Abstract
Context:
Seafood long-chain polyunsaturated omega-3 fatty acids (n-3 PUFAs) improve insulin sensitivity in animal experiments, but findings remain inconsistent in humans. Adiponectin is a robust marker for insulin sensitivity and adipocyte function. Whether n-3 PUFAs affect adiponectin in humans is unknown.
Objective:
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, the objective of the study was to perform a systematic review and meta-analysis of randomized, placebo-controlled clinical trials (RCTs) to determine the effect of n-3 PUFA consumption on circulating adiponectin in humans.
Data Sources:
MEDLINE, EMBASE, CABI (CAB abstracts), Cochrane Central Registry of Controlled Trials, ClinicalTrials.gov, SIGLE, and Faculty of 1000 were searched through to June 2012, supplemented with author contact and reference list searches.
Study Selection:
RCTs of either fish oil supplementation or isocaloric fish meal feeding that evaluated adiponectin as an outcome were selected for the study.
Data Extraction:
Two investigators independently extracted the data. Effect estimates were pooled using inverse-variance weighted, random-effects meta-analysis. Heterogeneity was assessed by the I2 and Q statistic. Prespecified sources of heterogeneity were investigated by meta-regression. Publication bias was assessed using funnel plots and Egger's test.
Data Synthesis:
Of 110 studies, 14 RCTs met inclusion criteria. Fourteen trial arms evaluated fish oil (fish oil, n = 682; placebo, n = 641). Fish oil increased adiponectin by 0.37 μg/mL [95% confidence interval (CI) 0.07; 0.67, P = .02]. Although effects in 11 of 14 trials were 0 or greater, statistical heterogeneity was evident (I2 = 72.9%), unexplained by n-3 PUFA dose or duration, study quality score, study location, or baseline body mass index (meta-regression P > .05 each). The funnel plot was asymmetric in favor of smaller trials with greater effects (Egger's P = .11); the fill-and-trim method suggested a theoretical pooled effect of 0.18 μg/mL (95% CI −0.15; +0.52, P = .28). Only 2 trial arms evaluated fish feeding (n = 136 intervention and 68 control subjects), for which the pooled effect on adiponectin was not statistically significant (−0.01μg/mL, 95% CI −0.65; 0.64, P = 0.99), although CIs were broad due to the small number of subjects.
Conclusions:
In placebo-controlled RCTs, fish oil moderately increases circulating adiponectin, although with unexplained heterogeneity as well as potential publication bias. These findings provide no evidence for harm and support possible benefits of n-3 PUFA consumption on insulin sensitivity and adipocyte function.
Increased consumption of seafood long-chain polyunsaturated omega-3 fatty acids (n-3 PUFAs) improves insulin sensitivity in animal studies (1–3). However, evidence for the effects of n-3 PUFA on insulin sensitivity in human subjects remains limited and inconclusive (4). The potential molecular mechanisms that may link n-3 PUFA intake and altered insulin sensitivity in humans also remain unclear.
Adiponectin is a protein produced by adipocyte cells that possess potent insulin-sensitizing and antiinflammatory properties. Adiponectin protects against development of type 2 diabetes and atherosclerosis in animal models (5), and higher circulating levels of adiponectin are associated with a lower risk of type 2 diabetes and coronary heart disease in prospective cohort studies (6, 7). In vitro and animal studies demonstrate that n-3 PUFA consumption increases the levels of these fatty acids in adipocytes and protects adipose tissue against inflammation and insulin resistance (8). Animal experimental studies have also consistently found that n-3 PUFA intake increases circulating levels of adiponectin (1–3).
Whether consumption of n-3 PUFAs affects circulating adiponectin in humans is not established. Such an effect would elucidate the biological pathways of effects of n-3 PUFAs on adipocyte function and insulin sensitivity. To address this gap in knowledge, we performed a systematic review and meta-analysis of randomized placebo-controlled clinical trials to determine the effect of n-3 PUFA intake on circulating adiponectin in humans.
Materials and Methods
We conducted this systematic review and meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines during all stages of design, implementation, analysis, and reporting (9). The prespecified study protocol is available from the authors upon request.
Search strategy and eligibility criteria
We searched multiple electronic databases from the earliest available online indexing year through June 2012, without language restrictions, including MEDLINE, EMBASE, CABI (CAB abstracts), Cochrane Central Registry of Controlled Trials, ClinicalTrials.gov, SIGLE, and Faculty of 1000. Key words included, among others, fatty acids, omega-3, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), fish oils, adiponectin, and clinical trial; the full search terms are available on request. Additional studies were identified through searching of electronically linked related articles on MEDLINE and hand searching of citation lists of full-text articles selected for review.
Studies were included if they were randomized placebo-controlled trials of oral fish oil or isocaloric fish meal feeding that evaluated adiponectin as an outcome. Studies were excluded if in pregnant women or if they had a concomitant intervention for which effects could not be separated (ie, factorial design studies could be included); were of less than 2 weeks' duration; or were observational, commentaries, reviews, case reports, nonrandomized studies, or duplicate publication from the same study.
Study selection and data extraction
One investigator (J.W.) screened the title and abstracts of all identified articles for eligibility. The full-text of remaining articles was subsequently assessed independently in duplicates by 2 investigators (J.W. and L.C.) to determine inclusion or exclusion. Discrepancies were resolved by consensus.
For studies that met inclusion criteria, data were extracted independently in duplicate by 2 investigators (J.W. and L.C.), using a standardized electronic form. Extracted data included author, publication year, study location (North America, Europe, and others), population characteristics (age, gender, ethnicity, and comorbidities), and study design characteristics including randomization method, blinding of investigators and participants, parallel or crossover design, washout period if crossover, dose of n-3 PUFA, content of placebo, duration of intervention, percentage of dropout, method of adiponectin measurement, and type of adiponectin measured. We extracted the mean concentration and error (SD, SE, or confidence intervals) of circulating adiponectin at study baseline, posttreatment, and/or change between baseline and posttreatment. Study quality was assessed using the instrument of Jadad et al (10), which incorporated 0 or 1 point for each of the following 5 criteria: 1) presence of randomization, 2) appropriate method of randomization, 3) presence of double blinding, 4) appropriate method of double blinding, and 5) description of withdrawals and dropouts. Trials were considered high quality if they obtained a score of 3 or higher. We contacted study authors to obtain relevant missing information and received responses for 7 of 15 studies contacted.
Statistical analysis
The primary end point was change in circulating adiponectin due to fish oil supplementation (or fish feeding). For crossover studies, we compared end-treatment circulating adiponectin in the control vs fish oil period. For parallel-design trials, we compared end-treatment changes in adiponectin in the control vs fish oil groups. Within-person changes were used when possible; otherwise, we used group means. The SD was extracted, or if not reported, calculated from SEs. Within-person changes and SD were available and used for 10 of the studies. For the remaining 4 studies, group means and SDs were used to calculate the change in adiponectin (11), and the SD of the change was imputed (12, 13), using a within-person correlation of 0.93 (based on observed serial measured of adiponectin taken 1 year apart (personal communication, Rimm E., April 2012). We performed sensitivity analyses assuming a lower within-person correlation of 0.50. We calculated the variance of the difference in mean change in adiponectin between fish oil and the placebo group, using the following equation: (SD of change in adiponectin2fish oil group/nfish oil group) + (SD of change in adiponectin2control group/ncontrol group). Details of the methods used for imputation of SD and calculation of treatment variance are given in Online Supplemental Materials, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Pooled effects were estimated using the inverse-variance weighted, random effects meta-analysis of DerSimonian and Laird (14). The I2 statistic and Q statistic were used to assess heterogeneity (15). Prespecified potential sources of heterogeneity were evaluated in stratified meta-analyses, with meta-regression examining whether effect estimates were significantly different. These included study location (Europe/North America vs others), duration of treatment (less than median; or equal to or greater than median of all studies), blinding (double vs single), major fatty acid of the placebo (linoleic acid vs oleic acid vs others), dose of n-3 PUFA (EPA + DHA, less than median; or equal to or greater than median of all studies), study quality score (low, 0–2; or high, 3–5), age (less than median; or equal to or greater than median of all studies), body mass index (BMI; less than median; or equal to or greater than median of all studies), and population (adults vs children/adolescents). We also assessed the duration of treatment (weeks), the dose of n-3 PUFA (grams per day), the quality score, age (years), BMI (kilograms per square meter), and gender (percentage male) as continuous covariates in the meta-regression analysis. Publication bias was assessed by visual inspection of funnel plots and statistically using Egger's and Begg's tests. We used the nonparametric trim-and-fill method to assess the potential influence of publication bias in sensitivity analyses and provide a theoretical pooled estimate accounting for estimated missing studies (16). All analyses were performed using STATA 10.0 (StataCorp, College Station, Texas), with a 2-sided alpha = .05.
Results
Search Results
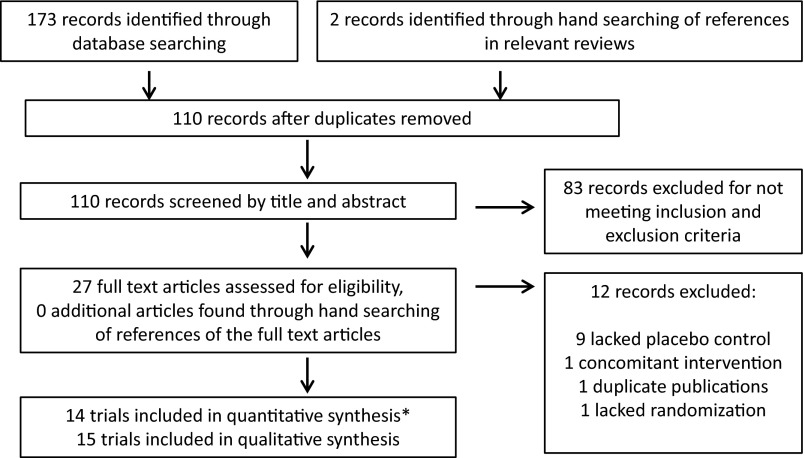
Of 110 identified reports, 83 were excluded based on initial review (Figure 1). The full-text articles of the remaining 27 reports were retrieved and reviewed. Of these, 12 were excluded because they lacked placebo control (n = 9) (17–25), had a concomitant intervention (n = 1) (26), were duplicate publications from the same study (n = 1) (27), or lacked randomization (n = 1) (28). One additional study, published only as an abstract, nominally met inclusion criteria but did not contain sufficient data for quantitative analysis (29), which could also not be obtained after repeated author requests, and was therefore excluded. After the final exclusions, 14 studies met the eligibility criteria and were included in this meta-analysis (29–43).
Figure 1.
Flow chart of the literature search, screening, and selection process for randomized, placebo-controlled trials that investigated the effects of fish oil or fish consumption on circulating adiponectin in humans. *, One appropriate trial [published as an abstract (29)] was not included in the quantitative synthesis due to insufficient information about changes in adiponectin. We included this study as part of our qualitative review of published trials.
Overview of trial characteristics
Each of the 14 trials provided an effect estimate for the effect of fish oil supplementation on circulating adiponectin (Table 1). In addition, 1 trial also evaluated how different types of fish meals (lean, fatty) influenced adiponectin (39). Thirteen were parallel design (3 of which included a 2 × 2 factorial design), and 1 used a crossover design. Most (n = 11) trials were double blind; in 3 trials, only the investigators were blinded. In total, the trial treated 682 subjects with fish oil (range 6–247 subjects per study),and 641 subjects with placebo (range 5–239 subjects per study). Eleven trials were in adults (mean age ranging from 25.4 to 70 years; median 44.7 years), and 3 were in children. Most trials were performed in Europe (n = 10) or North America (n = 1); 3 were from Asia, Central America, and Australia. Subjects were generally at least overweight (mean BMI > 25 kg/m2 in 11 trials), and 1 trial evaluated subjects with type 2 diabetes.
Table 1.
Characteristics of the 14 trials (16 Treatment Groups) Included in the Meta-Analysisa
| Author (Year) | Design | Blinding | EPA + DHA, nb,c | Control, nb | Mean Age, yd | Male, % | Mean BMI, kg/m2 | Population, Country | EPA + DHA, g/d | Duration, wk | Control Oil | Method for Adiponectin Measuremente | Dropout, % | Quality Scoref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al (2011) (30) | Parallel | Double | 55 | 54 | 0.76 | 53 | 17.7 | Healthy infants, Denmark | 1.25 | 39 | Sunflower | RIA | 14 | 5 |
| Dangardt et al (2012) (32) | Crossover | Double | 25 | 25 | 15.6 | 44 | 34.1 | Obese adolescents, Sweden | 1.22 | 12 | Medium-chain triglycerides | ELISA | 19 | 4 |
| Damsgaard et al (2008) (31) | Factorial | Double | 31 | 33 | 25.4 | 100 | 22.8 | Healthy men, Denmark | 2.9 | 8 | Olive | ELISA | 3 | 4 |
| Gammelmark et al (2012) (33) | Parallel | Double | 25 | 24 | 56.7 | 48 | 30.2 | Overweight/obese adults, Denmark | 1.12 | 6 | Olive | ELISA | 2 | 3 |
| Kabir et al (2007) (34) | Parallel | Double | 12 | 14 | 55 | 0 | 30 | Women with type 2 diabetes, France | 1.8 | 8 | Paraffin | RIA | 10 | 3 |
| Koh et al (2012) (35) | Parallel | Single | 50 | 49 | 54.7 | 59 | 25.3 | Adults with hypertriglyceridemia, Korea | 2 | 8 | NR | ELISA | 1 | 2 |
| Krebs et al (2006) (36) | Parallel | Double | 35 | 32 | 44.7 | 0 | 35 | Obese women, United Kingdom | 4.2 | 24 | Linoleic rich | RIA | 13 | 3 |
| Lopez-Alarcon et al (2011) (37) | Parallel | Single | 49 | 27 | 13.4 | 53 | 30.2 | Obese children /adolescents, Mexico | 0.9 | 4.3 | Sugar pill | RIA | 0 | 2 |
| Micallef and Garg (2009) (38) | Factorial | Double | 30 | 30 | 55.4 | 45 | 26.9 | Adults with hyperlipidemia, Australia | 1.44 | 3 | Sunflower | ELISA | 0 | 4 |
| Ramel et al (2008) (39) | Parallel | Single | 70 | 68 | 31.4 | 43 | 30.1 | Overweight/obese adults, Iceland | 2.1g | 8 | Sunflower | RIA | 14 | 2 |
| 74 | 1.1 | |||||||||||||
| 66 | 0.26g | |||||||||||||
| Rizza (2009) (40) | Parallel | Double | 26 | 24 | 29.9 | 50 | 26.2 | Healthy adults, Italy | 0.85 | 12 | Olive | ELISA | 0 | 2 |
| Sofi et al (2010) (41) | Parallel | Double | 6 | 5 | 54.4 | 82 | 29.3 | Adults with nonalcoholic fatty liver disease, Italy | 0.71 | 52 | Olive | Bioplex assay | 0 | 1 |
| Troseid et al (2009) (42) | Factorial | Double | 247 | 239 | 70 | 100 | 26.5 | Older men with hyperlipidemia, Norway | 1.32 | 156 | Linoleic rich | ELISA | 14 | 2 |
| Vargas et al (2011) (43) | Parallel | Double | 17 | 17 | 30 | 0 | 34.8 | Women with polycystic ovary syndrome, United States | 3.6 | 6 | Soybean | RIA | 13 | 2 |
Abbreviation: NR, not reported.
One additional study, published only as an abstract, nominally met inclusion criteria but did not contain sufficient data for quantitative analysis (29) and was therefore excluded from the meta-analysis. It was a randomized, double-blind, placebo-controlled, 2 × 2 factorial-design study. Subjects were randomized to receive either EPA (2 g/d) or vitamin E (0.4 g/d), or EPA (2 g/d) plus vitamin E (0.4 g/d), or placebo (corn oil) for 12 weeks. In the published abstract, the authors reported that both EPA and the EPA plus vitamin E treatment increased adiponectin (29).
Number of subjects who completed the intervention.
The study by Ramel et al (39) had a fish oil group (1.3 g/d EPA + DHA) and 2 fish meal feeding groups (cod, 0.26 g/d EPA + DHA; salmon, 2.1g/d EPA + DHA). All other studies used fish oil treatment.
When mean age was not specified, the median age was used.
The RIA measured all multimeric forms of adiponectin (low molecular weight, medium molecular weight, and high molecular weight) but not monomeric forms, whereas the ELISA and the Bioplex assay measured all multimeric and monomeric forms of adiponectin, except for the study by Dangardt et al (32), which measured high-molecular-weight adiponectin.
The components of the quality scores were: presence of randomization (yes = 1, no = 0), appropriate method of randomization (yes = 1, no = 0), presence of double blinding (yes = 1, no = 0), appropriate method of double blinding (yes = 1, no = 0), description of withdrawals and dropouts (yes = 1, no = 0); with an optimum possible score of 5.
Subjects in these intervention arms were given 150 g of cod (which provided 0.26 g EPA + DHA/d) or 150 g of salmon (which provided 2.1 g/d EPA + DHA) 3 times a week.
The median dose of n-3 PUFA (EPA + DHA) was 1.3 g/d (range 0.26–4.2 g/d), and the median duration of treatment was 8 weeks (range 3–156 weeks). Olive and sunflower oil were the most commonly used placebos. One trial measured high-molecular-weight adiponectin (32), whereas the others measured total (all multimeric forms) adiponectin; these measures are highly correlated (r = 0.89) (44). Dropout rates were low (median 10%; range 0%–19%) and generally similar between placebo and fish oil arms in each trial (not shown). Seven trials met at least 3 study quality criteria on the Jadad scale (10), whereas 7 satisfied 2 or less.
Effect of fish oil on circulating adiponectin
The individual trial results and the pooled estimate for the effect of fish oil on adiponectin are shown in Figure 2. Fish oil treatment increased adiponectin by 0.37 μg/mL (95% confidence interval [CI] 0.07, 0.67, P = .02). Between-study heterogeneity was evident (I2 = 72.9%, Q-statistic P < .001), although 11 of 14 trials suggested effects of 0 or greater, and only 2 suggested effects substantially less than 0. Among prespecified characteristics evaluated as potential sources of heterogeneity, the effect of fish oil on adiponectin appeared potentially stronger in trials of higher quality and in trials with younger or with obese subjects (Table 2). However, none of these potential interactions were statistically significant (P > .05 each). Even stratified by these factors, statistical heterogeneity remained in each pooled subgroup estimate (I2 = 48% to 81.3%, Q-statistic P < .001 to 0.1). In a sensitivity analysis assuming a within-person correlation in adiponectin levels of 0.50, rather than 0.93, results were very similar, with fish oil increasing adiponectin by 0.28 μg/mL (95% CI 0.01, 0.54, P = .04, I2 = 56.7%).
Figure 2.
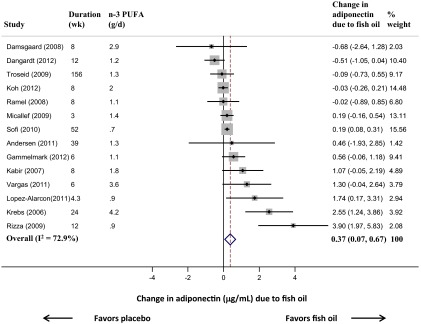
Change in circulating adiponectin resulting from fish oil consumption. There were 13 parallel-design trials and 1 crossover-design trial. Among the parallel trials, 3 were 2 × 2 factorial-design studies, none of which reported an interaction effect between fish oil and the second intervention (31, 38, 42). In the study by Troseid et al (42), the main effect of fish oil on adiponectin was obtained from the authors directly and used in the pooled meta-analysis. For the other 2 studies, this information was not available. To avoid duplication of statistics from these trials, we used a separate meta-analysis to initially obtain a pooled effect size and variance estimate within each of these studies, before using the trial-specific estimates in a second meta-analysis evaluating all studies. This approach was also applied to the study by Dangardt et al (32), which displayed results separately for males and females and did not report a significant interaction between gender and fish oil on adiponectin. The effect estimate (shaded squares) and 95% CI (lines extending from shaded squares) for each trial is shown. The DerSimonian and Laird method of the inverse-variance weighted, random effects meta-analysis was used to pool the results (14). The overall pooled estimate and 95% CI of the effect of fish oil on adiponectin is indicated by the dotted line and clear diamond, respectively. Interstudy heterogeneity was quantified y the I2 statistic (15).
Table 2.
Effect of Fish Oil on Circulating Adiponectin According to Prespecified Potential Sources of Heterogeneitya
| Number of Trials | Pooled Effect of Fish Oil on Adiponectin, μg/mL (95% CI) | Within Subgroup Heterogeneity |
Across Subgroup Meta-Regression, P Value | ||
|---|---|---|---|---|---|
| I2, % | Q-Statistic, P Value | ||||
| Overall | 14 | 0.37 (0.07, 0.67) | 72.9 | <.001 | |
| Location | |||||
| Europe/North America | 11 | 0.54 (0.07, 1.01) | 75.6 | <.001 | .79 |
| Others | 3 | 0.18 (−0.24, 0.59) | 63.2 | .07 | |
| Duration of treatment, wk | |||||
| <8 | 4 | 0.61 (0.05, 1.17) | 51.3 | .10 | .66 |
| ≥8 | 10 | 0.30 (−0.08, 0.68) | 77.3 | <.001 | |
| Blinding | |||||
| Double | 11 | 0.49 (0.09, 0.89) | 75.4 | <.001 | .73 |
| Single | 3 | 0.24 (−0.46, 0.94) | 57.9 | .09 | |
| Major fatty acid in placebo | |||||
| Linoleic acid | 6 | 0.55 (−0.09, 1.20) | 68.3 | .008 | .90 |
| Oleic acid | 4 | 0.72 (−0.20, 1.64) | 81.3 | .001 | |
| Others/not reported | 4 | 0.24 (−0.42, 0.91) | 73.9 | .009 | |
| Dose of n-3 PUFA, g/d | |||||
| <1.29 | 7 | 0.47 (−0.10, 1.04) | 76.7 | <.001 | .92 |
| ≥1.29 | 7 | 0.45 (−0.04, 0.93) | 72.3 | .001 | |
| Study quality | |||||
| Low, ≤2 | 7 | 0.34 (−0.04, 0.73) | 75.6 | <.001 | .76 |
| High, ≥3 | 7 | 0.47 (−0.16, 1.09) | 74.1 | .001 | |
| Age, y | |||||
| <38 | 7 | 0.77 (−0.26, 1.80) | 78.3 | <.001 | .77 |
| ≥38 | 7 | 0.28 (0.01, 0.56) | 70.2 | .003 | |
| Adult population | |||||
| No | 3 | 0.44 (−1.14, 2.01) | 72.7 | .03 | .74 |
| Yes | 11 | 0.41 (0.10, 0.72) | 73.6 | <.001 | |
| BMI, kg/m2b | |||||
| <30 | 6 | 0.16 (−0.14, 0.47) | 73.0 | .002 | .50 |
| ≥30 | 7 | 0.81 (0.05, 1.58) | 78.5 | <.001 | |
Duration of treatment, dose of n-3 PUFA, age, BMI, and baseline adiponectin were stratified based on the median value for all studies. When analyzed as continuous covariates, none of these factors were significant sources of heterogeneity (meta-regression, P all > .05).
Excluded the study of infants by Andersen et al (30).
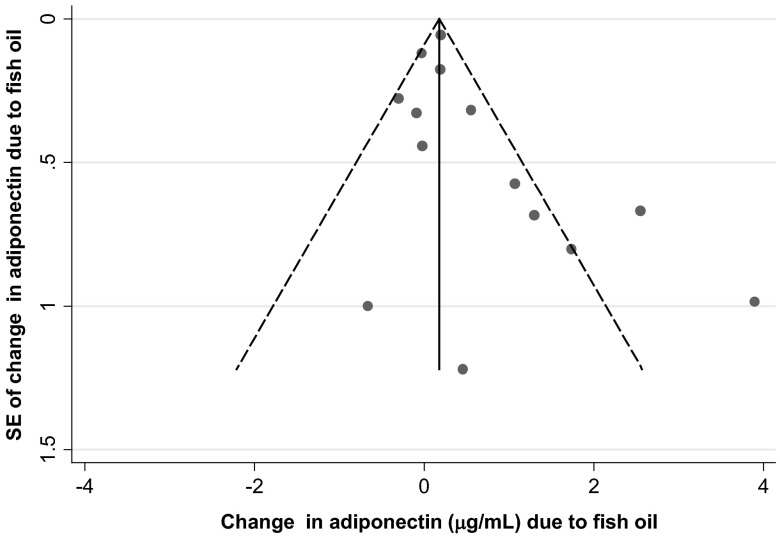
Visual inspection of a funnel plot identified slight asymmetry (Figure 3), indicative of potentially more small studies showing stronger effects. Neither Egger's (P = .11) nor Begg's (P = .08) tests achieved statistical significance, although nonsignificant trends were seen. The trim-and-fill correction analysis obtained a theoretical pooled estimate of 0.18 μg/mL (95% CI −0.15, +0.52, P = .28) after 3 theoretically unreported studies were added.
Figure 3.
Funnel plot of change in circulating adiponectin in randomized, placebo-controlled trials of fish oil consumption. The change in adiponectin due to fish oil (x-axis) is plotted against the SE of the change in adiponectin (y-axis) in each trial. The vertical line represents the overall pooled fixed-effect estimate, and the dashed lines indicate the expected 95% CI for a given SE.
Effect of fish meal feeding on circulating adiponectin
Only 2 trial arms in a single study evaluated fish meal feeding (39). Combining these arms, the pooled effect of fish meal feeding on adiponectin was not statistically significant (−0.01 μg/mL, 95% CI −0.65, +0.64, P = .99) although CIs were broad due to the small numbers of subjects.
Discussion
This systematic review and meta-analysis suggests that fish oil consumption increases circulating adiponectin. To our knowledge, this is the first demonstration that fish oil influences circulating adiponectin in randomized, placebo-controlled trials in humans. Our findings quantify the potential magnitude of the effect, an approximately 0.37 μg/mL increase due to fish oil consumption, as well as identify the presence of unexplained heterogeneity and evaluate and quantify potential effects of publication bias.
The overall pooled effect was modest: based on the observed association between adiponectin and type 2 diabetes (6), a 0.37-μg/mL increase in adiponectin would correspond to about 3% lower incidence of diabetes. Furthermore, between-study heterogeneity was evident, suggesting that fish oil supplementation may have stronger influence on adiponectin in some populations and weaker effects in others. In an exploratory analysis, we did not identify any factors that explained the heterogeneity between studies, although meta-regression analyses to confirm sources of heterogeneity can be limited by insufficient statistical power (45). We did not detect a dose-response relationship, but most trials (11 of 14) tested doses in a relatively narrow range of 0.7–2 g/d, limiting our ability to detect a dose-response across a wider range. Additionally, our findings cannot confirm that enhanced insulin sensitivity is a clinically relevant mechanism of cardiometabolic protection of fish or fish oil consumption.
Interestingly, fish consumption has been linked to modestly higher incidence of type 2 diabetes in some but not all studies (46) and to slight increases in fasting glucose concentrations in some randomized controlled trials (47). Experimental studies have suggested that such effects might relate to increased hepatic gluconeogenesis, possibly a result of decreased de novo lipogenesis and lower release of triglycerides as an energy substrate by the liver, rather than due to any adverse effects on insulin resistance (48, 49). Given the key role of adiponectin in pathways of insulin sensitivity and metabolic health, the present findings provide further support that fish oil consumption does not adversely influence adipocyte function or related metabolic pathways and in fact could favorably influence these pathways. Such effects are consistent with known beneficial effects of n-3 PUFAs on triglycerides as well as modest increases in high-density lipoprotein concentrations and low-density lipoprotein participle size (50–52), and our results highlight the need for additional studies to investigate the effect of fish oil supplementation on other metabolic outcomes such as inflammation, free fatty acids, and insulin resistance.
In cultured rat and human primary adipocytes, enrichment of EPA and DHA leads to increased synthesis and secretion of adiponectin (53, 54). In these experiments, antagonism of peroxisome proliferator-activated receptor-γ, a key nuclear receptor known to be bound by n-3 PUFA, blocked the adiponectin-increasing effects of n-3 PUFAs (53, 54). n-3 PUFAs may also enhance adiponectin synthesis by inhibiting transient receptor potential canonical calcium ion channels, which are expressed on mature adipocytes and regulate adiponectin production (55). These findings in cultured adipocytes are supported by in vivo animal models, in which higher intake of n-3 PUFAs increases tissue and circulating adiponectin (56). Interestingly, recent experimental data suggest that specific DHA-derived metabolites, resolvins D1 and D2, can also stimulate the production of adiponectin (57). Our findings provide support that these pathways identified in vitro and in vivo may have functional relevance to effects of n-3 PUFAs on adipocyte function and adiponectin levels in humans.
There were several strengths of our analysis. We systematically searched for all published and unpublished (ie, abstracts) studies with broad search terms across multiple databases and also contacted authors for clarification and additional data, which minimized potential for publication bias and misclassification. We limited our analysis to randomized, placebo-controlled trials, and most were also double blind, strengthening inference for a cause-and-effect relationship. Attrition bias was also unlikely, due to low dropout rates that were also similar for the placebo and fish oil treatment in each trial. Only 1 trial used a crossover design, and its 6-week washout period minimized carryover effects. We evaluated several prespecified sources of heterogeneity, including dose, duration, and study location.
Limitations should be considered. Despite the broad search criteria, publication bias remains a possibility. Although statistical tests for heterogeneity were not significant, asymmetry was apparent in the funnel plot, and a trim-and-fill sensitivity analysis suggested the effect of fish oil could be overestimated in our unadjusted pooled analysis. Conversely, funnel plot asymmetry could also be due to other factors (16), such as true biological heterogeneity (effect modification) between different identified trials. We also excluded 1 trial that reported increased adiponectin after fish oil treatment due to insufficient reported data (29); inclusion of this trial may have reduced asymmetry in the funnel plot. Several of the trials had lower study quality as assessed by the Jadad scale (10). Yet effects of fish oil on adiponectin were not significantly different in trials with higher or lower study quality scores. Thus, study quality may not have had a systematic influence on the pooled findings.
In conclusion, this systematic review and meta-analysis of randomized, placebo-controlled clinical trials suggests that fish oil supplementation moderately increases circulating adiponectin. These findings support potential beneficial effects of fish oil supplementation on pathways related to adipocyte health and adiponectin metabolism.
Supplementary Material
Acknowledgments
We thank the following authors for providing clarification on their published articles and/or additional unpublished data: Drs Francesco Sofi, Alfons Ramel, Inga Thorsdottir, Anders Gammelmark, Salwa Rizkalla, Mardia Lopez-Alarcon, Ingebjrg Seljeflot, Lotte Lauritzen, and Anders Andersen. We thank Dr Eric Rimm for providing the within-person adiponectin correlation based on data from the Health Professional Follow-Up Study. We also thank the National Heart, Lung, and Blood Institute (Bethesda, MD) and National Institutes of Health (Bethesda, MD) for funding support. Dr Jason Wu gratefully acknowledges Research Fellowship support from the National Heart Foundation of Australia. Author contributions are as follows: J.H.Y.W. and D.M. designed the research; J.H.Y.W. and L.E.C. conducted the research; J.H.Y.W. and D.M. performed the statistical analysis; J.H.Y.W. wrote the paper; J.H.Y.W. had primary responsibility for the final content; and J.H.Y.W., L.E.C., and D.M. had the responsibility for the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
This work was supported by National Heart, Lung, and Blood Institute Grant 1 R21 HL109924-01A1 (to D.M.). L.E.C. was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship. J.H.Y.W. was supported by an Australian National Heart Foundation Overseas Fellowship (O 08P 3859).
Disclosure Summary: J.H.Y.W. and L.E.C. have no conflict of interest to disclose. D.M. reports the following potential conflicts of interest: ad hoc travel reimbursement and/or honoraria for 1-time scientific presentations on diet and cardiometabolic diseases from SPRIM, Nutrition Impact, the International Life Sciences Institute, Bunge, Pollock Institute, and Quaker Oats; ad hoc consulting fees from McKinsey Health Systems Institute and Foodminds; Unilever North America Scientific Advisory Board; research grants from GlaxoSmithKline, Sigma Tau, Pronova, and the National Institutes of Health for an investigator-initiated, not-for-profit, RCT of fish oil supplements for the prevention of postsurgical complications; and royalties from UpToDate for an online chapter on fish oil.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- n-3 PUFA
- long-chain polyunsaturated omega-3 fatty acid.
References
- 1. Kalupahana NS, Claycombe K, Newman SJ, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–1922 [DOI] [PubMed] [Google Scholar]
- 2. Neschen S, Morino K, Dong J, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-α-dependent manner. Diabetes. 2007;56:1034–1041 [DOI] [PubMed] [Google Scholar]
- 3. Saraswathi V, Morrow JD, Hasty AH. Dietary fish oil exerts hypolipidemic effects in lean and insulin sensitizing effects in obese LDLR−/− mice. J Nutr. 2009;139:2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–146 [DOI] [PubMed] [Google Scholar]
- 5. Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin [published online August 15, 2012]. Heart Fail Rev. doi:10.1007/s10741-012-9337-8 [DOI] [PubMed] [Google Scholar]
- 6. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188 [DOI] [PubMed] [Google Scholar]
- 7. Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629 [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Periz A, Claria J. Resolution of adipose tissue inflammation. Sci World J. 2010;10:832–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 11. Alderson P, Greean S, eds. Cochrane Collaboration open learning material for reviewers. Available from http://www.cochrane-net.org/openlearning/index.htm Accessed May 1, 2012
- 12. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773 [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Greean S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, updated March 2011. Available from http://www.cochrane-handbook.org/ Accessed May 1, 2012
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–4562 [DOI] [PubMed] [Google Scholar]
- 17. Itoh M, Suganami T, Satoh N, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–1925 [DOI] [PubMed] [Google Scholar]
- 18. Mindrescu C, Gupta RP, Hermance EV, et al. Omega-3 fatty acids plus rosuvastatin improves endothelial function in South Asians with dyslipidemia. Vasc Health Risk Manag. 2008;4:1439–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizia-Stec K, Haberka M, Mizia M, et al. N-3 polyunsaturated fatty acid therapy improves endothelial function and affects adiponectin and resistin balance in the first month after myocardial infarction. Arch Med Sci. 2011;7:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nomura S, Inami N, Shouzu A, et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. 2009;20:16–22 [DOI] [PubMed] [Google Scholar]
- 21. Nomura S, Shouzu A, Omoto S, et al. Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J Atheroscler Thromb. 2009;16:83–90 [DOI] [PubMed] [Google Scholar]
- 22. Olza J, Mesa MD, Aguilera CM, et al. Influence of an eicosapentaenoic and docosahexaenoic acid-enriched enteral nutrition formula on plasma fatty acid composition and biomarkers of insulin resistance in the elderly. Clin Nutr (Edinburgh, Scotland). 2010;29:31–37 [DOI] [PubMed] [Google Scholar]
- 23. Patel JV, Lee KW, Tomson J, Dubb K, Hughes EA, Lip GYH. Effects of ω-3 polyunsaturated fatty acids on metabolically active hormones in patients post-myocardial infarction. Int J Cardiol. 2007;115:42–45 [DOI] [PubMed] [Google Scholar]
- 24. Romeo J, Warnberg J, Garcia-Marmol E, et al. Daily consumption of milk enriched with fish oil, oleic acid, minerals and vitamins reduces cell adhesion molecules in healthy children. Nutr Metab Cardiovasc Dis. 2011;21:113–120 [DOI] [PubMed] [Google Scholar]
- 25. Satoh N, Shimatsu A, Kotani K, et al. Highly purified eicosapentaenoic acid reduces cardio-ankle vascular index in association with decreased serum amyloid A-LDL in metabolic syndrome. Hypertens Res. 2009;32:1004–1008 [DOI] [PubMed] [Google Scholar]
- 26. Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS. Effect of dietary n-3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr. 2008;87:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarbolouki S, Djalali M, Dorosty AR, et al. Effects of eicosapentaenoic acid alone and combined with vitamin E on leptin, adiponectin, serum glycemic indices concentration in patients with type II diabetes mellitus. Obes Rev. 2010;11:819 [Google Scholar]
- 28. Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. Effects of N-3 fatty acids on hepatic triglyceride content in humans. J Invest Med. 2008;56:780–785 [DOI] [PubMed] [Google Scholar]
- 29. Sarbolouki S, Hashemi SB, Djalali M, et al. Evaluating serum adiponectin and glycemic indices concentrations and their effect in patients with type II diabetes on eicosapentaenoic acid with or without vitamin E. J Diabetes. 2011;3:10–11 [Google Scholar]
- 30. Andersen AD, Michaelsen KF, Hellgren LI, Trolle E, Lauritzen L. A randomized controlled intervention with fish oil versus sunflower oil from 9 to 18 months of age: exploring changes in growth and skinfold thicknesses. Pediatr Res. 2011;70:368–374 [DOI] [PubMed] [Google Scholar]
- 31. Damsgaard CT, Frokiaer H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr. 2008;138:1061–1066 [DOI] [PubMed] [Google Scholar]
- 32. Dangardt F, Chen Y, Gronowitz E, Dahlgren J, Friberg P, Strandvik B. High physiological omega-3 fatty acid supplementation affects muscle fatty acid composition and glucose and insulin homeostasis in obese adolescents. J Nutr Metab. 2012;2012:395757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gammelmark A, Madsen T, Varming K, Lundbye-Christensen S, Schmidt EB. Low-dose fish oil supplementation increases serum adiponectin without affecting inflammatory markers in overweight subjects. Nutr Res. 2012;32:15–23 [DOI] [PubMed] [Google Scholar]
- 34. Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–1679 [DOI] [PubMed] [Google Scholar]
- 35. Koh KK, Quon MJ, Shin KC, et al. Significant differential effects of omega-3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis. 2012;220:537–544 [DOI] [PubMed] [Google Scholar]
- 36. Krebs JD, Browning LM, McLean NK, et al. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes. 2006;30:1535–1544 [DOI] [PubMed] [Google Scholar]
- 37. Lopez-Alarcon M, Martinez-Coronado A, Velarde-Castro O, Rendon-Macias E, Fernandez J. Supplementation of n3 long-chain polyunsaturated fatty acid synergistically decreases insulin resistance with weight loss of obese prepubertal and pubertal children. Arch Med Res. 2011;42:502–508 [DOI] [PubMed] [Google Scholar]
- 38. Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204:476–482 [DOI] [PubMed] [Google Scholar]
- 39. Ramel A, Martinéz A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51:1261–1268 [DOI] [PubMed] [Google Scholar]
- 40. Rizza S, Tesauro M, Cardillo C, et al. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis. 2009;206:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sofi F, Giangrandi I, Cesari F, et al. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr. 2010;61:792–802 [DOI] [PubMed] [Google Scholar]
- 42. Troseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism. 2009;58:1543–1549 [DOI] [PubMed] [Google Scholar]
- 43. Vargas ML, Almario RU, Buchan W, Kim K, Karakas SE. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metab Clin Exp. 2011;60:1711–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Almeda-Valdes P, Cuevas-Ramos D, Mehta R, et al. Total and high molecular weight adiponectin have similar utility for the identification of insulin resistance. Cardiovasc Diabetol. 2010;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simmonds MC, Higgins JP. Covariate heterogeneity in meta-analysis: criteria for deciding between meta-regression and individual patient data. Stat Med. 2007;26:2982–2999 [DOI] [PubMed] [Google Scholar]
- 46. Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr Suppl. 2012;107(suppl 2):S214–S227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care. 1998;21:494–500 [DOI] [PubMed] [Google Scholar]
- 48. Berger A, Mutch DM, German JB, Roberts MA. Dietary effects of arachidonate-rich fungal oil and fish oil on murine hepatic and hippocampal gene expression. Lipids Health Dis. 2002;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Puhakainen I, Ahola I, Yki-Jarvinen H. Dietary supplementation with n-3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1995;61:121–126 [DOI] [PubMed] [Google Scholar]
- 50. Bernstein AM, Ding EL, Willett WC, Rimm EB. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr. 2012;142:99–104 [DOI] [PubMed] [Google Scholar]
- 51. Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol. 2009;136:4–16 [DOI] [PubMed] [Google Scholar]
- 52. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease—effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067 [DOI] [PubMed] [Google Scholar]
- 53. Banga A, Unal R, Tripathi P, et al. Adiponectin translation is increased by the PPARγ agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296:E480–E489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tishinsky JM, Ma DW, Robinson LE. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARγ-dependent manner in human adipocytes. Obesity (Silver Spring). 2011;19:262–268 [DOI] [PubMed] [Google Scholar]
- 55. Sukumar P, Sedo A, Li J, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moreno-Aliaga MJ, Lorente-Cebrian S, Martinez JA. Regulation of adipokine secretion by n-3 fatty acids. Proc Nutr Soc. 2010;69:324–332 [DOI] [PubMed] [Google Scholar]
- 57. Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin d1 and resolvin d2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.