Abstract
Background
Previous studies on gene-lifestyle interaction and obesity have mostly focused on the FTO gene and physical activity, while little attention has been paid to sedentary behavior as indicated by television (TV) watching.
Methods and Results
We analyzed interactions between TV watching, leisure-time physical activity and genetic predisposition in relation to body mass index (BMI) in 7740 women and 4564 men from 2 prospective cohorts: the Nurses’ Health Study and Health Professionals Follow-up Study. Data on physical activity and TV watching were collected 2 years prior to assessment of BMI. A weighted genetic risk score (GRS) was calculated on the basis of 32 established BMI-associated variants. In both women and men, the genetic associations with BMI strengthened with increased hours of TV watching. An increment of 10 points in the weighted GRS was associated with 0.8 [SE 0.4], 0.8 [0.2], 1.4 [0.2], 1.5 [0.2] and 3.4 [1.0] kg/m2 higher BMI across the 5 categories of TV watching (0-1, 2-5, 6-20, 21-40, and >40h/wk) (P for interaction=0.001). In contrast, the genetic association with BMI weakened with increased levels of physical activity. An increment of 10 points in the weighted GRS was associated with 1.5 [0.2], 1.3 [0.2], 1.2 [0.2], 1.2 [0.2] and 0.8 [0.2] kg/m2 higher BMI across the quintiles of physical activity. The interactions of TV watching and physical activity with genetic predisposition in relation to BMI were independent of each other.
Conclusions
Sedentary lifestyle indicated by prolonged TV watching may accentuate predisposition to elevated adiposity, whereas greater leisure-time physical activity may attenuate the genetic association.
Keywords: Body mass index, Genetics, Gene-lifestyle interaction, Television watching, Physical activity
Obesity and related comorbid conditions have become a serious threat to public health globally.1 It is well accepted that both genetic predisposition and environmental factors are important contributors to increasing body mass index (BMI).2 Recently, 32 BMI-predisposing loci have been established in a meta-analysis of genome wide association studies (GWASs).3 There is emerging evidence that high levels of physical activity could attenuate the genetic predisposition to increased BMI and obesity risk.4-10 However, to the best of our knowledge, no study has investigated modifying effect of television (TV) watching, the most common sedentary behavior in leisure time, on the genetic predisposition in relation to BMI. In the United States, the average daily TV watching time has recently been reported to be 5 hours, and about 3.5-4.0 hours of daily TV watching time was reported in several European countries and Australia.11 Epidemiologic studies have shown that sedentary lifestyle indicated by prolonged TV watching was associated with increased risk of obesity and related diseases, independent of physical activity levels.11-15 In addition, previous studies have focused on levels of total physical activity,4-10 while little attention has been paid to types of physical activity.
Therefore, in the current study, we examined whether leisure-time TV watching and physical activity modify the genetic predisposition to high BMI, estimated by a weighted genetic risk score (GRS) on basis of 32 established BMI loci, in women and men from two prospective cohorts: the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS).
METHODS
Study Participants
The NHS is a prospective cohort study of 121,700 female registered nurses who were 30 to 55 years old at study inception in 1976.16 The HPFS is a prospective cohort study of 51,529 U.S. male health professionals who were 40 to 75 years old at study inception in 1986.17 In both cohorts, information about medical history, lifestyle and disease has been collected biennially by self-administered questionnaires every 2 years since inception. Study samples for the current analysis included 7740 women and 4564 men of genetically inferred European ancestry from NHS and HPFS, with GWAS data that were initially designed to address various chronic diseases such as type 2 diabetes, coronary heart disease, kidney stone disease, open-angle glaucoma and breast cancer (NHS only). Population structure was investigated by principal component analysis, and participants clustered with the HapMap CEU samples were genetically inferred to have European ancestry. Details regarding the study design, genotyping quality control of these GWAS data have been reported elsewhere.18-22
Assessment of television watching and physical activity
Assessments of hours spent on TV watching have been described previously.13, 14 In 1992, NHS participants were asked to report their average weekly time spent watching TV (including videotapes). Starting from 1988, HPFS participants reported their average weekly time spent watching TV or videotapes on the biennial questionnaires. The response included 6 to 9 categories (ranging from 0 to >90h/wk). In the current analysis, 5 categories were coded consistently across all questionnaires (0-1, 2-5, 6-20, 21-40, and >40h/wk) in the NHS and HPFS participants.
Detailed assessments of physical activity in the NHS and HPFS were first obtained by questionnaires in 1986 and every 2 years thereafter except for 1990 in the NHS. Participants were asked to report the average amount of time they spent per week on leisure-time physical activities, including walking, jogging, running, bicycling, calisthenics or use of a rowing machine, lap swimming, squash or racquetball, and tennis. They were also asked about their usual walking pace. Based on this information, weekly energy expenditure in metabolic equivalent hours (METs) was calculated.23 We defined any physical activity requiring 6 METs or greater (a 6-fold or greater increase above resting metabolic rate) as vigorous, including activities as mentioned above except for walking. Walking requires an energy expenditure of 2-4.5 METs, depending on pace, and was therefore considered to be a light- to moderate-intensity activity. The reproducibility and validity of the physical activity questionnaire has been described elsewhere.24
Assessment of body mass index and covariates
Body weight was self-reported in the biennial questionnaires. Self-reported weights were highly correlated with measured weight (r=0.97 in men and women).25 BMI was calculated as body weight (kg)/height2 (m2). Information about anthropometric data, lifestyle factors, and menopausal status and postmenopausal hormone therapy (women only) was derived from the biennial questionnaires.16, 17 Detailed dietary information was collected from semi-quantitative food-frequency questionnaires 26, 27 administered in 1986 and every 4 years thereafter.
Genotyping
We selected 32 single nucleotide polymorphisms (SNPs) which showed significant genome-wide association with BMI in a recent meta-analysis.3 SNP genotyping and imputation have been described in detail elsewhere.18-22 Characteristics of the 32 SNPs were listed in Supplemental Table 1. Most of the SNPs were genotyped or had a high imputation quality score (MACH r2≥0.8).
Genetic risk Score Computation
The GRS was calculated on basis of the 32 established BMI-associated genetic variants by using a previously reported weighted method.3 Individual SNP was recoded as 0, 1, and 2 according to the number of risk alleles (BMI-increasing alleles). Each SNP was weighted by its relative effect size (β-coefficient) obtained from the reported meta-analysis data.3 We firstly created a weighted score using equation 1, where β is the β-coefficient of each individual SNP on BMI, and n is 32 in the current analysis.
| (1) |
We rescaled the weighted score to reflect the number of BMI-increasing allele using equation 2, where total number of SNPs is 32 and sum of the β-coefficients is 4.39 in the current analysis.
| (2) |
For all further analyses, we used this weighed GRS. There were 18 participants with genotype data missing on 4 or fewer of the 32 SNPs, and the weighted GRS for these participants were standardized to those for participants with completed genotype data by using a previously reported method:28 standardized GRS = GRS with missing genotypes/number of non-missing SNPs × total number of studied SNPs.
Statistical analyses
We analyzed the data prospectively with the assessments of TV watching or physical activity 2 years prior to the assessment of BMI. For example, the 1988 TV watching data in men and the 1992 TV watching data in women were related to the 1990 BMI and 1994 BMI, respectively. General linear regression models were applied to examine the main effect of the weighted GRS and its interaction with TV watching or physical activity on BMI based on first 2 years of follow-up data, adjusted for age, genotype data source (5 GWAS samples in women and 4 GWAS samples in men) and disease status (controls, or incident cases of type 2 diabetes, coronary heart disease, kidney stone disease, open-angle glaucoma or breast cancer). Because effect size of each BMI-predisposing variant is very modest, we estimated the genetic effect on BMI for each additional 10 points of the weighted GRS. Interactions between the weighted GRS and TV watching or physical activity were tested by including the respective interaction terms in the general linear regression models. In the multivariate analysis, we further adjusted for smoking (never, past or current), alcohol intake (0, 0.1-4.9, 5.0-9.9 10.0-14.9 or ≥15.0 g/day), menopausal status (pre or postmenopausal [never, past or current hormone use], women only), total energy intake (quintiles) and physical activity levels (quintiles). To further verify our findings, we used generalized linear models (PROC GENMOD in SAS software) with robust variance and accounting for within-individual repeated measures to test interaction between the weighted GRS and physical activity based on 10 years (1986-1996) of follow-up data in women and men. The repeated measures analysis for TV watching was only conducted in men during 1988-1996, since follow-up data on TV watching were not available in women. For missing data, the values from the closest prior cycle were carried forward for BMI, physical activity and TV watching. Given the possible confounding due to age-related weight change,29 we only used follow-up data up to 1996 in women and men in the repeated measures analyses as the mean age of our study samples was greater than 65 years old in 1998. We further examined the genetic predisposition to increased BMI according to joint classification of TV watching and physical activity, in which both variables were classified into 3 categories rather than 5 categories to achieve sufficient power. All analyses were conducted in women and men separately, and then pooled by inverse-variance–weighted, fixed-effects meta-analyses. All reported P-values are nominal and two-sided. Statistical analyses were performed in SAS 9.1 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Table 1 shows the characteristics of the study participants in 1986. The weighted GRS ranged from 13.1 to 43.4, and the mean (SD) was 29.2 (3.9), and it showed similar normal distribution in both women and men (Supplemental Figure 1). The correlation between TV watching and physical activity levels was minimal in women and men (r=-0.03 and −0.06, respectively).
Table 1.
Baseline (1986) characteristics of the study participants*
| Women n=7740 |
Men n=4564 |
|
|---|---|---|
| Age, y | 54.0 (6.6) | 55.3 (8.7) |
| BMI, kg/m2 | 25.9 (5.1) | 25.8 (3.4) |
| Genetic risk score | 29.2 (3.9) | 29.2 (3.9) |
| Television watching, h/wk | 13.6 (11.9) | 11.7 (8.7) |
| Physical activity, METs/wk | 14.1 (18.9) | 19.6 (26.8) |
| Vigorous activity, h/wk | 1.1 (2.1) | 1.4 (2.8) |
| Walking, h/wk | 1.9 (2.5) | 2.1 (2.7) |
| Alcohol consumption, g/d | 6.2 (10.7) | 11.9 (15.8) |
| Total energy intake, kcal/d | 1786 (521) | 2031 (612) |
| Current smokers, n (%) | 1394 (17.6) | 419 (9.3) |
| Postmenopausal hormone use, n (%) | 1758 (22.8) | - |
Television watching was assessed in 1992 for women and in 1988 for men. Data are presented as mean (SD) unless otherwise indicated.
Main effect analysis showed that each additional 10 points of the weighted GRS was associated with 1.6 (SE: 0.1) and 0.9 (0.1) kg/m2 higher in BMI in women and men, respectively, with a pooled effect size of 1.3 (0.1) kg/m2.
As shown in Table 2, prolonged TV watching accentuated the effect of the weighted GRS on BMI in women and men (both P for interaction ≤0.05). The genetic effect of the weighted GRS on BMI increased across categories of TV watching. In the pooled results from women and men (P for interaction=0.001), an increment of 10 points in the weighted GRS was associated with an increase of 0.8 (0.4) kg/m2 in BMI in participants with 0-1 h/wk of TV watching, and was associated with an increase of 3.4 (1.0) kg/m2 in BMI in participants with >40 h/wk of TV watching. Further adjustment for physical activity and other covariates did not change the results. Considering the stronger genetic effect on BMI in the highest category of TV watching (>40 h/wk) compared to other 4 categories, we performed a sensitivity analysis by excluding participants with 40 h/wk of TV watching, and the result remained significant (P for interaction=0.03). In repeated measures analysis in men, we observed similar interaction results (P for interaction=0.04). In sensitivity analyses, the interaction remained significant (all P for interaction<0.05) after exclusion of incident cases of type 2 diabetes, coronary heart disease, kidney stone disease, open-angle glaucoma or breast cancer.
Table 2.
Difference in BMI per 10 points of the weighted GRS according to average hours of TV watching*
| Average hours of TV watching per week |
P for Interaction | |||||
|---|---|---|---|---|---|---|
| 0-1 | 2-5 | 6-20 | 21-40 | ≥41 | ||
| Women | ||||||
| n | 439 | 1634 | 4003 | 1198 | 185 | |
| GRS (range) | 18.9-43.4 | 16.6-42.3 | 14.8-43.3 | 16.7-42.3 | 17.9-39.9 | |
| β (SE)† | ||||||
| Model 1‡ | 1.1 (0.6) | 1.3 (0.3) | 1.7 (0.2) | 1.6 (0.4) | 3.2 (1.2) | 0.05 |
| Model 2§ | 1.1 (0.6) | 1.2 (0.3) | 1.7 (0.2) | 1.6 (0.4) | 3.0 (1.1) | 0.05 |
| Men | ||||||
| n | 519 | 2231 | 1301 | 488 | 25 | |
| GRS (range) | 16.9-40.4 | 16.8-41.9 | 16.0-40.6 | 19.5-40.6 | 21.1-37.3 | |
| β (SE)† | ||||||
| Model 1‡ | 0.7 (0.4) | 0.7 (0.2) | 1.0 (0.2) | 1.3 (0.5) | 3.9 (2.1) | 0.04 |
| Model 2§ | 0.7 (0.4) | 0.7 (0.2) | 1.0 (0.2) | 1.3 (0.5) | 3.9 (2.2) | 0.05 |
| Pooled β (SE) † ¶ | ||||||
| Model 1‡ | 0.8 (0.4) | 0.8 (0.2) | 1.4 (0.2) | 1.5 (0.3) | 3.4 (1.0) | 0.001 |
| Model 2§ | 0.8 (0.4) | 0.8 (0.2) | 1.4 (0.2) | 1.5 (0.3) | 3.2 (1.0) | 0.001 |
Data are based on first 2 year of follow-up after the assessment of hours spent on TV watching in women (1992-1994) and men (1988-1990).
Difference in BMI (kg/m2) per 10 points of the weighted GRS.
Adjusted for age, genotype data source and disease status.
Further adjusted for physical activity, smoking, alcohol intake, menopausal status (women only) and total energy intake.
Results were pooled between women and men by inverse-variance–weighted, fixed-effects meta-analyses.
In contrast, leisure-time physical activity levels significantly attenuated the effect of the weighted GRS on BMI in both women and men (both P for interaction ≤0.001) (Table 3). The genetic effect of the weighted GRS on BMI decreased across the quintiles of physical activity. In the pooled results from women and men, an increment of 10 points in the weighted GRS was associated with an increase of 1.5 (0.2) kg/m2 in BMI in the lowest quintile of physical activity, and was associated with an increase of 0.8 (0.2) kg/m2 in BMI in the highest quintile of physical activity. After further adjustment for smoking, alcohol intake, menopausal status and total energy intake, the results were not significantly changed (P for interaction<0.001). In repeated measures analysis, similar interaction results between the weighted GRS and physical activity on BMI were observed. In sensitivity analysis, we observed similar interactions (all P for interaction<0.001) after exclusion of incident cases of chronic diseases.
Table 3.
Difference in BMI per 10 points of the weighted GRS according to the quintiles of physical activity
| Quintiles of physical activity |
P for interaction | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Women | ||||||
| METs/wk | 0-2.4 | 2.5-5.5 | 5.5-11.6 | 11.7-22.9 | ≥23.0 | |
| n | 1716 | 1506 | 1491 | 1473 | 1421 | |
| GRS (range) | 16.8-41.8 | 14.8-42.3 | 16.0-40.2 | 13.1-42.4 | 17.9-43.4 | |
| β (SE)* | ||||||
| 1986-1988† | 1.8 (0.3) | 1.8 (0.3) | 1.6 (0.3) | 1.5 (0.3) | 1.2 (0.3) | 0.001 |
| 1986-1996‡ | 2.1 (0.3) | 1.7 (0.2) | 1.6 (0.2) | 1.3 (0.2) | 1.3 (0.2) | 0.001 |
| Men | ||||||
| METs/wk | 0-2.7 | 2.8-7.6 | 7.7-16.2 | 16.3-31.3 | ≥31.4 | |
| n | 918 | 925 | 885 | 916 | 900 | |
| GRS (range) | 16.6-40.6 | 17.1-39.9 | 18.3-40.8 | 16.9-41.9 | 16.0-41.3 | |
| β (SE)* | ||||||
| 1986-1988† | 1.2 (0.3) | 0.7 (0.3) | 0.7 (0.3) | 1.0 (0.2) | 0.6 (0.3) | 0.001 |
| 1986-1996‡ | 1.5 (0.3) | 0.7 (0.2) | 0.8 (0.2) | 1.0 (0.2) | 0.5 (0.2) | 0.03 |
| Pooled β (SE) * § | ||||||
| 1986-1988† | 1.5 (0.2) | 1.3 (0.2) | 1.2 (0.2) | 1.2 (0.2) | 0.8 (0.2) | <0.001 |
| 1986-1996‡ | 1.8 (0.2) | 1.2 (0.2) | 1.2 (0.2) | 1.1 (0.1) | 0.8 (0.1) | <0.001 |
Difference in BMI (kg/m2) per 10 points of the weighted GRS, adjusted for age, genotype data source, and disease status.
Data are based on first 2 year of follow-up after the assessment of physical activity in women and men (1986-1988).
Data are based on 10 years of follow-up in women and men (1986-1996, repeated measures analysis)
Results were pooled between women and men by inverse-variance–weighted, fixed-effects meta-analyses.
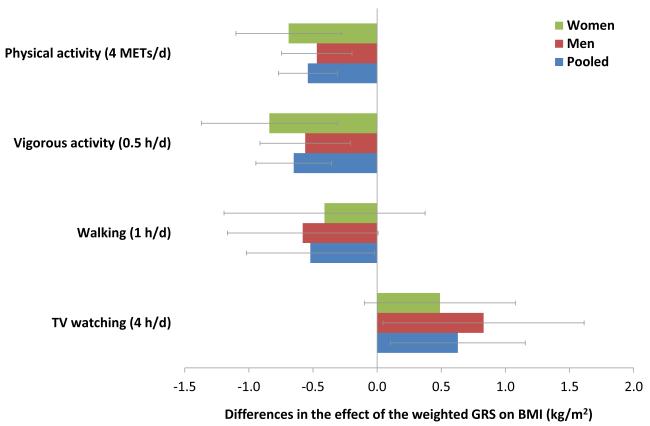
To further estimate the modifying effects of TV watching and physical activity on genetic predisposition to increased BMI, we conducted interaction analyses including TV watching (4 h/d), physical activity (4 METs/d [1 METs/d = 7 METs/wk], roughly equivalent to 1 h/d of brisk walking), vigorous activity (0.5 h/d) and walking (1 h/d) in continuous form (Figure 1). Each 4 h/d of TV watching was associated with a 0.6 (95% CI 0.1, 0.1.1) kg/m2 increase in the effect of each additional 10 points in the weighted GRS on BMI. In contrast, each 4 METs/d of leisure-time physical activity was associated with a 0.5 (0.3, 0.8) kg/m2 reduction in the effect of each additional 10 points in the weighted GRS on BMI. Vigorous activity (0.5 h/d) and walking (1 h/d) were both associated with a decreased effect of the weighted GRS on BMI (β [95% CI] for interaction = −0.6 [−0.3, −0.9] and 0.5 [−1.0, 0.0] kg/m2, respectively).
Figure 1. Differences in effect of the weighted GRS on BMI associated with physical activity and TV watching.
Data are based on the first 2 year of follow-up after the assessments of physical activity (1986-1988 in women and men) and TV watching (1992-1994 in women, 1988-1990 in men). Differences in genetic effect on BMI (beta [95% CI] for interaction) are reported for each additional 10 points of the weighted GRS associated with increased hours of total physical activity, vigorous activity, walking or TV watching, adjusted for age, genotype data source, disease status, smoking, alcohol intake, menopausal status (women only), total energy intake, vigorous activity (walking analysis only), walking (vigorous activity analysis only), and total physical activity (TV watching analysis only).
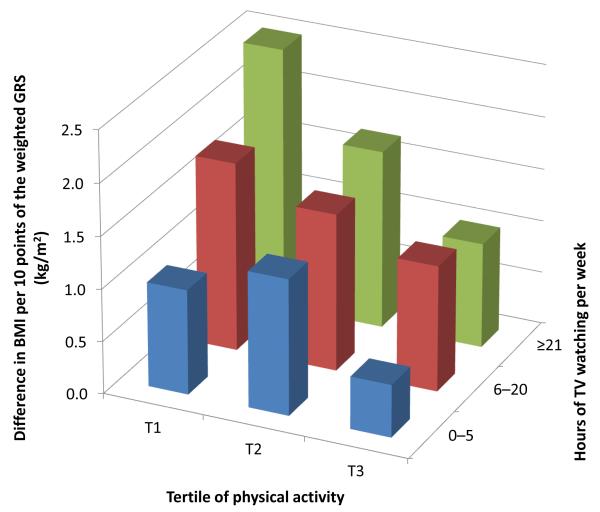
The modifying effects of TV watching and physical activity on genetic associations with BMI were independent of each other (Figure 2). Among individuals with the lowest tertile of physical activity and >21 h/wk of TV watching, an increment of 10 points in the weighted GRS was associated with an increase of 2.5 [95% CI 1.5, 3.5] kg/m2 in BMI (P<0.001), while the genetic effect was largely attenuated (β=0.5 [0.1, 1.0] kg/m2, P=0.03) among individuals with the highest tertile of physical activity and 0-5 h/wk of TV watching. Results were similar in women (β=2.5 [1.1, 3.9] vs 0.4 [−0.4, 0.8] kg/m2) and men (β= 2.5 [0.5, 4.5] vs 0.6 [0.0, 1.2] kg/m2) by comparing these 2 extreme groups separately.
Figure 2. Difference in BMI per 10 points of the weighted GRS according to joint classification of physical activity and TV watching.
Data are based on the first 2 years of follow-up after the both assessments of physical activity and TV watching in women (1992-1994) and men (1988-1990). Data are differences in BMI (kg/m2) per 10 points of the weighted GRS pooled from women and men, adjusted for age, genotype data source, disease status, smoking, alcohol intake, menopausal status (women only), and total energy intake.
The weighted GRS alone explained 1.5% of the variation in BMI (R2=0.015) for the combined data from women and men, consistent with the previously reported result that the established 32 BMI loci explained 1.45% of the variation in BMI.3 The R2 (measure of the goodness of fit) of the multivariate model including the weighted GRS and non-genetic factors was 0.14, and it slightly increased to 0.15 when the interaction terms (GRS-by-TV watching, or GRS-by-physical activity) was added in the model, though the improvement was statistically significant (P≤0.001).
DISCUSSION
In two large prospective cohorts of US women and men, we found that prolonged TV watching accentuated the genetic predisposition to high BMI, estimated by a weighted GRS on basis of 32 established BMI-predisposing variants, whereas greater leisure-time physical activity attenuated the genetic effects on BMI. Most of the previous cross-sectional studies reporting the interaction between physical activity and genetic susceptibility for obesity have focused on one single locus, the FTO gene,4-7, 9, 10, 30-35 but the results were inconsistent. One reason for the inconsistent results is that a relative small proportion of genetic susceptibility to obesity is explained by this one locus. Recently, a modest interaction between physical activity and FTO variants on obesity risk was reported by a meta-analysis.9 In our study, the genetic predisposition was estimated by 32 well-established BMI-associated variants,3 which captures the overall genetic susceptibility. This approach may be preferable in the interaction analysis between genetic predisposition and lifestyle factors. Our results were consistent with a recent study using similar approach by calculating a GRS on basis of 12 BMI-predisposing variants.8 Moreover, our data suggest that there is no particular threshold of physical activity levels attenuating the genetic effects on BMI.
One of our novel findings is that prolonged TV watching accentuates the genetic predisposition to increased BMI independent of physical activity levels. Our results are consistent with our previously reported independent associations of TV watching and physical activity with risk of overweight, obesity and related disease in men and women.12-14 These findings do not necessarily imply that TV watching per se causally interacts with genetic predisposition to increased BMI. Individuals who spent more time watching tended to have lower energy expenditure, as TV watching even results in lower energy expenditure than other sedentary activities.23 Prolonged TV watching is associated with increased food and total calorie intake because TV watching is often accompanied by eating.13, 36, 37 In addition, our previous studies also showed that individuals who spent more time watching TV tended to follow an unhealthy eating pattern13 associated with risk of obesity and related disease.38, 39 Taken together, an unhealthy lifestyle indicated by prolonged TV watching may accentuate the genetic predisposition to increased BMI and obesity risk. Further studies are needed to tease out the factors driving the observed interactions.
Our findings may have important public health implications, as we have shown that sedentary behaviors indicated by prolonged TV watching accentuated the genetic effects on BMI, whereas greater leisure-time physical activity attenuated the genetic predisposition to increased BMI. These results provide important evidence that deleterious effects of genetic factors could be modified (attenuated or accentuated) by lifestyle factors and challenge the common notion that genetic predisposition to obesity cannot be overcome. Our data strongly suggest that a more active lifestyle with both increased exercise and reduced sedentary behaviors in leisure time, especially TV watching, is critical to reduce obesity risk, especially among those with high genetic risk. Furthermore, we observed comparable magnitudes of reduction in the genetic effect on BMI with walking (1h/d) and vigorous activity (0.5 h/d), when total energy expenditure was similar. Though people need to spend more time on walking than on vigorous activity to have similar benefits, walking is highly accessible, readily adopted, and rarely associated with physical activity-related injury,40 especially for the middle-aged and older populations. In addition to increasing exercise levels, we should further emphasize reducing TV watching time since TV watching is the most commonly reported daily activity in free time in many populations around the world.11 It has been speculated that TV watching might have contributed to the obesity epidemic in the United States.14 Our data provide further evidence that this might be partly through exaggeration of the genetic predisposition to increased BMI.
The major strengths of this study included consistent findings in two independent and well-established prospective cohorts, detailed and multiple assessments of physical activity, TV watching and BMI, comprehensive coverage of established BMI associated genetic variants, and minimal population stratification. Our repeated measures analysis using longitudinally collected data should have reduced random-measurement error and enhanced the robustness of our findings. Several limitations need to be acknowledged. Physical activity, TV watching and BMI were self-reported, but these data have been well validated with high correlations with measured data.24, 25 Samples with incident cases of chronic diseases were included in the current analysis. However, this approach has been validated in the previous GWAS for BMI.3 Our sensitivity analyses with the exclusion of incident cases showed similar results. This is consistent with the results from a previous study where exclusion of individuals with present cardiovascular disease and cancer only slightly changed the observed interaction.8 Although our GRS captured the combined information from the established BMI-associated variants so far, it accounts for only a small amount of variation in BMI.3 It remains to be examined whether our results could be generalized to other ethnic groups.
In conclusion, our data provide consistent evidence from two independent cohorts that sedentary lifestyle indicated by prolonged TV watching may accentuate the genetic predisposition to high BMI. In contrast, increasing leisure-time physical activity may attenuate the genetic effect on BMI. These findings emphasize the important roles of both increasing exercise levels and reducing sedentary behaviors in public health efforts to prevent obesity, particularly in individuals who are more genetically predisposed to obesity.
Supplementary Material
Clinical Perspective.
Sedentary behavior and physical inactivity have independent effects on obesity and related cardiometabolic diseases. Previous studies on gene-lifestyle interaction and obesity have mostly focused on the FTO gene and physical activity, while little attention has been paid to sedentary behavior as indicated by television (TV) watching. In two prospective cohorts with 7740 women and 4564 men, we calculated a genetic risk score based on 32 established body mass index (BMI)-associated variants to capture the overall genetic susceptibility; and to examine its interactions with leisure-time TV watching and physical activity in relation to adiposity. Our results indicate that prolonged TV watching might accentuate genetic predisposition to elevated adiposity, whereas greater leisure-time physical activity might attenuate the genetic association. These findings suggest that deleterious effects of genetic factors could be modified by lifestyle factors and challenge the common lay perception of deterministic genetic predisposition to obesity. Our data provide new information in understanding the interplay between genes and environment, and also emphasize the important roles of both increasing exercise levels and reducing sedentary behaviors in public health efforts to prevent obesity and related cardiometabolic diseases, particularly in individuals who are more genetically predisposed to obesity.
Acknowledgements
We thank all the participants of the NHS and the HPFS for their continued cooperation.
Sources of Funding: This study was supported by grants DK091718, HL071981, HL073168, CA87969, CA49449, CA055075, HL34594, HL088521, U01HG004399, DK080140, 5P30DK46200, U54CA155626, DK58845, U01HG004728-02, EY015473, DK70756 and DK46200 from the National Institutes of Health, with additional support for genotyping from Merck Research Laboratories, North Wales, PA. Dr. Lu Qi is a recipient of the American Heart Association Scientist Development Award (0730094N). Dr. Pasquale is supported by Research to Prevent Blindness (NYC) and a Harvard Ophthalmology Scholar Award (Harvard Medical School) from the Harvard Glaucoma Center of Excellence.
Footnotes
Conflict of Interest Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–694. doi: 10.1111/j.1753-4887.2008.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan Ja, Magi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segre AV, Estrada K, Liang L, Nemesh J, Park J-H, Gustafsson S, Kilpelainen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga J-J, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JRB, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson J-O, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MTS, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AIF, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proenca C, Chen Y-DI, Chen C-M, Chines PS, Clarke R, Coin L, Connell J, Day INM, Heijer Md, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJC, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Graszler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen A-L, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jorgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, Konig IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaloy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimaki T, Lettre G, Liu J, Lokki M-L, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O’Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Pare G, Parker AN, Perola M, Pichler I, Pietilainen KH, Platou CGP, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstrale M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo M-L, Tardif J-C, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tonjes A, Tuomi T, van Meurs JBJ, van Ommen G-J, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CIG, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kahonen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Gronberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin M-R, Kaprio J, Karpe F, Khaw K-T, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PEH, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann HE, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, I McCarthy M, Hirschhorn JN, Ingelsson E, Loos RJF. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, Nielsen AL, Albrechtsen A, Borch-Johnsen K, Rasmussen SS, Clausen JO, Sandbaek A, Lauritzen T, Hansen L, Jørgensen T, Pedersen O, Hansen T. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 5.Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, O’Connell JR, Ducharme JL, Hines S, Sack P, Naglieri R, Shuldiner AR, Snitker S. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168:1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vimaleswaran KS, Li S, Zhao JH, Luan Ja, Bingham SA, Khaw K-T, Ekelund U, Wareham NJ, Loos RJF. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr. 2009;90:425–428. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- 7.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90:1418–1425. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Zhao JH, Luan Ja, Ekelund U, Luben RN, Khaw K-T, Wareham NJ, Loos RJF. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk Prospective Population Study. PLoS Med. 2010;7:e1000332. doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, Ahmad T, Mora S, Kaakinen M, Sandholt CH, Holzapfel C, Autenrieth CS, Hyppönen E, Cauchi S, He M, Kutalik Z, Kumari M, Stančáková A, Meidtner K, Balkau B, Tan JT, Mangino M, Timpson NJ, Song Y, Zillikens MC, Jablonski KA, Garcia ME, Johansson S, Bragg-Gresham JL, Wu Y, van Vliet-Ostaptchouk JV, Onland-Moret NC, Zimmermann E, Rivera NV, Tanaka T, Stringham HM, Silbernagel G, Kanoni S, Feitosa MF, Snitker S, Ruiz JR, Metter J, Larrad MT, Atalay M, Hakanen M, Amin N, Cavalcanti-Proença C, Grøntved A, Hallmans G, Jansson JO, Kuusisto J, Kähönen M, Lutsey PL, Nolan JJ, Palla L, Pedersen O, Pérusse L, Renström F, Scott RA, Shungin D, Sovio U, Tammelin TH, Rönnemaa T, Lakka TA, Uusitupa M, Rios MS, Ferrucci L, Bouchard C, Meirhaeghe A, Fu M, Walker M, Borecki IB, Dedoussis GV, Fritsche A, Ohlsson C, Boehnke M, Bandinelli S, van Duijn CM, Ebrahim S, Lawlor DA, Gudnason V, Harris TB, Sørensen TI, Mohlke KL, Hofman A, Uitterlinden AG, Tuomilehto J, Lehtimäki T, Raitakari O, Isomaa B, Njølstad PR, Florez JC, Liu S, Ness A, Spector TD, Tai ES, Froguel P, Boeing H, Laakso M, Marmot M, Bergmann S, Power C, Khaw KT, Chasman D, Ridker P, Hansen T, Monda KL, Illig T, Järvelin MR, Wareham NJ, Hu FB, Groop LC, Orho-Melander M, Ekelund U, Franks PW, Loos RJF. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8:e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad T, Lee IM, Paré G, Chasman DI, Rose L, Ridker PM, Mora S. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care. 2011;34:675–680. doi: 10.2337/dc10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality. JAMA. 2011;305:2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching PL, Willett WC, Rimm EB, Colditz GA, Gortmaker SL, Stampfer MJ. Activity level and risk of overweight in male health professionals. Am J Public Health. 1996;86:25–30. doi: 10.2105/ajph.86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical Activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 15.Dunstan DW, Barr ELM, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N. Television viewing time and mortality. Circulation. 2010;121:384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Manson JE, Hankinson SE. The Nursesá Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 18.Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, Berndt SI, Boerwinkle E, Chanock S, Chatterjee N, Couper D, Curhan G, Heiss G, Hu FB, Hunter DJ, Jacobs K, Jensen MK, Kraft P, Landi MT, Nettleton JA, Purdue MP, Rajaraman P, Rimm EB, Rose LM, Rothman N, Silverman D, Stolzenberg-Solomon R, Subar A, Yeager M, Chasman DI, van Dam RM, Caporaso NE. Genome-wide meta-analysis identifies regions on 7p21 AHR and 15q24 CYP1A2 as determinants of habitual caffeine consumption. PLoS Genet. 2011;7:e1002033. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF, Hoover RN, Thomas G, Chanock SJ. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi L, Cornelis MC, Kraft P, Stanya KJ, Linda Kao WH, Pankow JS, Dupuis Je, Florez JC, Fox CS, Paré G, Sun Q, Girman CJ, Laurie CC, Mirel DB, Manolio TA, Chasman DI, Boerwinkle E, Ridker PM, Hunter DJ, Meigs JB, Lee CH, Meta-Analysis of Glucose and Insulin-related traits Consortium (MAGIC) Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium. van Dam RM, Hu FB. Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet. 2010;19:2706–2715. doi: 10.1093/hmg/ddq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiggs JL, Hee Kang J, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W, DelBono E, Loomis S, Haines JL, Pasquale LR. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20:4707–4713. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MK, Pers TH, Dworzynski P, Girman CJ, Brunak Sr, Rimm EB. Protein interaction-based genome-wide analysis of incident coronary heart disease. Circ Cardiovasc Genet. 2011;4:549–556. doi: 10.1161/CIRCGENETICS.111.960393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr., Montoye HJ, Sallis JF, Paffenbarger RS., Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 28.Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, Cai T, Hunter DJ, Hu FB. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med. 2009;150:541–550. doi: 10.7326/0003-4819-150-8-200904210-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz JR, Labayen I, Ortega FB, Legry V, Moreno LA, Dallongeville J, Martinez-Gomez D, Bokor S, Manios Y, Ciarapica D, Gottrand F, De Henauw S, Molnar D, Sjostrom M, Meirhaeghe A, for the HSG Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: the HELENA Study. Arch Pediatr Adolesc Med. 2010;164:328–333. doi: 10.1001/archpediatrics.2010.29. [DOI] [PubMed] [Google Scholar]
- 31.Tan JT, Dorajoo R, Seielstad M, Sim XL, Ong RT-H, Chia KS, Wong TY, Saw SM, Chew SK, Aung T, Tai ES. FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes. 2008;57:2851–2857. doi: 10.2337/db08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cauchi Sp, Stutzmann F, Cavalcanti-Proença C, Durand E, Pouta A, Hartikainen A-L, Marre M, Vol S, Tammelin T, Laitinen J, Gonzalez-Izquierdo A, Blakemore A, Elliott P, Meyre D, Balkau B, Järvelin MR, Froguel P. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med. 2009;87:537–546. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- 33.Hakanen M, Raitakari OT, Lehtimäki T, Peltonen N, Pahkala K, Sillanmäki L, Lagström H, Viikari J, Simell O, Rönnemaa T. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab. 2009;94:1281–1287. doi: 10.1210/jc.2008-1199. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson A, Renström F, Lyssenko V, Brito E, Isomaa B, Berglund G, Nilsson P, Groop L, Franks P. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia. 2009;52:1334–1338. doi: 10.1007/s00125-009-1355-2. [DOI] [PubMed] [Google Scholar]
- 35.Demerath EW, Lutsey PL, Monda KL, Linda Kao WH, Bressler J, Pankow JS, North KE, Folsom AR. Interaction of FTO and physical activity level on adiposity in African-American and European-American adults: the ARIC Study. Obesity. 2011;19:1866–72. doi: 10.1038/oby.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vereecken CA, Todd J, Roberts C, Mulvihill C, Maes L. Television viewing behaviour and associations with food habits in different countries. Public Health Nutr. 2006;9:244–250. doi: 10.1079/phn2005847. [DOI] [PubMed] [Google Scholar]
- 37.Wiecha JL, Peterson KE, Ludwig DS, Kim J, Sobol A, Gortmaker SL. When children eat what they watch: impact of television viewing on dietary intake in youth. Arch Pediatr Adolesc Med. 2006;160:436–442. doi: 10.1001/archpedi.160.4.436. [DOI] [PubMed] [Google Scholar]
- 38.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 39.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136:201–209. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 40.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women. JAMA. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.