Abstract
Organisms that use ammonium as the sole nitrogen source discriminate between [15N] and [14N] ammonium. This selectivity leaves an isotopic signature in their biomass that depends on the external concentration of ammonium. To dissect how differences in discrimination arise molecularly, we examined a wild-type (WT) strain of Escherichia coli K12 and mutant strains with lesions affecting ammonium-assimilatory proteins. We used isotope ratio mass spectrometry (MS) to assess the nitrogen isotopic composition of cell material when the strains were grown in batch culture at either high or low external concentrations of NH3 (achieved by controlling total NH4Cl and pH of the medium). At high NH3 (≥0.89 µM), discrimination against the heavy isotope by the WT strain (−19.2‰) can be accounted for by the equilibrium isotope effect for dissociation of NH4+ to NH3 + H+. NH3 equilibrates across the cytoplasmic membrane, and glutamine synthetase does not manifest an isotope effect in vivo. At low NH3 (≤0.18 µM), discrimination reflects an isotope effect for the NH4+ channel AmtB (−14.1‰). By making E. coli dependent on the low-affinity ammonium-assimilatory pathway, we determined that biosynthetic glutamate dehydrogenase has an inverse isotope effect in vivo (+8.8‰). Likewise, by making unmediated diffusion of NH3 across the cytoplasmic membrane rate-limiting for cell growth in a mutant strain lacking AmtB, we could deduce an in vivo isotope effect for transport of NH3 across the membrane (−10.9‰). The paper presents the raw data from which our conclusions were drawn and discusses the assumptions underlying them.
Fractionation of heavy and light isotopes of nitrogen (15N vs. 14N), carbon (13C vs. 12C), and hydrogen (D vs. H) can provide information about metabolic pathways and reaction mechanisms within living organisms (1–4). For example, fractionation between 15N and 14N during incorporation of ammonium N in a single-celled organism like Escherichia coli is determined by the rate-limiting step for assimilating it into glutamate, the precursor of 88% of cellular nitrogen-containing material (5). All nitrogen assimilated into this central metabolic intermediate goes on to be incorporated into cell material. Transfers from glutamate to other molecules are direct. Although transfers from glutamine, including transfers to glutamate, involve deamidation, the NH3 released is carried directly to the assimilatory catalytic site through a tunnel and hence, cannot be protonated or diffused away (6). The overall ratio of 15N to 14N in biomass is, thus, controlled by steps at or before assimilation of ammonium N into glutamate.
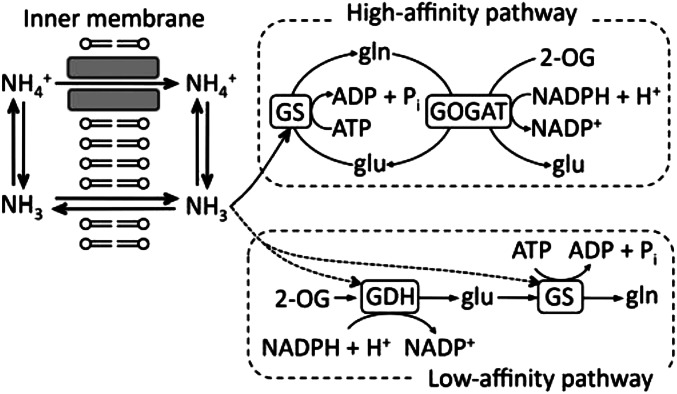
In E. coli, the proteins participating in early and potentially rate-determining steps in the incorporation of NH4+ into glutamate are (i) AmtB, its only membrane channel for NH4+ (7), (ii) glutamine synthetase (GS), the first enzyme of the high-affinity ammonium-assimilatory pathway, (iii) glutamate synthase [glutamine(amide) 2-oxoglutarate amino transferase (GOGAT)], and (iv) glutamate dehydrogenase (GDH), the first enzyme of the low-affinity ammonium-assimilatory pathway (Fig. 1) (reviewed in ref. 8). To study effects of these proteins on the in vivo fractionation of ammonium N, we used WT and genetic mutant strains in which one or more was lacking or defective and studied these strains at high or low external concentrations of NH3. To decrease the concentration of external NH3 and still achieve significant cell yield, we lowered both the total concentration of NH4Cl and the pH of the medium. Although E. coli lives in the human gut, which is nitrogen-rich, it also survives in fresh and brackish water, in which supplies of available ammonium can be limited (9, 10). Moreover, it acidifies its own environment by fermentation. Accordingly, the conditions that we have chosen to study the behavior of E. coli at low external NH3 are pertinent to its normal life cycle.
Fig. 1.
Schematic diagram of nitrogen assimilation from ammonium by E. coli K12.
Ammonium (NH4+ + NH3) is the optimal nitrogen source for E. coli (i.e., the source which yields most rapid growth). Ammonium (pKa = 9.25) enters cells in two forms (Fig. 1). NH3, which is ∼2% of total ammonium at pH 7.4, crosses the cell membrane by unmediated diffusion, a process that cannot be altered genetically. When the pH is decreased to 5.5, NH3 is only ∼0.02% of total ammonium. NH4+, which is the bulk of total ammonium at both pH 7.4 and pH 5.5, can enter the cells if and only if the AmtB channel is expressed and functional. Its expression is controlled at the transcriptional level and regulated largely by the free-pool concentration of glutamine in the cell interior (8), whereas its activity is controlled by the regulatory protein GlnK, largely in response to the free-pool concentration of the precursor metabolite 2-oxoglutarate, which is an intermediate in the tricarboxylic acid cycle (ref. 11 and references cited therein). Expression of AmtB increases as the glutamine concentration declines, and the channel is activated as the concentration of 2-oxoglutarate rises.
Within the cell, N is assimilated into glutamate, the organic precursor of most cellular nitrogen, by a high-affinity cycle and a low-affinity enzyme (Fig. 1). The high-affinity cycle is constituted by the exquisitely regulated GS and by GOGAT. The low-affinity enzyme is biosynthetic (NADPH-dependent) GDH (8). Use of 1 mol ATP per glutamate synthesized in the GS/GOGAT cycle drives assimilation of N even at extremely low concentrations of ammonium, but it is apparently detrimental when ammonium is abundant and energy is limiting (12). Both GS and GDH use NH3 as their substrate, because bond formation requires the lone pair of electrons on the N of NH3 (6, 13). Hence, NH4+ must be dissociated to NH3 + H+ before either enzyme can use it. An equilibrium isotope effect associated with this spontaneous process leads to depletion of 15N in NH3 relative to NH4+. Its magnitude is −19.2‰ (weighted mean, SE = 0.4‰, n = 3) (14). That is, at equilibrium, 15N/14N in NH3 is 19.2 parts per thousand lower than that ratio in NH4+.
To control the site of rate limitation, we used well-characterized mutant strains (Table 1). One such strain (ΔamtB) lacked AmtB. A second strain (AmtBΔC-term) had a poorly active AmtB channel, in which the normal membrane pores lacked the usual carboxyl-terminal cytoplasmic extensions (15, 16). A third strain (ΔgdhA::kan) lacked GDH, the low-affinity ammonium-assimilatory enzyme. The last two strains (gltD::kan and gltD::kanΔamtB) lacked GOGAT (Table 1) (8). We did not use a mutant lacking GS, because this strain is auxotrophic for glutamine and requires that glutamine be added to the medium in high concentrations, even in the presence of high concentrations of ammonium (17). We studied the mutant strains and their parental strain, which is a physiologically robust E. coli K12 WT (18, 19), under both ammonium-excess and -limiting conditions. We determined doubling time, cell yield, residual ammonium in the medium, and isotopic fractionation associated with incorporation of ammonium into cell material (Materials and Methods). When the absence or alteration of one protein increased the doubling time of the strain (i.e., decreased the growth rate), we could determine the rate-limiting step in transport or assimilation.
Table 1.
Bacterial strains
| Strain: genotype | Phenotype |
| NCM3722: E. coli K12 WT | WT |
| NCM4199: amtB, tesB::kan | AmtBΔC-term* |
| NCM4453: gltD::kan | GOGAT−† |
| NCM4454: ΔgdhA::kan | GDH−† |
| NCM4590: ΔamtB | AmtB− |
| NCM4701: gltD::kanΔamtB | GOGAT−, AmtB− |
All strains were constructed in the background of a physiologically robust E. coli K12 WT strain, NCM3722 (Materials and Methods) (18, 19).
Provided by Dalai Yan, Indiana University School of Medicine, Indianapolis.
Results
Parental WT Strain.
The doubling time was 50 min at all values of c0, the initial external concentration of ammonia (note, NH3 not NH3 + NH4+) (Fig. 2A and Table 2). For 0.89 ≤ c0 ≤ 280 µM, measured isotopic fractionations (εb) ranged from −16.1‰ to −23.8‰ (mean and SD; −19.2 ± 2.6‰, n = 7), with negative values of ε indicating depletion of 15N in the biomass relative to the dissolved inorganic N in the medium (Table 2). At lower concentrations (c0 ≤ 200 nM; i.e., at 1.0 or 0.5 mM total ammonium and pH 5.5), the isotopic fractionation decreased to −8.1‰ or −5.4‰, respectively (Fig. 2B and Table 2). AmtB is highly expressed, even when the concentration of external NH3 is 5–10 times higher, namely 1 μM (5 mM total ammonium at pH 5.5) (11); however, its activity is not needed for optimal growth, and it is inhibited by the regulatory protein GlnK (20–22). AmtB is not expressed when external NH3 is 10 μM (0.5 mM total ammonium at pH 7.4) or higher (11, 23).
Fig. 2.
(A) Growth and (B) determination of εb for assimilation of external ammonium (c0 = 0.089 µM NH3, 0.5 mM total NH4Cl, 0.1% glucose, pH 5.5) into biomass in WT (□) and AmtB− strains (△). Cultures in B were different from cultures in A, which were sampled more frequently.
Table 2.
Summary of cultures and measured isotopic fractionations
| Experiment | Strain* | c0 (NH3)† (μM) | DT‡ (min) | n§ | εb¶ (‰) |
| 17 | NCM3722 | 280 | 50 | 8 | −20.4 ± 2.1 |
| 23 | NCM3722 | 280 | 50 | 7 | −16.8 ± 1.9 |
| 16 | NCM3722 | 140 | 50 | 8 | −16.1 ± 0.7 |
| 22 | NCM3722 | 140 | 50 | 7 | −18.2 ± 1.1 |
| 8 | NCM3722 | 70 | 50 | 9 | −19.1 ± 1.4 |
| 10 | NCM3722 | 7 | 50 | 5 | −23.8 ± 1.4 |
| 5 | NCM3722 | 0.89 | 50 | 6 | −20.0 ± 1.0 |
| 26 | NCM3722 | 0.18 | 50 | 6 | −8.1 ± 0.3 |
| 2 | NCM3722 | 0.089 | 50 | 5 | −5.4 ± 0.3 |
| 9 | NCM4590 | 70 | 50 | 9 | −19.9 ± 1.7 |
| 11 | NCM4590 | 7 | 50 | 4 | −22.2 ± 2.9 |
| 3 | NCM4590 | 0.89 | 50 | 8 | −20.3 ± 0.5 |
| 27 | NCM4590 | 0.18 | >100 | 5 | −30.2 ± 0.7 |
| 1 | NCM4590 | 0.089 | >200 | 6 | −29.9 ± 0.9 |
| 18 | NCM4454 | 280 | 50 | 9 | −22.1 ± 0.7 |
| 21 | NCM4454 | 280 | 50 | 8 | −19.4 ± 2.2 |
| 19 | NCM4454 | 140 | 50 | 7 | −25.4 ± 2.9 |
| 20 | NCM4454 | 140 | 50 | 8 | −18.8 ± 0.5 |
| 15 | NCM4454 | 70 | 50 | 7 | −19.3 ± 1.4 |
| 14 | NCM4454 | 7 | 50 | 4 | −20.4 ± 1.4 |
| 6 | NCM4454 | 0.89 | 50 | 8 | −23.9 ± 0.4 |
| 7 | NCM4454 | 0.089 | 50 | 5 | −6.1 ± 0.6 |
| 13 | NCM4453 | 70 | 50 | 9 | −10.3 ± 1.0 |
| 12 | NCM4453 | 7 | 65 | 5 | −11.2 ± 0.4 |
| 29 | NCM4453 | 7 | 65 | 5 | −11.5 ± 0.5 |
| 28 | NCM4701 | 7 | 75 | 5 | −10.3 ± 0.1 |
| 24 | NCM4199 | 0.89 | 50 | 8 | −23.3 ± 1.2 |
| 25 | NCM4199 | 0.089 | 110 | 4 | −17.2 ± 0.6 |
| 30 | NCM4199 | 0.089 | 110 | 4 | −17.8 ± 0.4 |
Strain number (phenotypes listed in Table 1).
Concentrations of NH3 determined from total concentrations of NH4Cl, which were 71-fold higher than the concentrations of NH3 at pH 7.4 and 5,620-fold higher at pH 5.5. Cultures with c0 ≤ 0.89 were grown at pH 5.5.
Doubling time.
Number of points used in the determination of εb.
Reported uncertainty is the SE of the slope derived from the regression described in Calculations.
GDH− Strain.
The GDH− strain (ΔgdhA::kan) lacks the low-affinity pathway for ammonium assimilation and hence, is completely dependent on the high-affinity pathway for synthesis of glutamate and glutamine. Its doubling time was indistinguishable from that of WT under all conditions of nitrogen availability, and its isotopic fractionations were very similar to those of the WT strain (Table 2). For 0.89 ≤ c0 ≤ 280 µM, εb ranged from −18.8‰ to −25.4‰ (mean and SD; −21.3 ± 2.6‰, n = 7). For co = 89 nM, εb decreased to −6.1‰. Because the ranges of εb for cells having or lacking GDH overlap, these observations strongly support earlier reports that the GS/GOGAT cycle is the primary means for incorporating ammonium into biomass at all concentrations of NH3 (12, 24, 25).
GOGAT− Strains.
The GOGAT− strain (gltD::kan) lacks the high-affinity ammonium-assimilatory cycle and depends on the linear, low-affinity pathway (biosynthetic NADPH-dependent GDH) for synthesis of glutamate. GS converts ∼12% of the glutamate product of GDH to glutamine to meet biosynthetic needs. When initial external concentrations of NH3 were decreased from 70 to 7 μM, the doubling time of the strain increased from 50 to 65 min (Table 2 and Fig. S1A), and hence, the activity of GDH [E. coli has only a biosynthetic GDH (26)] was apparently rate-limiting for cell growth under these conditions. The weighted-mean isotopic fractionation was −11.2‰ (SE = 0.3‰, n = 3) at both concentrations of NH3 (Fig. S1D). This strain did not grow at all at c0 ≤ 1 μM (23).
In agreement with its dependence on the low-affinity enzyme GDH for synthesis of glutamate, gltD::kan has an abnormally low internal free-pool concentration of glutamate at low NH3 (27). The gltD::kan strain also has an unusually high free-pool concentration of glutamine (27–29), the primary metabolic indicator of nitrogen sufficiency and hence, the primary metabolic regulator of the transcriptional response to nitrogen availability (8, 27). This strain fails to express a number of proteins under the control of nitrogen regulatory protein C (NtrC), which is active at low internal concentrations of glutamine (8, 30).
Although we presumed that AmtB, which is one of the proteins controlled by NtrC, was poorly expressed (23, 31), we constructed a double-mutant strain (gltD::kanΔamtB) to be certain that AmtB was completely absent. The doubling time of the gltD::kanΔamtB strain was slightly longer than the doubling time of the gltD::kan strain, but N and C utilization rates (Fig. S1 B and C) and the isotopic fractionation was unchanged at −10.3‰ (Table 2 and Fig. S1D).
AmtB− and AmtB-Defective Strains.
Finally, the AmtB− strain (ΔamtB) lacks the NH4+ channel. For acquisition of ammonium, it must depend on unmediated diffusion of NH3 across the cytoplasmic membrane. At high external concentrations of NH3, both the doubling time of the ΔamtB strain and its isotopic fractionation were identical to those of the WT and the ΔgdhA strain (Table 2). In fact, under these conditions, the WT does not transcribe the glnKamtB operon (11, 23, 31). When the external concentration of NH3 was decreased to 1 μM, the ΔamtB strain grew very slowly; its doubling time was initially 200 min, and growth slowed even more as the strain consumed ammonium and thereby decreased the external NH3 concentration further (11, 23). The AmtB− strain also failed to consume all of the NH3 supplied in the media and it displayed a high C/N ratio in its biomass (see Supporting Information and Figs. S2 and S3). Under these conditions, the weighted-mean isotopic fractionation was −30.1‰ (SE = 0.6‰, n = 2), markedly larger than the value observed in other strains.
For the strain in which the AmtB protein was modified at the carboxyl terminal (AmtBΔC-term) (15, 16), both the doubling time and isotopic fractionation at c0 = 0.89 µM did not differ from those of the WT, GDH−, and AmtB− strains (Table 2). However, when c0 was decreased to 89 nM, AmtBΔC-term had a doubling time of 110 min, more than two times as long as that of the WT and approximately one-half as long as that of ΔamtB. Under this condition, the activity of AmtB seemed to be rate-limiting for growth, and the weighted-mean εb was −17.6‰ (SE = 0.3‰, n = 2).
Process-Related Summary of Isotopic Observations.
Table 2 includes 11 different observations of εb for cells equilibrating NH3 by diffusion and having both the low- and high-affinity pathways for its assimilation (Fig. 1). These observations include seven WT cultures with 0.89 ≤ c0 ≤ 280 µM, three ΔamtB cultures with 0.89 ≤ c0 ≤ 70 µM, and one AmtBΔC-term strain with c0 = 0.89 µM. Values of εb range from −16.1‰ to −23.8‰. The weighted mean is −19.6‰ (SE = 0.7‰). There were seven different observations for cells equilibrating NH3 by diffusion and lacking the low-affinity GDH pathway for its assimilation. For these ΔgdhA cultures, as for WT, 0.89 ≤ c0 ≤ 280 µM. Values of εb range from −18.8‰ to −25.4‰. The weighted mean is −22.2‰ (SE = 0.9‰). In total, there are 18 different observations of εb for cells equilibrating NH3 by diffusion and using the high-affinity GS/GOGAT cycle to incorporate ammonium N into organic molecules. Values of εb range from −16.1‰ to −25.4‰. The weighted mean is −21.1‰ (SE = 0.6). Variations of εb are not correlated with c0 (r2 = 0.13). Three values of εb were obtained for cells relying on the AmtB channel for transport of NH4+ and assimilating ammonium N by GS + GOGAT. In those cases (two WT cultures with c0 = 0.089 µM and c0 = 0.18 µM and one ΔgdhA culture with c0 = 0.089 µM), εb ranged from −5.4‰ to −8.1‰. In four cases, cells incorporated N using GS + GOGAT but had either no AmtB channel or an AmtB channel that was impaired by deletion of the C-terminal extensions. When AmtB was entirely absent (two cases, c0 = 0.18 µM and c0 = 0.089 µM), weighted-mean εb was −30.1‰. When AmtB was only modified (c0 = 0.089 µM; two cases), the weighted-mean εb was −17.6‰. Finally, when cells lacked GOGAT (three cultures of ΔgltD and one culture of ΔgltDamtB::kan), values of εb varied from −10.3‰ to −11.5‰ with a weighted mean of −10.5‰ (SE = 0.2‰).
Discussion
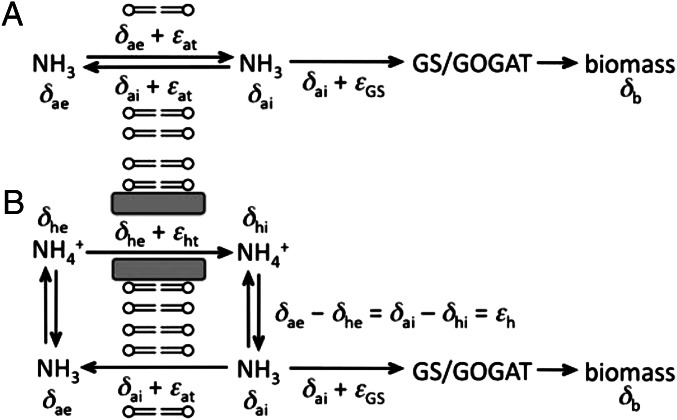
When external concentrations of NH3 exceed 0.18 µM, NH3 can rapidly equilibrate across the cytoplasmic membrane of many bacteria by unmediated diffusion, and NH4+ channels are not expressed (7, 11, 23, 32–34). The interior pool of NH3 (in equilibrium with intracellular NH4+) has only one input: diffusion of NH3 from the external medium. Although it has three potential outputs—assimilation of NH3 by GS, assimilation of NH3 by GDH, and leakage from the cell—our results are not significantly affected by the absence of GDH (Process-Related Summary of Isotopic Observations), which reduces the outputs to two. The isotopic budget is summarized in Fig. 3A. Balancing the input against the outputs, we can write
where the δ-values are defined in Fig. 3A, εat is the isotope effect associated with transport of NH3 across the membrane, and g is the fraction of the input that is incorporated into biomass. When equilibration of NH3 across the membrane is rapid in comparison to the rate of assimilation, g → 0 (n.b.; g is not the amount of NH3 that is assimilated but instead the fraction that is assimilated) and δae = δai. Because δb = δai + εGS, the isotopic composition of the external NH3 is related to the isotopic composition of the NH3 within the cells by δae = δb − εGS. Because essentially all external N is in the form of NH4+, δae = δe + εh, where δe is the measured isotopic composition of the N supplied to the medium and εh is the equilibrium isotope effect relating NH4+ and NH3. Finally, recalling that measured values of εb are equal to δb − δe, we obtain εGS = εb − εh. As noted above and summarized in Table 3, 18 experiments yielded εb ∼ −21.1‰. The 95% confidence interval of that value overlaps with the 95% confidence interval for εh. Accordingly, there is no evidence for fractionation by GS.
Fig. 3.
Flows of N across the cell membrane and within cells. Isotopic compositions are denoted by δ-terms, and isotope effects are denoted by ε-terms. In subscripts, a is NH3, b is biomass, e is external, h is NH4+ or protonation, i is internal, and t is transport. Isotopic compositions of the N fluxes are then given by expressions such as δhe + εht, which indicates the isotopic composition of the ammonium being transported by the AmtB channel. (A) Cells not using the AmtB channel to actively transport NH4+. (B) Cells in which the AmtB channel is used.
Table 3.
Process-related isotope effects
| Process | Related experiments | Result* |
| 1. Assimilation of NH3 by GS | WT, c0 ≥ 0.89 µM, n = 7 | 95% Confidence intervals: |
| For g → 0 | AmtB−, c0 ≥ 0.89 µM, n = 3 |
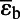 = −21.1 ± 1.3‰ = −21.1 ± 1.3‰ |
| εGS = εb − εh | AmtB∆C-term, c0 = 0.89 µM, n = 1 |
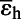 = −19.2 ± 1.7‰ = −19.2 ± 1.7‰ |
| GDH−, c0 ≥ 0.89 µM, n = 7 | εGS ∼ 0 | |
| 2. Transmembrane transport of NH3 | AmtB−, c0 < 0.2 µM, n = 2 |
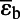 = −30.1 ± 0.5‰ = −30.1 ± 0.5‰ |
| For g → 1 | εat = −10.9 ± 0.7‰ | |
| εat = εb − εh | ||
| 3. Transport of NH4+ by AmtB | WT, c0 = 0.089 µM, n = 1 |
 = −5.5 ± 0.3‰ = −5.5 ± 0.3‰ |
| εht = εb + (1 − g)εat | GDH−, c0 = 0.089 µM, n = 1 | For g = 0.2 ± 0.05, εht = −14.1 ± 0.8‰ |
| 4. Transport of NH4+ by altered AmtB | AmtB∆C-term, c0 = 0.089 µM, n = 2 |
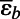 = −17.6 ± 0.3‰ = −17.6 ± 0.3‰ |
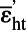 = εb + (1 − g)εat = εb + (1 − g)εat
|
For g = 0.5 ± 0.1, 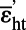 = −23.1 ± 1.2‰ = −23.1 ± 1.2‰ |
|
| 5. Assimilation of NH3 by GDH | GOGAT−, AmtB−, c0 = 7 µM, n = 1 |
 = −10.5 ± 0.2‰ = −10.5 ± 0.2‰ |
| εGDH = εb − εh | GOGAT−, c0 ≥ 7 µM, n = 3 | εGDH = 8.8 ± 0.4‰ |
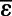 denotes weighted mean. Indicated uncertainties are SEs except for process 1, where 95%confidence intervals are specified.
denotes weighted mean. Indicated uncertainties are SEs except for process 1, where 95%confidence intervals are specified.
An alternative interpretation, with g appreciably greater than zero, is not tenable. Specifically, 18 experiments yielding εb ≅ εh could then be explained only if (i) g, εGS, and εat happened in all cases to have values satisfying the relationship g = εGS/(εGS − εat) and (ii) g was independent of the external concentration of NH3. Additionally, a well-documented experimental study of nitrogen and carbon isotope effects associated with glutamine synthetase from E. coli found a near-zero nitrogen isotope effect of −0.7 ± 0.6‰ (mean and SD, n = 7) (35).
It is unlikely that GS limits growth, because it is synthesized in excess when supplies of NH3 are plentiful; also, its catalytic activity is regulated downward by covalent modification (36–38). It is more likely that the flux of N into glutamate, the most plentiful intermediate in central nitrogen metabolism, is limited by the capacity of GOGAT. No isotopic fractionation is associated with GOGAT, because practically all of the amide N in gln is transferred to 2-oxoglutarate to produce glu.
When external NH3 concentrations are below 0.89 µM, E. coli K12 depends on the ammonium channel AmtB to maintain an optimal growth rate. Cells lacking this channel (ΔamtB) depend on uncatalyzed transport of NH3 across the membrane. At c0 ≤ 0.2 µM, growth of the ΔamtB strain is extremely slow, and the rate decreases as the external concentration of NH3 declines (Fig. 2A and Table 2). The mass balance described by Eq. 1 applies, but the conditions differ from the conditions just discussed. Instead, g ≠ 0, and because GS imposed no fractionation (even when supplies of NH3 were abundant), we know that δai = δb and εGS = 0. Making these substitutions and simplifying, we obtain δae = δb − gεat. Substituting δae = δe + εh and εb = δb − δe leads to εb = gεat + εh. At the limit in which growth is limited by transport of NH3 and g → 1, εat = εb − εh. If the slowest growing cultures (Table 2, experiments 1 and 27) represent that case, εat = −30.1 + 19.2 = −10.9 ± 0.7‰ (SE from combining 0.5 and 0.4 in quadrature). This relatively large value suggests that transport of NH3 across the membrane is limited by some process other than simple diffusion. Polar interactions within the membrane may play a role. Rishavy and Cleland (39) commented that the isotope effect in a related case “could easily be 2%” (i.e., 20‰; almost two times the value estimated here) (39).
For cells with a normal AmtB channel (WT or ΔgdhA strain) and c0 = 0.089 µM, εb was observed as low as −5.4 ± 0.3‰, much lower than the value of a strain lacking the channel (ΔamtB). The corresponding mass balance is shown schematically in Fig. 3B and expressed mathematically in Eq. 2:
Here, εht is the isotope effect associated with transport of NH4+ by the AmtB channel, and substitutions introduced above have been adopted where appropriate (δhe = δe, δai = δb). Simplifying gives εb = εht − (1 − g)εat. A recent quantitative study of AmtB function (11) indicates that g is ∼ 0.2. For εb = −5.4‰, adopting εat ∼ −10.9‰ (see above), we find εht = −14.1‰. If an uncertainty of 0.05 is assigned to g, the estimated SE of εht is 0.7‰. Notably, the reduced fractionation at low values of c0, a condition that may be encountered in nature, derives from not only fractionations associated with the AmtB channel but also, an interplay between fractionations associated with εht and εat.
When the WT strain was grown with a slightly higher c0 = 0.18 µM, the observed fractionation increased to −8.1‰. The AmtB channel also functions at this NH3 concentration, because an AmtB− strain continues to grow suboptimally. Hence, Eq. 2 applies. Using εht = −14.1 ± 0.7‰ and solving for g, we find g = 0.45 ± 0.1. When AmtB functions, the internal ammonium concentration is held constant, and hence, there is less leakage of NH3 at higher external NH3 concentrations (11).
For cells with an altered AmtB channel lacking the cytoplasmic C-terminal extensions (16), εb = −17.6 ± 0.3‰ (weighted mean and SE) at c0 = 0.089 μM. The fraction of N assimilated by GS is 0.5 (11), and solving as above yields 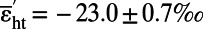 (where a prime is used to denote the altered channel). If the uncertainty assigned to g is doubled (to 0.1), the SE increases only to ±1.2‰. Hence, the isotope effect for the mutant AmtB channel is significantly larger than that for the WT channel. The mutant channel is known to lack coordination between the function of its individual monomers and have other unusual properties (40).
(where a prime is used to denote the altered channel). If the uncertainty assigned to g is doubled (to 0.1), the SE increases only to ±1.2‰. Hence, the isotope effect for the mutant AmtB channel is significantly larger than that for the WT channel. The mutant channel is known to lack coordination between the function of its individual monomers and have other unusual properties (40).
To make E. coli dependent on the low-affinity pathway for assimilation of ammonium, we inactivated GOGAT. This inactivation eliminates the GOGAT cycle and makes the organism dependent on biosynthetic GDH. At an external NH3 concentration of 10 μM, GDH activity already limits the growth rate of the GOGAT− strain, and the strain does not grow at all at c0 = 1 μM. The fractionation observed for the GOGAT− strains with c0 ≥ 7 µM is −10.5‰. Assuming that NH3 inside and outside the cell was in equilibrium, such as for the WT, ΔgdhA, and ΔamtB strains at the same concentrations, it follows that internal NH3 was depleted in 15N by 19.2‰ relative to external dissolved inorganic N and therefore, that the observation of εb = −10.5‰ requires inverse fractionation of 15N by GDH (i.e., enrichment of the product relative to the reactant) with εGDH = 8.7 ± 0.4‰. An inverse isotope effect has also been reported for bovine liver GDH (41).
Conclusion.
Our studies of E. coli K12 have yielded in vivo isotope effects as summarized in Table 3. To our knowledge, the isotope effect for transport of NH3 is the first for a biological membrane. A previous measurement was made in vitro with a membrane filter (14).
The equality of εb for the ΔghdA and WT strains at all external concentrations of NH3 confirms the finding of Yuan et al. (24, 25) that the GOGAT cycle is the major means for assimilation of NH3 by E. coli K12 at not only low but also, high concentrations. Although the GOGAT cycle is widespread in bacteria and archaea (8, 42, 43), whereas the occurrence of biosynthetic GDH seems to be more restricted (44), important examples of organisms naturally lacking GOGAT have recently come to light (45). Determining εb for the abundant ocean archaean Nitrosopumilis maritima will be particularly interesting, because it not only lacks GOGAT and depends on GDH for ammonium assimilation but also, oxidizes ammonium extracellularly as its primary energy source.
We hope that the present results will help future workers to correlate environmental genomic data with isotopic variations observed in nature.
Comparison with Earlier Work.
Studying the γ-proteobacterium Vibrio harveyi, a close evolutionary relative of E. coli K12, Hoch et al. (46) also found that isotopic fractionation between external ammonium and cell material varied with external ammonium availability. Now, by using AmtB− strains, we have determined that the decrease in εb from ∼ −20‰ to −4‰ that Hoch et al. (46) observed as external ammonium was dropped from an initial concentration of ∼500 to ∼25 μM (pH 7.4), and it did, as Hoch et al. (46) proposed, depend on the activity of an active ammonium channel (46). Specifically, εb ∼ −5‰ results from (i) acquisition of NH4+ rather than NH3 by the growing cells, (ii) an isotope effect associated with transport of NH4+ by the AmtB channel (εht ∼ −14‰), (iii) equilibration of NH4+ and NH3 inside the cell, (iv) an absence of isotopic fractionation during assimilation of NH3 by GS, and (v) leakage of 15N-depleted NH3 from the cells.
Given the very large εb characteristic of E. coli AmtB− strains at low external NH3 (−30‰), which seems to result from rate-limiting diffusion of NH3 across the cytoplasmic membrane, it is tempting to speculate that the fractionation observed in V. harveyi as the external concentration of ammonium decreased from 182 to 107 μM in a single experiment [εb = −26.5‰ (46)] may indicate the precise range of external ammonium concentrations at which unmediated diffusion of NH3 becomes limiting in WT V. harveyi just before activation of its AmtB channel. In E. coli, expression of AmtB occurs in response to a decrease in the internal free-pool concentration of glutamine, whereas activation requires, in addition, an increase in the pool concentration of its precursor metabolite 2-oxoglutarate (11). The latter occurs at lower external NH3 concentrations than the former. Activation requires release of the inhibitory gating protein GlnK (20–22).
Finally, we think that the decrease in εb from −21‰ to −14‰, which Hoch et al. (46) observed above 5 mM external ammonium, is an artifact of growth inhibition (doubling time increased from the optimal of 84 min to 138 min at high ammonium). Whatever the explanation, the εb of −14‰ cannot be characteristic of GDH as Hoch et al. (46) proposed, because the NADH-dependent GDH that they characterized is a catabolic enzyme (46). The genome sequence of V. harveyi is now available and it apparently lacks a biosynthetic, NADPH-dependent GDH. Moreover, we find that the biosynthetic, NADPH-dependent GDH of E. coli contributes very little to ammonium assimilation even at 20 mM ammonium, their highest concentration and our highest concentration.
Materials and Methods
Bacterial Strains and Cultures.
NCM3722 (18) was the parental strain for all genetic mutant strains used in this work (Table 1). Additional details of strain construction are in SI Materials and Methods. For growth experiments, bacterial cultures were grown on the minimal medium by Neidhardt et al. (47) in MOPS buffered medium, pH 7.4, with 0.1% glucose as sole carbon source and NH4Cl as sole nitrogen source. For experiments at pH 5.5, cultures were additionally adapted to low pH in minimal medium buffered with MES at pH 5.5. Growth and doubling time were determined by measuring OD at 420 nm.
Ammonia Assay.
Residual ammonium in cell-free supernatants was assayed in a GDH catalyzed reaction (AA0100 kit; Sigma). In the assay, 2-oxoglutarate is reduced to l-glutamate by GDH using ammonium as substrate and NADPH as the cofactor providing reducing equivalents. Oxidation of NADPH is measured by a change in absorbance at 340 nm.
Sample Preparation and Isotopic Analyses.
Bacterial cell samples were taken at various points during growth and removed from the supernatant by high-speed centrifugation. The cell-free supernatant was frozen at −80 °C for later measurement of residual ammonium, glucose, and final pH. The cells were washed two times in medium without additional glucose or ammonium and dried in air overnight. Uniform amounts of 2 mg dry weight, yielding 0.8 mg carbon and 0.2 mg nitrogen, were transferred into preweighed tin capsules (part number 240–053-00; Costech Analytical Technologies, Inc.). Capsules containing only the reactant glucose and ammonium chloride used in the media were also prepared. All samples were analyzed at the University of California at Berkeley Center for Stable Isotope Biogeochemistry. δ15N and %N were determined by using a Europa 2020 mass spectrometer coupled to an automatic elemental analyzer (48). The isotopic abundance parameters are defined as follows:
where 15R ≡ 15N/14N and the isotopic standard for nitrogen is N2 in air, for which 15R = 0.0036765 (49). Values of δ15N express the relative difference between the isotope ratio in the sample and the standard, expressed in parts per thousand (‰). A value of δ15N = −12.2‰, for example, indicates that 15Rsample is 0.0036316. The precision of the analyses, expressed as the SD of a single observation and based on five pairs of duplicates and four sets of triplicates collected during the analyses (thus, 13 degrees of freedom), is 0.06‰.
Calculations.
The objective of the isotopic analyses is to determine εb, the overall isotope effect associated with the assimilation of N. The isotope effect is most simply expressed by the isotopic difference between the starting pool of inorganic N in the medium and the first increment of biomass formed after inoculation. In mathematical terms, the isotopic difference is expressed as a ratio of isotope ratios. Because the isotope ratios are very similar, a notation is used that expresses the difference in terms of parts per thousand:
where 15Re0 is the ratio of 15N to 14N in the initial medium and 15Rb0 is the same ratio in the first increment of biomass. As growth proceeds, the measured isotopic compositions of both the medium and the biomass change as a result of preferential transfer of either 15N or 14N (depending on the sign of the isotope effect) from the medium to the biomass. Measurements of εb must take this change into account.
Here, we use the regression of δb on [f/(1 − f)] · lnf (1), thus fitting the observations to a linear equation of the form:
where δb is the measured δ15N of the biomass, δ0 is the measured δ15N of the medium at t = 0, and f is the fraction of ammonium unused. If, for example, εb = −18.8‰, it indicates that 15N is assimilated and used to produce biomass 18.8 parts per thousand more slowly than 14N.
Values of δ0 vary between experiments depending on the batch of NH4Cl that was used. Specific values are, for experiments 1–5, 3.25 ± 0.19‰ (mean and SD, n = 8); for experiments 6–15, 1.43 ± 0.06‰ (n = 3); for experiments 16 and 17, 1.12 ± 0.19‰ (n = 2); for experiments 18 and 19, 0.96 ± 0.19‰ (n = 2); for experiments 20 and 21, 1.15 ± 0.21‰ (n = 2); for experiments 22–24, 1.36 ± 0.09‰ (n = 3); and for experiments 25–29, 0.91 ± 0.08‰ (n = 5).
Uncertainties in εb, calculated from the variance about the regression and expressed as SEs of the slope, are reported in Table 2 and range from 0.1‰ to 2.9‰. Where weighted means are reported, the weighting factor is the inverse variance. The reported SEs of weighted means are conventional or dispersion-corrected, whichever is greater. Uncertainties reported for calculated isotope effects are derived by conventional propagation of errors.
Supplementary Material
Acknowledgments
We thank Stefania Mambelli, Paul Brooks, Todd Dawson, and the Center for Stable Isotope Biogeochemistry for contributing reagents and analytical tools. We also thank Kwang-Seo Kim, Dalai Yan, National BioResource Project-E.coli at NIG, and Fred Blattner for contributing bacterial strains. We thank Dalai Yan, Minsu Kim, and Terrence Hwa for their contributions to our understanding of nitrogen metabolism and its regulation. This work was supported by National Institutes of Health Grant GM38361 (to S.K.). J.M.H. thanks Woods Hole Oceanographic Institution for support as an emeritus scientist. Dedicated by S.K. to her former colleague, Wally van Heeswijk, who discovered GlnK, the world's most widespread regulatory protein and a key component of the system investigated in this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216683110/-/DCSupplemental.
References
- 1.Mariotti A, et al. Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes. Plant Soil. 1981;62(3):413–430. [Google Scholar]
- 2.Stanley SM. Relation of Phanerozoic stable isotope excursions to climate, bacterial metabolism, and major extinctions. Proc Natl Acad Sci USA. 2010;107(45):19185–19189. doi: 10.1073/pnas.1012833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valentine DL. Isotopic remembrance of metabolism past. Proc Natl Acad Sci USA. 2009;106(31):12565–12566. doi: 10.1073/pnas.0906428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Gillespie AL, Sessions AL. Large D/H variations in bacterial lipids reflect central metabolic pathways. Proc Natl Acad Sci USA. 2009;106(31):12580–12586. doi: 10.1073/pnas.0903030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wohlhueter R, Schutt H, Holzer H. In: The Enzymes of Glutamine Metabolism. Prusiner S, Stadtman ER, editors. New York: Academic; 1973. pp. 45–64. [Google Scholar]
- 6.Raushel FM, Thoden JB, Holden HM. Enzymes with molecular tunnels. Acc Chem Res. 2003;36(7):539–548. doi: 10.1021/ar020047k. [DOI] [PubMed] [Google Scholar]
- 7.Fong RN, Kim K-S, Yoshihara C, Inwood WB, Kustu S. The W148L substitution in the Escherichia coli ammonium channel AmtB increases flux and indicates that the substrate is an ion. Proc Natl Acad Sci USA. 2007;104(47):18706–18711. doi: 10.1073/pnas.0709267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda TP, Shauger AE, Kustu S. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol. 1996;259(4):589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 9.Hartl DL, Dykhuizen DE. The population genetics of Escherichia coli. Annu Rev Genet. 1984;18(1984):31–68. doi: 10.1146/annurev.ge.18.120184.000335. [DOI] [PubMed] [Google Scholar]
- 10.van Elsas JD, Semenov AV, Costa R, Trevors JT. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011;5(2):173–183. doi: 10.1038/ismej.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M, et al. Need-based activation of ammonium uptake in Escherichia coli. Mol Syst Biol. 2012;8(2012):616. doi: 10.1038/msb.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helling RB. Why does Escherichia coli have two primary pathways for synthesis of glutamate? J Bacteriol. 1994;176(15):4664–4668. doi: 10.1128/jb.176.15.4664-4668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman SC. In: The Enzymes of Glutamine Metabolism. Prusiner S, Stadtman ER, editors. New York: Academic; 1973. pp. 319–330. [Google Scholar]
- 14.Hermes JD, Weiss PM, Cleland WW. Use of nitrogen-15 and deuterium isotope effects to determine the chemical mechanism of phenylalanine ammonia-lyase. Biochemistry. 1985;24(12):2959–2967. doi: 10.1021/bi00333a023. [DOI] [PubMed] [Google Scholar]
- 15.Severi E, Javelle A, Merrick M. The conserved carboxy-terminal region of the ammonia channel AmtB plays a critical role in channel function. Mol Membr Biol. 2007;24(2):161–171. doi: 10.1080/09687860601129420. [DOI] [PubMed] [Google Scholar]
- 16.Inwood WB, Hall JA, Kim K-S, Fong R, Kustu S. Genetic evidence for an essential oscillation of transmembrane-spanning segment 5 in the Escherichia coli ammonium channel AmtB. Genetics. 2009;183(4):1341–1355. doi: 10.1534/genetics.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kustu SG, McFarland NC, Hui SP, Esmon B, Ames GF. Nitrogen control of Salmonella typhimurium: Co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons E, Freeling M, Kustu S, Inwood W. Using genomic sequencing for classical genetics in E. coli K12. PLoS One. 2011;6(2):e16717. doi: 10.1371/journal.pone.0016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soupene E, et al. Physiological studies of Escherichia coli strain MG1655: Growth defects and apparent cross-regulation of gene expression. J Bacteriol. 2003;185(18):5611–5626. doi: 10.1128/JB.185.18.5611-5626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Heeswijk WC, et al. An additional PII in Escherichia coli: A new regulatory protein in the glutamine synthetase cascade. FEMS Microbiol Lett. 1995;132(1–2):153–157. doi: 10.1111/j.1574-6968.1995.tb07825.x. [DOI] [PubMed] [Google Scholar]
- 21.Javelle A, Severi E, Thornton J, Merrick M. Ammonium sensing in Escherichia coli. Role of the ammonium transporter AmtB and AmtB-GlnK complex formation. J Biol Chem. 2004;279(10):8530–8538. doi: 10.1074/jbc.M312399200. [DOI] [PubMed] [Google Scholar]
- 22.Conroy MJ, et al. The crystal structure of the Escherichia coli AmtB-GlnK complex reveals how GlnK regulates the ammonia channel. Proc Natl Acad Sci USA. 2007;104(4):1213–1218. doi: 10.1073/pnas.0610348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soupene E, He L, Yan D, Kustu S. Ammonia acquisition in enteric bacteria: Physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc Natl Acad Sci USA. 1998;95(12):7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J, Bennett BD, Rabinowitz JD. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat Protoc. 2008;3(8):1328–1340. doi: 10.1038/nprot.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J, Fowler WU, Kimball E, Lu W, Rabinowitz JD. Kinetic flux profiling of nitrogen assimilation in Escherichia coli. Nat Chem Biol. 2006;2(10):529–530. doi: 10.1038/nchembio816. [DOI] [PubMed] [Google Scholar]
- 26.Blattner FR, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277(5331):1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 27.Csonka LN, Ikeda TP, Fletcher SA, Kustu S. The accumulation of glutamate is necessary for optimal growth of Salmonella typhimurium in media of high osmolality but not induction of the proU operon. J Bacteriol. 1994;176(20):6324–6333. doi: 10.1128/jb.176.20.6324-6333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan D, Ikeda TP, Shauger AE, Kustu S. Glutamate is required to maintain the steady-state potassium pool in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93(13):6527–6531. doi: 10.1073/pnas.93.13.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan D. Protection of the glutamate pool concentration in enteric bacteria. Proc Natl Acad Sci USA. 2007;104(22):9475–9480. doi: 10.1073/pnas.0703360104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahel G, Zelenetz AD, Tyler BM. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Heeswijk WC, et al. An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol Microbiol. 1996;21(1):133–146. doi: 10.1046/j.1365-2958.1996.6281349.x. [DOI] [PubMed] [Google Scholar]
- 32.Boussiba S, Dilling W, Gibson J. Methylammonium transport in Anacystis nidulans R-2. J Bacteriol. 1984;160(1):204–210. doi: 10.1128/jb.160.1.204-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrade SLA, Einsle O. The Amt/Mep/Rh family of ammonium transport proteins. Mol Membr Biol. 2007;24(5–6):357–365. doi: 10.1080/09687680701388423. [DOI] [PubMed] [Google Scholar]
- 34.Loqué D, von Wirén N. Regulatory levels for the transport of ammonium in plant roots. J Exp Bot. 2004;55(401):1293–1305. doi: 10.1093/jxb/erh147. [DOI] [PubMed] [Google Scholar]
- 35.Stoker PW. 1994. Heavy-atom isotope effects for enzymes of glutamine metabolism. PhD thesis (Univ of Nebraska, Lincoln, NE)
- 36.Jiang P, Ninfa AJ. A source of ultrasensitivity in the glutamine response of the bicyclic cascade system controlling glutamine synthetase adenylylation state and activity in Escherichia coli. Biochemistry. 2011;50(50):10929–10940. doi: 10.1021/bi201410x. [DOI] [PubMed] [Google Scholar]
- 37.Okano H, Hwa T, Lenz P, Yan D. Reversible adenylylation of glutamine synthetase is dynamically counterbalanced during steady-state growth of Escherichia coli. J Mol Biol. 2010;404(3):522–536. doi: 10.1016/j.jmb.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Rhee SG, Chock PB, Stadtman ER. Regulation of Escherichia coli glutamine synthetase. Adv Enzymol Relat Areas Mol Biol. 1989;62(1989):37–92. doi: 10.1002/9780470123089.ch2. [DOI] [PubMed] [Google Scholar]
- 39.Rishavy MA, Cleland WW. 13C, 15N, and 18O equilibrium isotope effects and fractionation factors. Can J Chem. 1999;77(5–6):967–977. [Google Scholar]
- 40.Inwood WB, et al. Epistatic effects of the protease/chaperone HflB on some damaged forms of the Escherichia coli ammonium channel AmtB. Genetics. 2009;183(4):1327–1340. doi: 10.1534/genetics.109.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss PM, Chen CY, Cleland WW, Cook PF. Use of primary deuterium and 15N isotope effects to deduce the relative rates of steps in the mechanisms of alanine and glutamate dehydrogenases. Biochemistry. 1988;27(13):4814–4822. doi: 10.1021/bi00413a035. [DOI] [PubMed] [Google Scholar]
- 42.Dincturk HB, Cunin R, Akce H. Expression and functional analysis of glutamate synthase small subunit-like proteins from archaeon Pyrococcus horikoshii. Microbiol Res. 2011;166(4):294–303. doi: 10.1016/j.micres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Dincturk HB, Knaff DB. The evolution of glutamate synthase. Mol Biol Rep. 2000;27(3):141–148. doi: 10.1023/a:1007107909619. [DOI] [PubMed] [Google Scholar]
- 44.Hudson RC, Daniel RM. L-glutamate dehydrogenases: Distribution, properties and mechanism. Comp Biochem Physiol B. 1993;106(4):767–792. doi: 10.1016/0305-0491(93)90031-y. [DOI] [PubMed] [Google Scholar]
- 45.Walker CB, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA. 2010;107(19):8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoch M, Fogel M, Kirchman D. Isotope fractionation associated with ammonium uptake by a marine bacterium. Limnol Oceanogr. 1992;37(7):1447–1459. [Google Scholar]
- 47.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks PD, Geilmann H, Werner RA, Brand WA. Improved precision of coupled delta13C and delta15N measurements from single samples using an elemental analyzer/isotope ratio mass spectrometer combination with a post-column six-port valve and selective CO2 trapping; improved halide robustness of the combustion reactor using CeO2. Rapid Commun Mass Spectrom. 2003;17(16):1924–1926. doi: 10.1002/rcm.1134. [DOI] [PubMed] [Google Scholar]
- 49.Coplen TB, Krouse HR, Bohlke JK. Reporting of nitrogen-isotope abundances. Pure Appl Chem. 1992;64(6):907–908. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.