Abstract
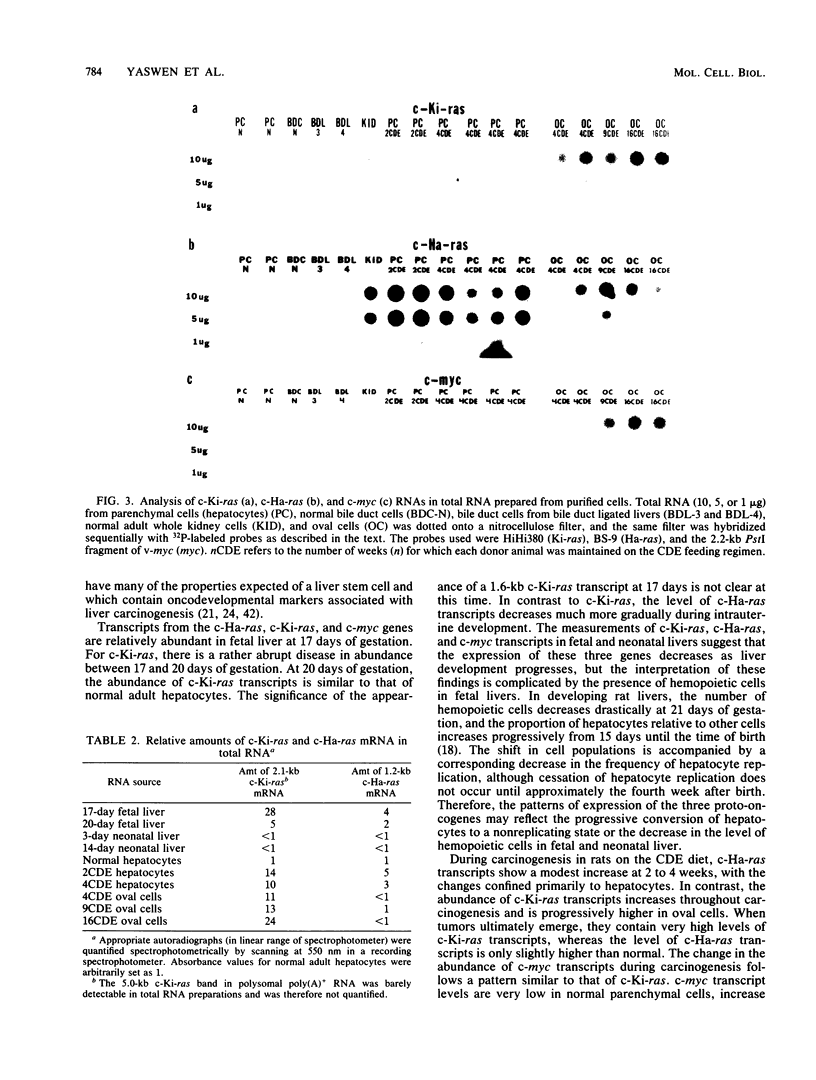
We examined the expression of six proto-oncogenes in (i) whole rat liver and isolated liver cell populations during the course of hepatocarcinogenesis induced by a choline-deficient diet containing 0.1% ethionine and (ii) fetal rat liver at different stages of development. The abundance of c-Ki-ras, c-Ha-ras, and c-myc transcripts in polysomal polyadenylated RNA from liver cells increased by 2 weeks after the start of the carcinogenic diet. c-Ki-ras and c-myc expression remained elevated during the 35 weeks of the diet, whereas c-Ha-ras transcripts increased transiently. A primary tumor sampled at 35 weeks after the carcinogenic diet was started contained high levels of both c-Ki-ras and c-myc RNA. The abundance of c-src transcripts was unchanged throughout carcinogenesis; c-abl and c-mos transcripts were not detected in either preneoplastic or neoplastic livers. To determine which cell types within the liver contained proto-oncogene transcripts, we isolated hepatocytes, oval cells, and bile duct cells from normal and preneoplastic livers. The results indicate that proto-oncogenes are expressed differentially in these cell types during hepatocarcinogenesis and that the expression of c-Ki-ras and c-myc is high in oval cells throughout carcinogenesis. In developing livers, c-Ki-ras, c-Ha-ras, and c-myc transcript levels were high at 17 days of gestation but reached the low values characteristic of adult rat livers between 20 days of gestation and 3 days after birth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Lemire J. M., Halvorson H. O. Physiological control of repressible acid phosphatase gene transcripts in Saccharomyces cerevisiae. Mol Cell Biol. 1983 May;3(5):839–853. doi: 10.1128/mcb.3.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corcos D., Defer N., Raymondjean M., Paris B., Corral M., Tichonicky L., Kruh J., Glaise D., Saulnier A., Guguen-Guillouzo C. Correlated increase of the expression of the c-ras genes in chemically induced hepatocarcinomas. Biochem Biophys Res Commun. 1984 Jul 18;122(1):259–264. doi: 10.1016/0006-291x(84)90468-6. [DOI] [PubMed] [Google Scholar]
- Craig R. W., Bloch A. Early decline in c-myb oncogene expression in the differentiation of human myeloblastic leukemia (ML-1) cells induced with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 Feb;44(2):442–446. [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempo K., Chisaka N., Yoshida Y., Kaneko A., Onoé T. Immunofluorescent study on alpha-fetoprotein-producing cells in the early stage of 3'-methyl-4-dimethylaminoazobenzene carcinogenesis. Cancer Res. 1975 May;35(5):1282–1287. [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Furth M. E., Scolnick E. M. Mouse cells contain two distinct ras gene mRNA species that can be translated into a p21 onc protein. Mol Cell Biol. 1982 Nov;2(11):1339–1345. doi: 10.1128/mcb.2.11.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Fausto N., Schultz-Ellison G., Atryzek V., Goyette M. Distribution and specificity of sequences in polyadenylated nuclear RNA of normal, regenerating, and neoplastic liver. J Biol Chem. 1982 Mar 10;257(5):2200–2206. [PubMed] [Google Scholar]
- Fausto N., Shank P. R. Oncogene expression in liver regeneration and hepatocarcinogenesis. Hepatology. 1983 Nov-Dec;3(6):1016–1023. doi: 10.1002/hep.1840030621. [DOI] [PubMed] [Google Scholar]
- Ghoshal A. K., Ahluwalia M., Farber E. The rapid induction of liver cell death in rats fed a choline-deficient methionine-low diet. Am J Pathol. 1983 Dec;113(3):309–314. [PMC free article] [PubMed] [Google Scholar]
- Giambarresi L. I., Katyal S. L., Lombardi B. Promotion of liver carcinogenesis in the rat by a choline-devoid diet: role of liver cell necrosis and regeneration. Br J Cancer. 1982 Nov;46(5):825–829. doi: 10.1038/bjc.1982.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillouzo A., Belanger L., Beaumont C., Valet J. P., Briggs R., Chiu J. F. Cellular and subcellular immunolocalization of alpha1-fetoprotein and albumin in rat liver. Reevaluation of various experimental conditions. J Histochem Cytochem. 1978 Nov;26(11):948–959. doi: 10.1177/26.11.82574. [DOI] [PubMed] [Google Scholar]
- Guillouzo A., Weber A., Le Provost E., Rissel M., Schapira F. Cell types involved in the expression of foetal aldolases during rat azo-dye hepatocarcinogenesis. J Cell Sci. 1981 Jun;49:249–260. doi: 10.1242/jcs.49.1.249. [DOI] [PubMed] [Google Scholar]
- Hayner N. T., Braun L., Yaswen P., Brooks M., Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):332–338. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kuhlmann W. D. Localization of alpha1-fetoprotein and DNA-synthesis in liver cell populations during experimental hepatocarcinogenesis in rats. Int J Cancer. 1978 Mar 15;21(3):368–380. doi: 10.1002/ijc.2910210319. [DOI] [PubMed] [Google Scholar]
- Makino R., Hayashi K., Sato S., Sugimura T. Expressions of the c-Ha-ras and c-myc genes in rat liver tumors. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1092–1102. doi: 10.1016/0006-291x(84)90887-8. [DOI] [PubMed] [Google Scholar]
- Mintz B., Fleischman R. A. Teratocarcinomas and other neoplasms as developmental defects in gene expression. Adv Cancer Res. 1981;34:211–278. doi: 10.1016/s0065-230x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Adamson E. D., Tremblay J. M., Müller D., Cline M. J., Verma I. M. Transcription of c-onc genes c-rasKi and c-fms during mouse development. Mol Cell Biol. 1983 Jun;3(6):1062–1069. doi: 10.1128/mcb.3.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Minase T., Onhoe T. Demonstration of glucose 6-phosphatase activity in the oval cells of rat liver and the significance of the oval cells in azo dye carcinogenesis. Cancer Res. 1974 Dec;34(12):3379–3386. [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Petropoulos C., Andrews G., Tamaoki T., Fausto N. alpha-Fetoprotein and albumin mRNA levels in liver regeneration and carcinogenesis. J Biol Chem. 1983 Apr 25;258(8):4901–4906. [PubMed] [Google Scholar]
- Potter V. R. Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br J Cancer. 1978 Jul;38(1):1–23. doi: 10.1038/bjc.1978.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scholla C. A., Tedeschi M. V., Fausto N. Gene expression and the diversity of polysomal messenger RNA sequences in regenerating liver. J Biol Chem. 1980 Apr 10;255(7):2855–2860. [PubMed] [Google Scholar]
- Sell S., Leffert H. L. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982 Jan-Feb;2(1):77–86. doi: 10.1002/hep.1840020113. [DOI] [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R. M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978 Apr;38(4):1092–1098. [PubMed] [Google Scholar]
- Sirica A. E., Cihla H. P. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984 Aug;44(8):3454–3466. [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriel J. Cancer, retrodifferentiation, and the myth of Faust. Cancer Res. 1976 Nov;36(11 Pt 2):4269–4275. [PubMed] [Google Scholar]
- Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982 Jun;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen P., Hayner N. T., Fausto N. Isolation of oval cells by centrifugal elutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):324–331. [PubMed] [Google Scholar]