Abstract
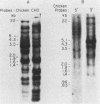
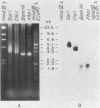
Chinese hamster ovary cells contain a complex family of ca. 16 unique alpha-tubulin sequences and a similar multiplicity of beta sequences. To examine which members of this multigene family are expressed, we constructed cDNA libraries from two Chinese hamster ovary cell lines according to the method of H. Okayama and P. Berg (Mol. Cell. Biol. 3:280-289, 1983). Each library consisted of 5.5 X 10(5) transformants and contained a high percentage of full-length tubulin clones. Three different alpha-tubulin genes were identified by sequence analysis of the 3' noncoding regions of these tubulin clones. The relative abundance of the transcripts corresponding to the three genes was estimated by gene-specific dot blotting of 96 cDNA alpha-tubulin clones and was found to be 71, 24, and 5%. There is little homology in the 3' noncoding sequences of these genes; however, a strong interspecies homology exists in this region for two of the Chinese hamster ovary genes with the two alpha-tubulin genes previously described in other systems. The third Chinese hamster ovary gene, with an expression frequency of 24%, is unique in that its 3' noncoding region is unlike that of the other mammalian alpha-tubulin genes. In addition, limited sequence data from the coding region of this gene indicates it codes for a unique alpha-tubulin protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandraki D., Ruderman J. V. Evolution of alpha q- and beta-tubulin genes as inferred by the nucleotide sequences of sea urchin cDNA clones. J Mol Evol. 1983;19(6):397–410. doi: 10.1007/BF02102315. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Tolson N., Ling V. The redistribution of fluoresceinated concanavalin A in Chinese hamster ovary cells and in their colcemid-resistant mutants. Exp Cell Res. 1980 Mar;126(1):75–85. doi: 10.1016/0014-4827(80)90472-3. [DOI] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Isolation of a taxol-resistant Chinese hamster ovary cell mutant that has an alteration in alpha-tubulin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4388–4391. doi: 10.1073/pnas.78.7.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Revertants of a Chinese hamster ovary cell mutant with an altered beta-tubulin: evidence that the altered tubulin confers both colcemid resistance and temperature sensitivity on the cell. Mol Cell Biol. 1982 Jun;2(6):720–729. doi: 10.1128/mcb.2.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Pittenger M. F., Feramisco J. R. Elevation of tubulin levels by microinjection suppresses new tubulin synthesis. Nature. 1983 Oct 20;305(5936):738–740. doi: 10.1038/305738a0. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Ling V. Microtubules in colcemid-resistant mutants of CHO cells. Exp Cell Res. 1981 Mar;132(1):147–155. doi: 10.1016/0014-4827(81)90091-4. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J., Dudley L. Tubulin isotypes and the multigene tubulin families. Int Rev Cytol. 1983;85:147–173. doi: 10.1016/s0074-7696(08)62372-4. [DOI] [PubMed] [Google Scholar]
- Elliott E. M., Ling V. Selection and characterization of Chinese hamster ovary cell mutants resistant to melphalan (L-phenylalanine mustard). Cancer Res. 1981 Feb;41(2):393–400. [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S. Resistance to the microtubule inhibitor podophyllotoxin: selection and partial characterization of mutants in CHO cells. Somatic Cell Genet. 1981 Jan;7(1):59–71. doi: 10.1007/BF01544748. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Payvar F., Spector D., Schimke R. T., Robinson H. L., Payne G. S., Bishop J. M., Varmus H. E. Heterogeneity of genetic loci in chickens: analysis of endogenous viral and nonviral genes by cleavage of DNA with restriction endonucleases. Cell. 1979 Oct;18(2):347–359. doi: 10.1016/0092-8674(79)90054-0. [DOI] [PubMed] [Google Scholar]
- Keates R. A., Sarangi F., Ling V. Structural and functional alterations in microtubule protein from Chinese hamster ovary cell mutants. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5638–5642. doi: 10.1073/pnas.78.9.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Ling V., Aubin J. E., Chase A., Sarangi F. Mutants of Chinese hamster ovary (CHO) cells with altered colcemid-binding affinity. Cell. 1979 Oct;18(2):423–430. doi: 10.1016/0092-8674(79)90061-8. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Measurement of DNA length by gel electrophoresis. Anal Biochem. 1979 Dec;100(2):319–323. doi: 10.1016/0003-2697(79)90235-5. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Warr J. R., Flanagan D. J., Anderson M. Mutants of Chinese hamster ovary cells with altered sensitivity to taxol and benzimidazole carbamates. Cell Biol Int Rep. 1982 May;6(5):455–460. doi: 10.1016/0309-1651(82)90117-5. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Chow L. T., Wefald F. C., Cowan N. J. Structure of two human alpha-tubulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(1):96–100. doi: 10.1073/pnas.79.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]