as tumor oncolytics for the treatment of melanoma, breast cancer, and prostate cancer (http://clinicaltrials.gov/show/NCT00636558) as well as glioma and small cell lung cancer.31,32 However, viruses within the cardiovirus genus have only recently been realized as potential therapeutics.6,30 Controversy surrounding whether cardioviruses infect humans and exist naturally in humans has recently been addressed with the identification of a broad group of TMEV-like viruses that do not clearly produce clinical illness.33 Although our findings use the murine cardiovirus TMEV, the sequence homology between TMEV and these human cardioviruses is extensive, suggesting that the principles identified here may be applicable to the development of human cardioviruses as therapeutics.
Seneca Valley virus has been developed and used as an oncolytic virus therapy for the treatment of small cell lung cancer and retinoblastoma.30,32 Although its origin is not known, it is believed to have originated in swine, suggesting that species barriers for infectivity and use as therapeutics for human disease may not be critical within this genus of picornavirus.34 TMEV was originally identified as a pathogenic virus in mice35 and no cases of clinical disease have been attributed to TMEV infection in humans despite being extensively investigated in open laboratories since its discovery over 70 years ago. However, this does not necessarily preclude it for use as an attenuated live vaccine because TMEV has been shown to infect several human cell lines in culture.23,24,36 Here, we have extended this finding to include human primary fibroblasts, further validating the potential for its use as a live attenuated vaccine. Finally, engineered TMEV may provide advantages over other human picornaviruses in that pre-existing antibodies that promote antibody-dependent enhancement of infection or that quickly neutralize virus may not be present to this murine virus.37 These findings may be valuable for the development of new variants of this virus that could be used in future immunotherapies.
The data in this report demonstrate the potential for using TMEV as immunotherapy that can be used in a safe and directed manner. This attenuated virus retains a limited ability to replicate in vivo and provides a therapy that can drive strong CD8+ T-cell immunity. Furthermore, this live virus vector promotes antitumor immunity that may be more potent than currently available therapies. The use of this safe and effective live virus vaccine lends promise to the future use of this and other cardioviruses as immunotherapy in clinical settings.
Materials and Methods
Mice, cell lines, and reagents. C57BL/6, BALB/cByJ, CB6F1/J, FVB/NJ, B6.129S7-Rag1tm1Mom/J, and C57BL/6-Tg(TcrαTcrβ)1100Mjb/J (OT-1) mice were purchased from Jackson Laboratory (Bar Harbor, ME). B6.SJL (CD45.1 congenic) mice were purchased from the National Cancer Institute mouse repository (Frederick, MD). Human CD45 and human SLAM transgenic mice deficient for the mouse IFNα/β receptor38,39 were kindly provided by Dr Roberto Cattaneo (Mayo Clinic, Rochester, MN). Mice were infected intracerebrally (2 × 104 plaque-forming units) or intraperitoneally (2 × 105 plaque-forming units) with wild-type or modified TMEV viruses. All animals were housed in the Mayo Clinic Department of Comparative Medicine and cared for according to institutional and NIH guidelines for animals use and care.
BHK, L929, P815, B16-OVA, and TUBO cell lines were maintained in DMEM (GIBCO Invitrogen, Grand Island, NY) containing 10% fetal bovine serum (GIBCO Invitrogen). Media for B16-OVA was supplemented with 10 mg/ml G418 (Life Technologies, Carlsbad, CA) to maintain expression of the ovalbumen transgene. Fibroblasts from C57BL/6 mice were generated as described previously.40 Human dermal fibroblasts were obtained from Lonza (Basel, Switzerland) and were maintained in RPMI (GIBCO) supplemented with 10% fetal bovine serum. MTT assays for cell death were performed on cells as described.41
PerCP-labeled anti-CD45 and allophycocyanin-labeled antimouse CD8 were purchased from BD Biosciences (San Diego, CA). PE-labeled tetramers for H-2Kd/p66, H-2Kd/RSV, H-2Kb/OVA257, and H-2Db/E749 were either purchased directly or custom manufactured by Beckman Coulter (Brea, CA). PE-labeled H-2Db/VP2121 tetramers were kindly provided by the NIH Tetramer Core at Emory University (Atlanta, GA).
Generation of TMEV expression plasmids and infectious virus. To generate the eurkaryotic expression vector for generating modified TMEV, we recloned the ClaI/XbaI fragment from the DAFL3 genomic clone for the Daniel's strain of TMEV18 into the eukaryotic expression vector pci-Neo (Promega, Madison, WI). Before cloning, we introduced a new ClaI site into the multiple cloning site of pci-Neo to facilitate direct ligation into the vector. This vector has a CMV promoter sequence that allows robust expression of insert fragments after transfection into eukaryotic cells. Modified TMEV vectors with epitope insertions were generated by using site-directed mutagenesis (QuikChange II Site-Directed Mutagenesis Kit; Agilent, Santa Clara, CA) to introduce the nucleotides 822–869 (Accession no. V00383.1) encoding amino acids 253 through 268 from chicken ovalbumin (AAB59956) and nucleotides 292–336 (NM_017003.2) encoding amino acids 63 through 77 from the rat Her2/neu oncogene (NP058699). All custom made oligonucleotides were manufactured by Integrated DNA Technologies (Carolville, IA). All virus encoding plasmids were verified by sequencing at the Mayo Clinic Advanced Genomics Technology Center.
To generate infectious virus, we transfected 30 µg of vector DNA that contained the wild-type or modified virus sequences into 107 BHK cells using electroporation. Media was changed on day 1 and 4 after transfection and cells were monitored daily for signs of cytopathic effect. By day 7, the generation of productive virus infection was noted by the presence of mostly rounded cells floating in media with few cells remaining adherent. Cells and supernatant were collected, sonicated, and spun to yield a clarified supernatant. Supernatants were titered by plaque assay before use in vaccine experiments. Viruses were tested for stability by serial passage on BHK monolayers. After seven passages on BHK monolayers, the p3-OVA8 and XhoI-OVA8 viruses maintained the modified insert and no wild-type virus genome was detected by reverse transcriptase PCR across the epitope insertion sites.
Quantitation of cytokines and virus. To determine the levels of viral RNA, we used real-time reverse transcriptase PCR as described previously.6 Briefly, RNA was isolated from cells or CNS homogenates using Trizol (Invitrogen, Carlsbad, CA), cDNA was generated using the Superscript III cDNA synthesis kit (Invitrogen) and reaction was set up according to the Fast SyBR Green Master Mix Kit (Applied Biosystems, Foster City, CA). We used the ΔΔCt method to calculate fold change over uninfected samples using actin as a reference.42 We used the following primer sets to analyze virus and cytokines: mouse and human actin (F-5′CTGGCACCACACCTTCTACAATGAGCTG and R-5′GCACAGCTTCTCTTTGATGTCACGCACGATTTC), viral protein 2 (VP2) of TMEV (F-5′TGGTCGACTCTGTGGTTACG and R-5′GCCGGTCTTGCAAAGATAGT), mouse IL-6 (F-5′CAAAGCCAGAGTCCTTCAGAGAGATACA and R-5′ AGGAGAGCATTGGAAATTGGGGTAGGAA), mouse IFNβ (F-5′ TGGGTGGAATGAGACTATTGTTGTAC and R-5′TGAGGACATCTCCCACGTCAATCTTT), and mouse IFNα (F-5′ CCCTCCTAGACTCATTCTGCA and R-5′CTCTGACCACCTCCCAGGCAC). The primers for IFNα amplified a conserved region that is shared between IFNα 1, 2, 5, 6, 7, 9, 11, 12, and 13. The primers used for amplification of human genes were: human IL-6 (F-5′GGTACATCCTCGACGGCATCT and R-5′GTGCCTCTTTGCTGCTTTCACAC) and human IFNβ (F-5′AGGATTCTGCATTACCTGAAGG and R-5′GGCTAGGAGATCTTCAGTTTCG).
To verify modified TMEV insert fidelity by reverse transcriptase PCR, we amplified sequences within the leader sequence between positions 963 and 1288 of the cloned DA strain of TMEV18 using the following primers: forward (5′GAGACACGTCGCAAGTGCTTTACACCTG) and reverse (5′GTTCCATGACAATATCGCGTACGAGCG). These primers amplified a 326 base pair fragment using the wild-type pci-DA virus and included the cloned insert sequence in modified TMEV strains. Amplified fragments were gel purified and sequenced to verify sequence identity.
Brains and spinal cord from mice infected with pci-DA, p3-OVA8, or XhoI-OVA8 were homogenized, sonicated, and clarified and tested for viral titer using plaque assays described previously.43
Lymphocyte analysis. CFSE staining and analysis of OT-1 cells was performed as described previously.15 Briefly, OT-1 CD8+ T cells were purified by magnetic column isolation using the mouse CD8a+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Congenic CD45.1 mice received 1 × 107 purified CD8+ T cells labeled with CFSE by intravenous transfer and were intraperitoneally infected with wild-type, p3-OVA8, or XhoI-OVA8 virus. After 48 hours, splenocytes were isolated and analyzed by flow cytometry for CD45.2, CD8, and CFSE content using FloJo Software (Tree Star, Ashland, OR).
To assess IFNγ responsiveness, CD8+ T cells were isolated from Balb/c splenocytes 14 days after infection with TMEV viruses. Cells were assessed by ELISPOT as described previously.19 The p66 peptide (TYVPANASL) and β-catenin peptide (SYLDSGIHS) were manufactured by Elim Biopharm (Hayward, CA).
Tumor experiments. B16-OVA tumor experiments were performed as described previously.6 To assess protection from TUBO outgrowth, we transferred 2 × 105 cells into the right flank of Balb/c mice that were given TMEV vaccines 7 days before cell transfer. We monitored tumor growth by measuring tumor in two dimensions. Tumor index was calculated by the following formula: (W × H)1/2.
Statistics. All data were analyzed by one-way or two-way analysis of variance. Multiple comparisons were performed using the Holm-Sidak method for normally distributed data and by Dunn's method for nonparametric data. Data with two groups were compared by Student's t-test. The Kaplan–Meier survival analysis was performed by the Gehan-Breslow method with pairwise comparisons analyzed by the Holm-Sidak method. Significance was determined by P < 0.05. All statistical analysis was performed using SigmaStat3.1 software (Systat Software, San Jose, CA).
Acknowledgments
This work was supported by grants from the National Institutes of Health (5R01CA104996-08) and the Department of Defense (W81XWH-12-1-1-0059, K.L.K. and L.K.). The authors declared no conflict of interest.
References
- Andino R, Silvera D, Suggett SD, Achacoso PL, Miller CJ, Baltimore D, et al. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994;265:1448–1451. doi: 10.1126/science.8073288. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Pagarigan R, Mena I, Feuer R, Whitton JL. Using recombinant coxsackievirus B3 to evaluate the induction and protective efficacy of CD8+ T cells during picornavirus infection. J Virol. 2001;75:2377–2387. doi: 10.1128/JVI.75.5.2377-2387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold GF, Resnick DA, Smith AD, Geisler SC, Holmes AK, Arnold E. Chimeric rhinoviruses as tools for vaccine development and characterization of protein epitopes. Intervirology. 1996;39:72–78. doi: 10.1159/000150477. [DOI] [PubMed] [Google Scholar]
- Altmeyer R, Escriou N, Girard M, Palmenberg A, van der Werf S. Attenuated Mengo virus as a vector for immunogenic human immunodeficiency virus type 1 glycoprotein 120. Proc Natl Acad Sci USA. 1994;91:9775–9779. doi: 10.1073/pnas.91.21.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sato S, Kim JI, Roos RP. Theiler'. s virus as a vector for foreign gene delivery. J Virol. 1995;69:3171–3175. doi: 10.1128/jvi.69.5.3171-3175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelko KD, Girtman MA, Mitsunaga Y, Mendez-Fernandez YV, Bell MP, Hansen MJ, et al. Theiler'. s murine encephalomyelitis virus as a vaccine candidate for immunotherapy. PLoS ONE. 2011;6:e20217. doi: 10.1371/journal.pone.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne AT, Dobrikova EY, Schmidt S, Gromeier M. Genetically stable picornavirus expression vectors with recombinant internal ribosomal entry sites. J Virol. 2002;76:8966–8972. doi: 10.1128/JVI.76.17.8966-8972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignuzzi M, Wendt E, Andino R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat Med. 2008;14:154–161. doi: 10.1038/nm1726. [DOI] [PubMed] [Google Scholar]
- van Pesch V, van Eyll O, Michiels T. The leader protein of Theiler'. s virus inhibits immediate-early alpha/beta interferon production. J Virol. 2001;75:7811–7817. doi: 10.1128/JVI.75.17.7811-7817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricour C, Delhaye S, Hato SV, Olenyik TD, Michel B, van Kuppeveld FJ, et al. Inhibition of mRNA export and dimerization of interferon regulatory factor 3 by Theiler'. s virus leader protein. J Gen Virol. 2009;90 Pt 1:177–186. doi: 10.1099/vir.0.005678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay A, Brahic M, Bureau JF. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler'. s virus. J Virol. 1994;68:4049–4052. doi: 10.1128/jvi.68.6.4049-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz J, David CS. Susceptibility to Theiler'. s virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Tonks P, Welsh CJ, Nash AA. The role of CD8+T cells in the acute and chronic phases of Theiler'. s murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992;73 (Pt 7):1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Mendez-Fernandez YV, Johnson AJ, Rodriguez M, Pease LR. Clearance of Theiler'. s virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur J Immunol. 2003;33:2501–2510. doi: 10.1002/eji.200324007. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, Njenga MK, Logan WC, Wettstein PJ. The balance between persistent virus infection and immune cells determines demyelination. J Immunol. 1996;157:5699–5709. [PubMed] [Google Scholar]
- Johnson AJ, Njenga MK, Hansen MJ, Kuhns ST, Chen L, Rodriguez M, et al. Prevalent class I-restricted T-cell response to the Theiler'. s virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J Virol. 1999;73:3702–3708. doi: 10.1128/jvi.73.5.3702-3708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calenoff MA, Badshah CS, Dal Canto MC, Lipton HL, Rundell MK. The leader polypeptide of Theiler'. s virus is essential for neurovirulence but not for virus growth in BHK cells. J Virol. 1995;69:5544–5549. doi: 10.1128/jvi.69.9.5544-5549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos RP, Stein S, Ohara Y, Fu JL, Semler BL. Infectious cDNA clones of the DA strain of Theiler'. s murine encephalomyelitis virus. J Virol. 1989;63:5492–5496. doi: 10.1128/jvi.63.12.5492-5496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–1334. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Mancheñ, o U, Riezu-Boj JI, Larraga A, Ochoa MC, Alignani D, et al. CD8 T cell priming in the presence of IFN-a renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J Immunol. 2012;189:3299–3310. doi: 10.4049/jimmunol.1102495. [DOI] [PubMed] [Google Scholar]
- Sikora AG, Jaffarzad N, Hailemichael Y, Gelbard A, Stonier SW, Schluns KS, et al. IFN-alpha enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Kong WP, Roos RP. The leader peptide of Theiler'. s murine encephalomyelitis virus is a zinc-binding protein. J Virol. 1995;69:8076–8078. doi: 10.1128/jvi.69.12.8076-8078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So EY, Kim BS. Theiler'. s virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia. 2009;57:1216–1226. doi: 10.1002/glia.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, So EY, Park H, Kim BS. Replication of Theiler'. s virus requires NF-kappa B-activation: higher viral replication and spreading in astrocytes from susceptible mice. Glia. 2008;56:942–953. doi: 10.1002/glia.20668. [DOI] [PubMed] [Google Scholar]
- Palma JP, Kwon D, Clipstone NA, Kim BS. Infection with Theiler'. s murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-kappaB activation: potential role for the initiation of demyelinating disease. J Virol. 2003;77:6322–6331. doi: 10.1128/JVI.77.11.6322-6331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agol VI. Molecular mechanisms of poliovirus variation and evolution. Curr Top Microbiol Immunol. 2006;299:211–259. doi: 10.1007/3-540-26397-7_8. [DOI] [PubMed] [Google Scholar]
- Pilipenko EV, Gmyl AP, Agol VI. A model for rearrangements in RNA genomes. Nucleic Acids Res. 1995;23:1870–1875. doi: 10.1093/nar/23.11.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Kim DY, Hyun BH, Bae YS. Novel design architecture for genetic stability of recombinant poliovirus: the manipulation of G/C contents and their distribution patterns increases the genetic stability of inserts in a poliovirus-based RPS-Vax vector system. J Virol. 2002;76:1649–1662. doi: 10.1128/JVI.76.4.1649-1662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Nam JH. Application of attenuated coxsackievirus B3 as a viral vector system for vaccines and gene therapy. Hum Vaccin. 2011;7:410–416. doi: 10.4161/hv.7.4.14422. [DOI] [PubMed] [Google Scholar]
- Wadhwa L, Hurwitz MY, Ché, vez-Barrios P, Hurwitz RL. Treatment of invasive retinoblastoma in a murine model using an oncolytic picornavirus. Cancer Res. 2007;67:10653–10656. doi: 10.1158/0008-5472.CAN-07-2352. [DOI] [PubMed] [Google Scholar]
- Goetz C, Dobrikova E, Shveygert M, Dobrikov M, Gromeier M. Oncolytic poliovirus against malignant glioma. Future Virol. 2011;6:1045–1058. doi: 10.2217/fvl.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Poirier JT, Senzer NN, Stephenson J, Jr, Loesch D, Burroughs KD, et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res. 2011;17:888–895. doi: 10.1158/1078-0432.CCR-10-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Greninger AL, Kanada K, Kwok T, Fischer KF, Runckel C, et al. Identification of cardioviruses related to Theiler'. s murine encephalomyelitis virus in human infections. Proc Natl Acad Sci USA. 2008;105:14124–14129. doi: 10.1073/pnas.0805968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers-Lalic D, Hoeben RC. Non-human viruses developed as therapeutic agent for use in humans. Rev Med Virol. 2011;21:227–239. doi: 10.1002/rmv.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. Spontaneous encephalomyelitis of mice–. a new virus disease. Science. 1934;80:122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- Stavrou S, Feng Z, Lemon SM, Roos RP. Different strains of Theiler'. s murine encephalomyelitis virus antagonize different sites in the type I interferon pathway. J Virol. 2010;84:9181–9189. doi: 10.1128/JVI.00603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman W, Martina BE, Rimmelzwaan GF, Gruters RA, Osterhaus AD. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27:505–512. doi: 10.1016/j.vaccine.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CS, Frenzke M, Leonard VH, Welstead GG, Richardson CD, Cattaneo R. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J Virol. 2010;84:3033–3042. doi: 10.1128/JVI.01559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rü, licke T, Volpe P, Buchholz CJ, Hourcade D, et al. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A. Establishment of fibroblast cultures. Curr Protoc Cell Biol. 2001;Chapter 2:Unit 2.1. doi: 10.1002/0471143030.cb0201s00. [DOI] [PubMed] [Google Scholar]
- Pavelko KD, Howe CL, Drescher KM, Gamez JD, Johnson AJ, Wei T, et al. Interleukin-6 protects anterior horn neurons from lethal virus-induced injury. J Neurosci. 2003;23:481–492. doi: 10.1523/JNEUROSCI.23-02-00481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz JL, Powell HC, Lampert PW. Neonatal infection with the Daniels strain of Theiler'. s murine encephalomyelitis virus. Lab Invest. 1983;49:672–679. [PubMed] [Google Scholar]