Abstract
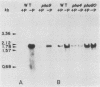
A DNA fragment bearing the PHO8 gene, which encodes repressible alkaline phosphatase of Saccharomyces cerevisiae, was cloned. Northern hybridizations with the PHO8 DNA as probe indicated that the PHO8 transcript is 1.8 kilobases in length and is more abundant in cells grown in low-phosphate medium than in high-phosphate medium. The pho9 mutant, whose phenotype is defective in the activity of repressible alkaline phosphatase, produced as much of the PHO8 transcript as did the PHO9+ cells. Hence, the PHO9 product should act at the post-transcriptional level. The pho4 mutant could not derepress the PHO8 transcript, whereas the pho80 mutant could, irrespective of the amount of Pi in the medium, as has been suggested by genetic study.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attias J., Bonnet J. L. A specific alkaline p-nitrophenylphosphatase activity from baker's yeast. Biochim Biophys Acta. 1972 May 12;268(2):422–430. doi: 10.1016/0005-2744(72)90338-5. [DOI] [PubMed] [Google Scholar]
- Attias J., Bonnet J. L., Sauvagnargues J. C. Séparation et étude partielle de deux phosphatases alcalines de levure. Biochim Biophys Acta. 1970 Aug 15;212(2):315–321. doi: 10.1016/0005-2744(70)90212-3. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Clark D. W., Tkacz J. S., Lampen J. O. Asparagine-linked carbohydrate does not determine the cellular location of yeast vacuolar nonspecific alkaline phosphatase. J Bacteriol. 1982 Nov;152(2):865–873. doi: 10.1128/jb.152.2.865-873.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J. A., Hu A. S. The separation and partial characterization of L-histidinol phosphatase and an alkaline phosphatase of Saccharomyces cerevisiae. J Biol Chem. 1969 Mar 25;244(6):1645–1650. [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W., Zubenko G. S., Parker R. R. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):665–677. doi: 10.1093/genetics/102.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Toh-e A., Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Onishi H. R., Tkacz J. S., Lampen J. O. Glycoprotein nature of yeast alkaline phosphatase. Formation of active enzyme in the presence of tunicamycin. J Biol Chem. 1979 Dec 10;254(23):11943–11952. [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To-E A., Ueda Y., Kakimoto S. I., Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Nakamura H., Oshima Y. A gene controlling the synthesis of non specific alkaline phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1976 Mar 25;428(1):182–192. doi: 10.1016/0304-4165(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Inouye S., Oshima Y. Structure and function of the PHO82-pho4 locus controlling the synthesis of repressible acid phosphatase of Saccharomyces cerevisiae. J Bacteriol. 1981 Jan;145(1):221–232. doi: 10.1128/jb.145.1.221-232.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Kaneko Y., Akimaru J., Oshima Y. An insertion mutation associated with constitutive expression of repressible acid phosphatase in Saccharomyces cerevisiae. Mol Gen Genet. 1983;191(3):339–346. doi: 10.1007/BF00425743. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Tada S., Oshima Y. 2-micrometers DNA-like plasmids in the osmophilic haploid yeast Saccharomyces rouxii. J Bacteriol. 1982 Sep;151(3):1380–1390. doi: 10.1128/jb.151.3.1380-1390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Mutations in PEP4 locus of Saccharomyces cerevisiae block final step in maturation of two vacuolar hydrolases. Proc Natl Acad Sci U S A. 1983 Jan;80(2):510–514. doi: 10.1073/pnas.80.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]