Abstract
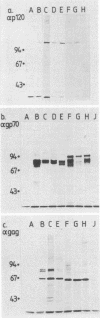
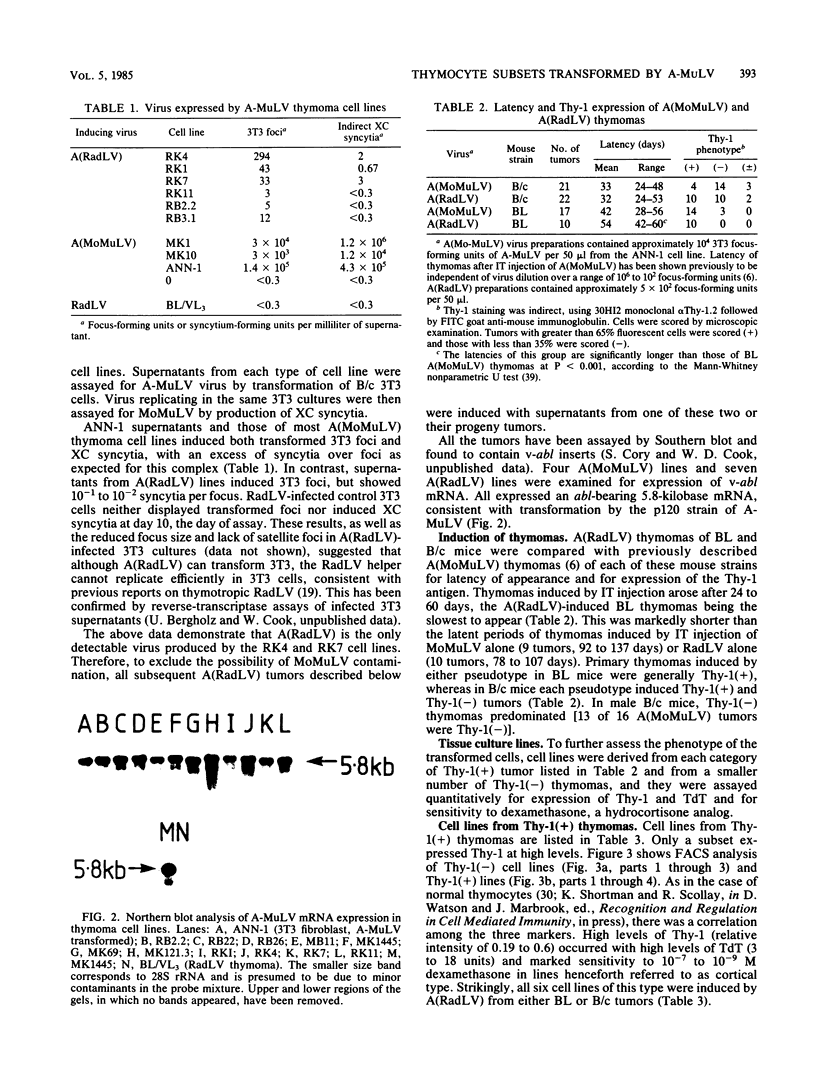
The infectious complex of Abelson murine leukemia virus was altered by replacing its usual helper virus, Moloney leukemia virus, with radiation leukemia virus (RadLV). After intrathymic injection of the Abelson-RadLV complex, thymomas arose rapidly, as described previously for injection of the Abelson-Moloney complex. Cell lines were derived from thymomas induced by each Abelson virus complex and were classified according to normal thymus cell phenotypes. Each virus complex induced some cell lines which were like a 0.7% subpopulation of murine thymocytes in that they failed to express the Thy-1 cell-surface antigen. These lines are thus far indistinguishable from some Abelson-derived bone marrow transformants classified as pre-B cells. However, the Abelson-Moloney complex induced some cell lines which expressed low levels of Thy-1 and which shared most markers with immature blast cells of the thymic medulla, whereas the Abelson-RadLV complex induced some lines which were clearly like thymic cortex blast cells. Thus, Abelson virus can induce thymoma cell lines of at least two, and possibly three, distinct phenotypes corresponding to normal thymocyte blast subsets, the determination of which can be influenced by helper virus sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Barton R., Goldschneider I., Bollum F. J. The distribution of terminal deoxynucleotidyl transferase (TdT) among subsets of thymocytes in the rat. J Immunol. 1976 Feb;116(2):462–468. [PubMed] [Google Scholar]
- Boniver J., Declève A., Finn O. J., Honsik C., Lieberman M., Kaplan H. S. Detection of infectious centers in C57BL/Ka lymphoid cell populations infected in vitro by the radiation leukemia virus. Cancer Res. 1980 Mar;40(3):544–549. [PubMed] [Google Scholar]
- Boss M., Greaves M., Teich N. Abelson virus-transformed haematopoietic cell lines with pre-B-cell characteristics. Nature. 1979 Apr 5;278(5704):551–553. doi: 10.1038/278551a0. [DOI] [PubMed] [Google Scholar]
- Cook W. D. Rapid thymomas induced by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2917–2921. doi: 10.1073/pnas.79.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Copper G. M. Transformation by subgenomic fragments of Rous sarcoma virus DNA. Cell. 1980 Apr;19(4):863–870. doi: 10.1016/0092-8674(80)90077-x. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Hines D. L., Gragowski L. Abelson murine leukemia virus: effect of helper virus from regressing Friend virus on leukemia development. Leuk Res. 1983;7(2):251–260. doi: 10.1016/0145-2126(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Hogarth P. M., Crewther P. E., McKenzie I. F. Description of a Qa-2 like alloantigen (Qa-m2). Eur J Immunol. 1982 May;12(5):374–379. doi: 10.1002/eji.1830120504. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Rosenberg N., Cotellessa A., Baltimore D. Leukemogenicity of clonal isolates of murine leukemia viruses. J Natl Cancer Inst. 1978 Jun;60(6):1473–1476. doi: 10.1093/jnci/60.6.1473. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S., Lieberman M. The role of lymphoid and haematopoietic target cells in viral lymphomagenesis of C57BL/Ka mice. II. Neoplastic transformation of bone marrow-derived cells in the thymic microenvironment. Nouv Rev Fr Hematol Blood Cells. 1976;17(1-2):301–317. doi: 10.1007/978-3-642-66312-3_21. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Manteuil-Brutlag S., Liu S. L., Kaplan H. S. Radiation leukemia virus contains two distinct viral RNAs. Cell. 1980 Mar;19(3):643–652. doi: 10.1016/s0092-8674(80)80041-9. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., Strominger J., Parkman R., Kaplan D., Schwaber J., Rosenberg N., Scher C. D. Abelson virus-transformed lymphocytes: null cells that modulate H-2. Cell. 1977 Nov;12(3):683–690. doi: 10.1016/0092-8674(77)90268-9. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. Abelson leukemia virus. Curr Top Microbiol Immunol. 1982;101:95–126. doi: 10.1007/978-3-642-68654-2_5. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983 Apr;130(4):1627–1633. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scher C. D. Effect of pseudotype on Abelson virus and Kirsten sarcoma virus-induced leukemia. J Exp Med. 1978 Apr 1;147(4):1044–1053. doi: 10.1084/jem.147.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Scollay R., Shortman K. Thymocyte subpopulations: an experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 1983 Sep;5(5-6):245–295. [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Simon I., Löhler J., Jaenisch R. Virus-specific transcription and translation in organs of BALB/Mo mice: comparative study using quantitative hybridization, in situ hybridization, and immunocytochemistry. Virology. 1982 Jul 15;120(1):106–121. doi: 10.1016/0042-6822(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Molecular and cellular biology of Abelson virus transformation. Curr Top Microbiol Immunol. 1983;103:127–146. doi: 10.1007/978-3-642-68943-7_6. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]