Abstract
Attenuated measles viruses (MV) are assessed in clinical trials for their capacity to preferentially infect and kill tumor cells. We recently showed that MV-infected tumor cells are able to activate tumor antigen cross-presentation by myeloid and plasmacytoid dendritic cells. Thus, MV-based antitumor virotherapy may stimulate antitumor immune response.
Keywords: cytotoxic CD8+ T cell, measles virus vaccine, myeloid DC, oncolytic virus, plasmacytoid DC, tumor antigen
Antitumor virotherapy using oncolytic viruses (OV) is currently assessed in numerous clinical trials.1 This therapeutic approach consists of the use of attenuated and/or genetically modified replication-competent virus to preferentially infect and kill tumor cells, with limited infection, if any, of healthy cells.
Among the different oncolytic viruses, vaccine-attenuated strains of measles virus (MV) are promising candidates for cancer therapy.2 MV is an enveloped and negative, non-segmented, single-strand RNA virus belonging to the Morbillivirus genus of the Paramyxoviridae family. Attenuated MV strains use the CD46 molecule as a main entry receptor into cells.3 This molecule negatively regulates the complement system and is frequently overexpressed by tumor cells to escape lysis by the complement system.4 Thus, MV oncolytic activity has been reported for numerous types of human cancers in vitro, and in immunodeficient mouse models of human tumor xenografts.2 Phase I clinical trials of anti-tumor virotherapy using “Edmonston” attenuated strain of MV are currently underway for ovarian cancer, glioma, myeloma, mesothelioma and squamous cell cancer of the head and neck.1
The antitumor activity of MV attenuated strains is due to their ability to preferentially infect and kill tumor cells. In addition, the capacity of MV-infected tumor cells to activate cells from the immune response, especially dendritic cells (DC), may also play a role. Indeed, we previously reported that mesothelioma tumor cells infected by the Schwarz attenuated strain of MV are able to induce monocyte-derived DC (Mo-DC) maturation in vitro, while UV-irradiated tumor cells are not.5 This maturation is induced by danger-associated molecular patterns (DAMP) released by MV-infected tumor cells, since the MV alone is not able to induce maturation of Mo-DC. We also showed that MV-infected tumor cells express the heat-shock proteins (HSP) HSP70 and gp96, which are not expressed by UV-irradiated tumor cells. These HSP could be at least partially responsible for Mo-DC maturation. Finally, we observed that Mo-DC exposed to MV-infected tumor cells, but not UV-irradiated ones, cross-prime a CD8+ T lymphocyte response specific for mesothelin, a tumor-associated antigen (TAA) expressed by mesothelioma tumor cells. Similar results were reported by Donnelly et al., who showed that melanoma tumor cells infected by the Edmonston strain of MV are able to activate Mo-DC, that can then cross-prime a CD8+ T lymphocyte response with a cytotoxic activity against melanoma tumor cells.6 Moreover, they observed that MV-infected melanoma tumor cells release DAMP such as HMGB1, or inflammatory cytokines such as type I IFN (IFNα and IFNβ), IL-6 and IL-8, which participate in Mo-DC maturation. Altogether, these studies show that MV infection of tumor cells induce an immunologic cell death capable of stimulating myeloid DC, notably their capacity to cross-prime antitumoral T cell responses.
We extended our study by looking at the effects of MV-infected tumor cells on plasmacytoid DC (pDC).7 This subset of DC is also known as IFN-producing cells, which produce a huge quantity of type I IFN (IFNα, IFNβ, IFNω) when exposed to viruses, notably Influenza virus, Herpes Simplex virus and HIV.8 Their expression of Toll-like receptors (TLR), in particular TLR-7 and TLR-9, allow them to detect viral nucleic acids. They also participate to the activation of NK and T cell responses. In cancer immunotherapy, there is an increasing interest for immunostimulatory molecules, such as CpG and Imiquimod, which are able to activate myeloid DC and/or pDC.9
In our recent in vitro study, we showed using the Schwarz strain of MV engineered to express the enhanced-green fluorescent protein that MV does not infect pDC, whereas it infects and kills different tumor cell lines (melanoma, mesothelioma and lung adenocarcinoma).7 We also observed that pDC exposed to MV-infected tumor cells express the maturation marker CD83, and produce huge amounts of IFNα. This IFNα production can be completely inhibited by the use of the TLR-7 specific inhibitor IRS661. Thus pDC activation is probably due to the triggering of TLR-7 by MV single-strand RNA. IFNα, and more generally type I IFN, have positive effects on the antitumor immune response.10 By confocal microscopy and flow cytometry, we observed that pDC internalized fragments of MV-infected tumor cells. To determine if this internalization allowed pDC to cross-present tumor antigens, we used a CD8+ T cell clone obtained from a melanoma tumor biopsy and specific for a peptide of New York esophageal-1 squamous cell carcinoma antigen (NY-ESO-1) presented in the HLA-A*0201 context. NY-ESO-1 is a TAA expressed by different types of cancer and not expressed by normal tissue except testis. We showed that HLA-A*0201pos pDC exposed to HLA-A*0201neg/NY-ESO-1pos melanoma tumor cells are able to activate IFN-γ production by the NY-ESO-1 specific CD8+ T cell clone. A similar cross-presentation was observed with mo-DC. In contrast, UV-irradiated tumor cells were not able to induce NY-ESO-1 cross-presentation by pDC or Mo-DC, nor IFNα production by pDC.
Taken together, these results show that attenuated strains of MV induce an immunogenic cell death of tumor cells capable of activating myeloid and plasmacytoid DC (Fig. 1). It also allows the release of tumor antigens for cross-presentation by both subsets of DC. Thus, attenuated MV are promising antitumor agents with interesting effects on the antitumor immune response.
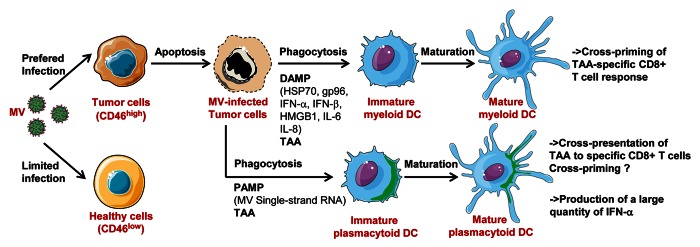
Figure 1. Attenuated measles virus (MV) antitumoral virotherapy activates myeloid and plasmacytoid dendritic cells, notably their capacity to cross-present tumor-associated antigens (TAA): MV preferentially infects tumor cells due to their overexpression of CD46. MV-infected tumor cells are lysed. They induce maturation of myeloid DC, mainly by DAMP expression (HSP70, gp96, IFNα, IFNβ, HMGB1, IL-6, IL-8), and maturation of plasmacytoid DC, mainly by MV single-strand RNA, a pathogen associated molecular pattern (PAMP) that triggers TLR7 expressed by pDC. DC internalized fragments of MV-infected tumor cells and cross-present TAA to specific CD8+ T cells. It has been demonstrated for myeloid DC that this cross-presentation can result in cross-priming, whereas this has not been demonstrated for pDC. pDC also produce large amount of IFN-α in response to tumor infected cells. This figure has been made using Servier Medical Art.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24212
References
- 1.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillerme JB, Grégoire M, Tangy F, Fonteneau JF. Antitumor virotherapy by attenuated measles virus (MV) Biology. 2013;2:587–602. doi: 10.3390/biology2020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–26. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 4.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–23. doi: 10.1016/S0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 5.Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F, Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–92. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20:7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillerme JB, Boisgerault N, Roulois D, Ménager J, Combredet C, Tangy F, et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res. 2013;19:1147–58. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 8.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 9.Tel J, van der Leun AM, Figdor CG, Torensma R, de Vries IJ. Harnessing human plasmacytoid dendritic cells as professional APCs. Cancer Immunol Immunother. 2012;61:1279–88. doi: 10.1007/s00262-012-1210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aricò E, Belardelli F. Interferon-α as antiviral and antitumor vaccine adjuvants: mechanisms of action and response signature. J Interferon Cytokine Res. 2012;32:235–47. doi: 10.1089/jir.2011.0077. [DOI] [PubMed] [Google Scholar]


