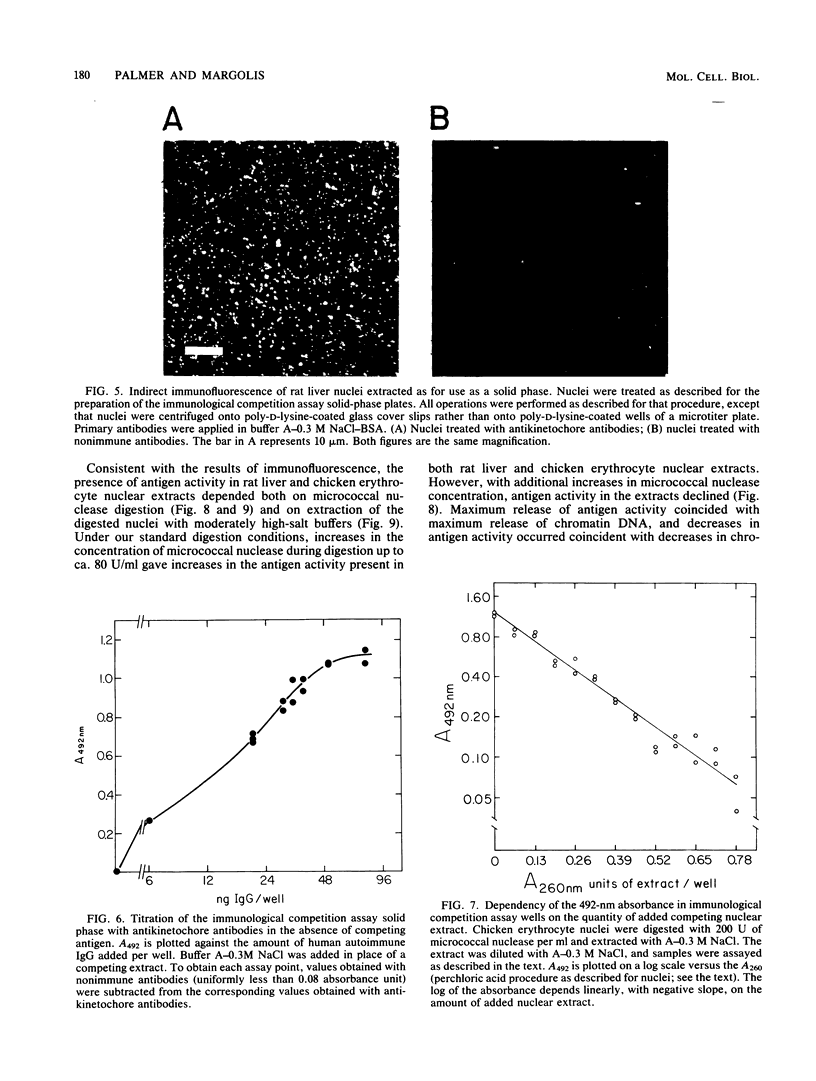
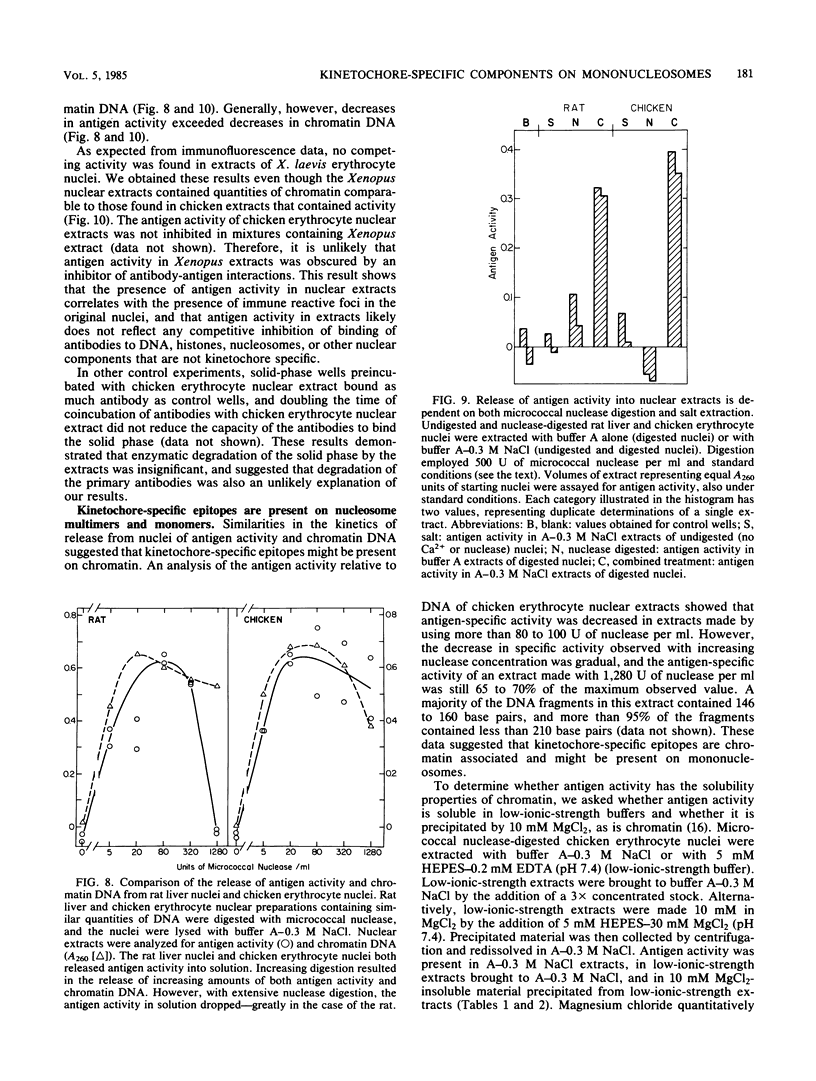
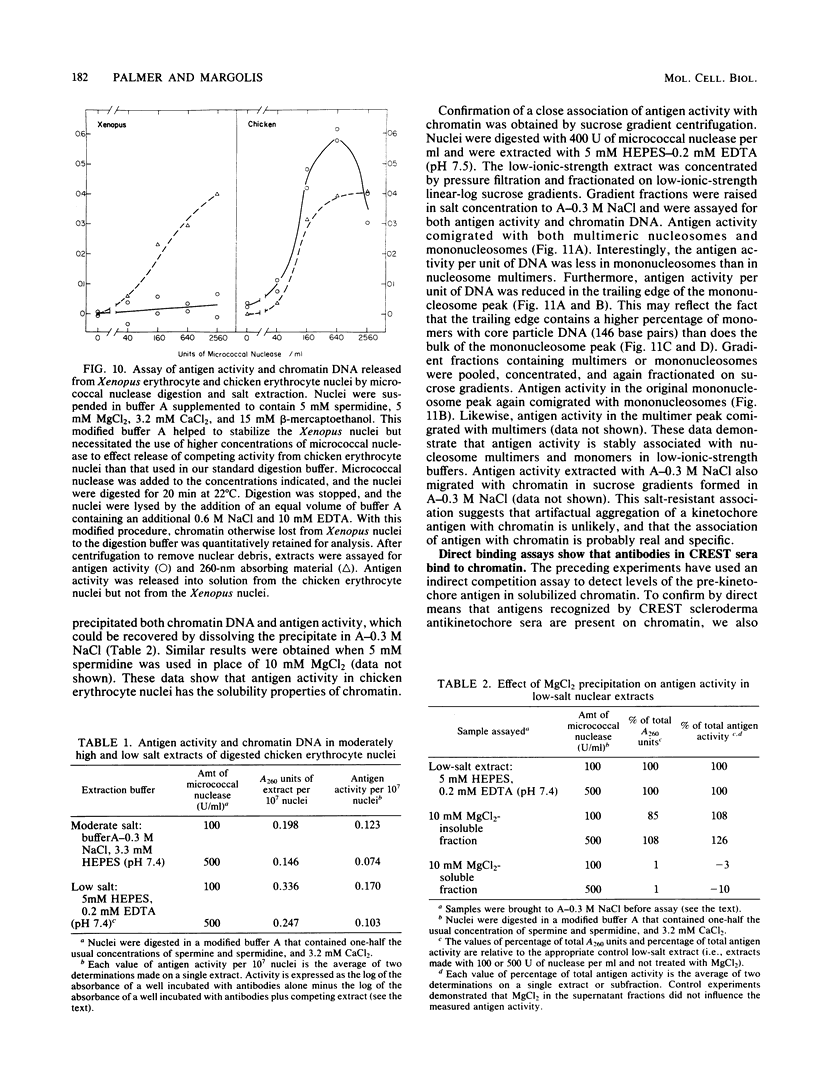
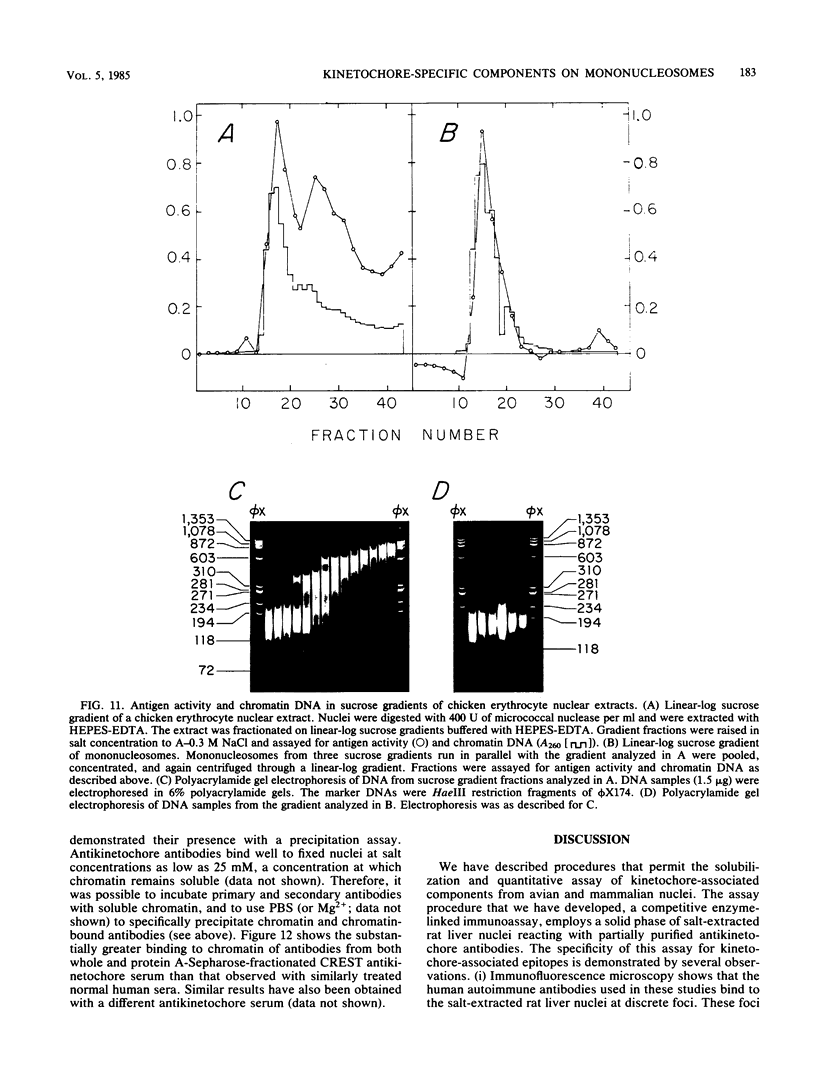
Abstract
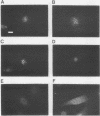
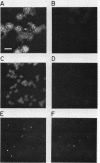
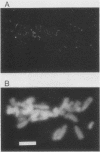
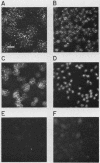
We have developed a competitive enzyme-linked immunosorbent assay for solubilized kinetochore components, using human CREST (calcinosis, Raynaud's phenomenon, esophageal dysfunction, sclerodactyly, telangiectasia) scleroderma autoimmune antibodies specific for these kinetochore elements. Using this quantitative assay, we found interphase persistent or "pre-kinetochore" components in low- and moderately high-salt (375 mM salt) extracts of micrococcal nuclease-digested rat liver and chicken erythrocyte nuclei. The release of antigen activity from nuclei under these conditions has been correlated with loss of pre-kinetochore foci as determined by immunofluorescence microscopy. Combined biochemical and competition assay analysis of chicken erythrocyte nuclear extracts indicates that pre-kinetochore components are tightly bound to chromatin of mononucleosome size. The conclusions based on competition assay data are supported by a direct binding assay, which confirms that antigens recognized by CREST sera are present on chromatin. These results raise the possibility that the kinetochore-specific chromosomal antigen(s) we have detected substitutes for "standard" mononucleosome components, such as histone H1. Furthermore, they suggest approaches to the isolation of kinetochore-specific DNA sequences from higher eucaryotes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S., Carbon J. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell. 1982 Jun;29(2):305–317. doi: 10.1016/0092-8674(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Dieden J. D., Kapp L. N., Sedat J. W. Rapid isolation of metaphase chromosomes containing high molecular weight DNA. J Cell Biol. 1979 Apr;81(1):255–259. doi: 10.1083/jcb.81.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal Biochem. 1970 Nov;38(1):56–64. doi: 10.1016/0003-2697(70)90155-7. [DOI] [PubMed] [Google Scholar]
- Brenner S., Pepper D., Berns M. W., Tan E., Brinkley B. R. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981 Oct;91(1):95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Cox S. M., Pepper D. A. Structure of the mitotic apparatus and chromosomes after hypotonic treatment of mammalian cells in vitro. Cytogenet Cell Genet. 1980;26(2-4):165–174. doi: 10.1159/000131438. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Schenk E. A., Olmsted J. B. Human anticentromere antibodies: distribution, characterization of antigens, and effect on microtubule organization. Cell. 1983 Nov;35(1):331–339. doi: 10.1016/0092-8674(83)90236-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan N., Cooke C., Rothfield N. The kinetochore is part of the metaphase chromosome scaffold. J Cell Biol. 1984 Jan;98(1):352–357. doi: 10.1083/jcb.98.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., McIntosh J. R. Structural polarity of kinetochore microtubules in PtK1 cells. J Cell Biol. 1981 May;89(2):338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler M. J., Kinsella T. D. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am J Med. 1980 Oct;69(4):520–526. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Heins J. N., Suriano J. R., Taniuchi H., Anfinsen C. B. Characterization of a nuclease produced by Staphylococcus aureus. J Biol Chem. 1967 Mar 10;242(5):1016–1020. [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. The subunit structure of chromatin: characteristics of nucleohistone and nucleoprotamine from developing trout testis. FEBS Lett. 1974 Nov 1;48(1):156–159. doi: 10.1016/0014-5793(74)81086-0. [DOI] [PubMed] [Google Scholar]
- Hozier J. C., Furcht L. T., Wendelshafer-Grabb G. Structure of human chromosomes visualized at the electron microscopic level. Chromosoma. 1981;82(1):55–64. doi: 10.1007/BF00285749. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Evidence for two levels of DNA folding in histone-depleted HeLa interphase nuclei. J Mol Biol. 1982 Apr 5;156(2):309–324. doi: 10.1016/0022-2836(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Laemmli U. K. Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell. 1982 May;29(1):171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Moroi Y., Hartman A. L., Nakane P. K., Tan E. M. Distribution of kinetochore (centromere) antigen in mammalian cell nuclei. J Cell Biol. 1981 Jul;90(1):254–259. doi: 10.1083/jcb.90.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R. B. Mitosis. Adv Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Palmer D., Snyder L. A., Blumenfeld M. Drosophila nucleosomes contain an unusual histone-like protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2671–2675. doi: 10.1073/pnas.77.5.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper D. A., Brinkley B. R. Tubulin nucleation and assembly in mitotic cells: evidence for nucleic acids in kinetochores and centrosomes. Cell Motil. 1980;1(1):1–15. doi: 10.1002/cm.970010102. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps J. D., Tippit D. H., Porter K. R. Rethinking mitosis. Cell. 1982 Jul;29(3):729–744. doi: 10.1016/0092-8674(82)90435-4. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Riley D., Weintraub H. Conservative segregation of parental histones during replication in the presence of cycloheximide. Proc Natl Acad Sci U S A. 1979 Jan;76(1):328–332. doi: 10.1073/pnas.76.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H., Witt P. L. Structure of the mammalian kinetochore. Chromosoma. 1981;82(2):153–170. doi: 10.1007/BF00286101. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Frazier G. R. Statistical analysis of radioligand assay data. Methods Enzymol. 1975;37:3–22. doi: 10.1016/s0076-6879(75)37003-1. [DOI] [PubMed] [Google Scholar]
- Rosok M. J., Rohrschneider L. R. Increased phosphorylation of vinculin on tyrosine does not occur during the release of stress fibers before mitosis in normal cells. Mol Cell Biol. 1983 Mar;3(3):475–479. doi: 10.1128/mcb.3.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Puigdomènech P., Eder G., Lurz R. Stability and reversibility of higher ordered structure of interphase chromatin: continuity of deoxyribonucleic acid is not required for maintenance of folded structure. Biochemistry. 1980 Jun 10;19(12):2544–2554. doi: 10.1021/bi00553a002. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Telzer B. R., Haimo L. T. Decoration of spindle microtubules with Dynein: evidence for uniform polarity. J Cell Biol. 1981 May;89(2):373–378. doi: 10.1083/jcb.89.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loghem E., Frangione B., Recht B., Franklin E. C. Staphylococcal protein A and human IgG subclasses and allotypes. Scand J Immunol. 1982 Mar;15(3):275–278. doi: 10.1111/j.1365-3083.1982.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Whittaker R. G., Blanchard B. J., Ingram V. M. A simple purification procedure for monomeric nucleosomes. Anal Biochem. 1979 Jan 15;92(2):420–425. doi: 10.1016/0003-2697(79)90680-8. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Turnbull D., Mullins J. M., McIntosh J. R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980 Apr;126(2):397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]