Differentiating xylem tissues of both hybrid poplar and Japanese cypress exhibits almost identical transport properties of coniferin, a monolignol glucoside, suggesting the involvement of a common endomembrane H+/coniferin antiport mechanism in the lignifying tissues of woody plants.
Abstract
Lignin biosynthesis is an essential physiological activity of vascular plants if they are to survive under various environmental stresses on land. The biosynthesis of lignin proceeds in the cell wall by polymerization of precursors; the initial step of lignin polymerization is the transportation of lignin monomers from the cytosol to the cell wall, which is critical for lignin formation. There has been much debate on the transported form of the lignin precursor, either as free monolignols or their glucosides. In this study, we performed biochemical analyses to characterize the membrane transport mechanism of lignin precursors using angiosperms, hybrid poplar (Populus sieboldii × Populus grandidentata) and poplar (Populus sieboldii), as well gymnosperms, Japanese cypress (Chamaecyparis obtusa) and pine (Pinus densiflora). Membrane vesicles prepared from differentiating xylem tissues showed clear ATP-dependent transport activity of coniferin, whereas less than 4% of the coniferin transport activity was seen for coniferyl alcohol. Bafilomycin A1 and proton gradient erasers markedly inhibited coniferin transport in hybrid poplar membrane vesicles; in contrast, vanadate had no effect. Cis-inhibition experiments suggested that this transport activity was specific for coniferin. Membrane fractionation of hybrid poplar microsomes demonstrated that transport activity was localized to the tonoplast- and endomembrane-rich fraction. Differentiating xylem of Japanese cypress exhibited almost identical transport properties, suggesting the involvement of a common endomembrane-associated proton/coniferin antiport mechanism in the lignifying tissues of woody plants, both angiosperms and gymnosperms.
Lignin is essential for efficient water flow from roots to leaves and terminal buds, resistance to biotic attack, and contributions to compressive strength in vascular plants (Whetten et al., 1998). Lignification proceeds in three steps: (1) biosynthesis of lignin precursors in the cell; (2) transport of the precursors to the cell wall; and (3) dehydrogenative polymerization of the precursors in the cell wall (Boudet et al., 1995; Whetten and Sederoff, 1995). Although the biosynthesis and dehydrogenative polymerization of lignin precursors have been intensely studied (Neish, 1968; Higuchi, 1985; Whetten et al., 1998; Boerjan et al., 2003), the transport mechanism of lignin precursors has not been determined.
Monolignols (e.g. p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol) are thought to be the main and direct precursors of lignin. Monolignols are produced from the general phenylpropanoid pathway, and the final step of monolignol synthesis is catalyzed by cinnamyl alcohol dehydrogenase (Whetten et al., 1998; Boerjan et al., 2003; Supplemental Fig. S1). It has been suggested that this enzyme is localized in the cytosol in woody plants as well as in herbal model plants (Takabe et al., 2001); that is, the completion of each lignin building unit, such as coniferyl alcohol or sinapyl alcohol, occurs in the cytosol. Feeding experiments using radiolabeled monolignol intermediates clearly showed the incorporation of the radiolabel into the lignin in the cell wall (Pickett-Heaps, 1968; Wooding, 1968; Takabe et al., 1985; Kaneda et al., 2008). These results indicate that lignin precursors are transported from the cytosol to the cell wall across the plasma membrane (Supplemental Fig. S1). Several transport mechanisms of lignin precursors across the plasma membrane can be hypothesized and classified into four patterns: passive diffusion, active primary transport, active secondary transport, and vesicle transport by the endomembrane system (Supplemental Fig. S2).
There has been a debate for more than 50 years over whether monolignols are transported as free forms or their derivatives, such as glucosides. Coniferin, a coniferyl alcohol glucoside, has been detected in the cambial sap of many tree species (Whetten et al., 1998). Feeding radiolabeled coniferin to xylem tissue demonstrated the incorporation of radioactivity into the cell wall lignin (Terashima and Fukushima, 1988; Tsuji and Fukushima, 2004), indicating that coniferin serves as a precursor for lignification. Several other studies have reported that coniferin may be a storage form that is accumulated in the vacuole, from which it is utilized when and where lignification occurs (Whetten et al., 1998; Samuels et al., 2002; Morikawa et al., 2010). Conventional methods such as autoradiography that are often applied to studies of lignin biosynthesis could not identify the direct substrate transported; thus, the transport mechanism is still unknown.
Recently, biochemical experiments using microsomal fractions from rosette leaves of Arabidopsis (Arabidopsis thaliana) indicated the possible involvement of an ATP-binding cassette (ABC)-like transporter in the transport of lignin precursors (Miao and Liu, 2010). In Arabidopsis leaves, however, lignification occurs in only a limited number of cells at the leaf vein, and the majority of microsomes are derived from mesophyll cells.
Hence, we used differentiating xylem tissues, in which most cells are actively lignifying, of several tree species as plant materials suitable for monolignol transport experiments. We found that the main transport activity is a proton (H+) gradient-dependent transport of coniferin across the tonoplast and endomembrane compartments, which is common to both hybrid poplar (Populus sieboldii × Populus grandidentata) and Japanese cypress (Chamaecyparis obtusa). The transport of free monolignols is less than 4% that of coniferin in these tree species. Our results suggested that an H+/coniferin antiporter on the tonoplast and endomembrane is commonly involved in the transport of monolignol glucoside in the differentiating xylem tissues of woody plants, in most cells of which active lignification proceeds.
RESULTS
Membrane Vesicle Preparation from Differentiating Xylem under Lignification
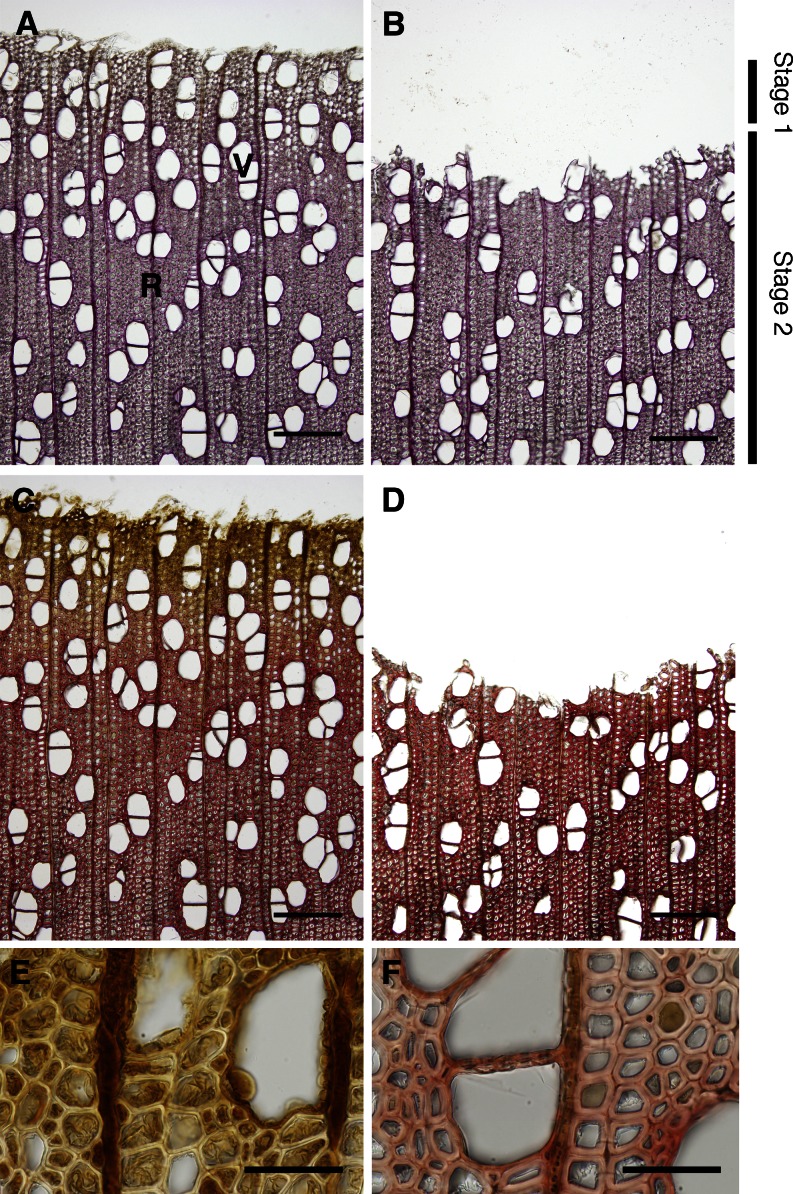
We used tree species to investigate monolignol transport because high lignification activity takes place in differentiating xylem tissues. We harvested differentiating xylem tissues of a 35-year-old hybrid poplar in early July, a time at which most active lignification occurs. Figure 1 shows the differentiating xylem of the hybrid poplar used in this study before and after scraping. Almost all cambial zone was removed when the bark was peeled off. The Wiesner reaction (Fig. 1, A and B) showed the lignin distribution as red-purple staining, and the Mäule reaction (Fig. 1, C–F) revealed syringyl lignin distribution highlighted by red-purple staining. Both color reactions in scraped tissues gradually increased toward the mature side. The scraped xylem contained fibers and vessels in the secondary wall formation stage and a few radial parenchyma cells. We obtained the scraped xylem from four tree species (i.e., hybrid poplar and poplar [Populus sieboldii] as representative angiosperms, and Japanese cypress and pine [Pinus densiflora] as representative gymnosperms). The frozen xylem tissues were homogenized and washed, and the membrane vesicles was prepared as described in “Materials and Methods.”
Figure 1.
Histochemical analyses of the extent of lignification of hybrid poplar. After bark removal, differentiating xylem was scraped and gathered. Images in A, C, E, and F were taken before scraping the differentiating xylem, and those in B and D were taken after scraping. A and B, Wiesner reaction of sections showing lignin distribution as red-purple staining. C to F, Mäule reaction of sections showing syringyl lignin distribution as red-purple staining. E and F show scraped xylem magnified images of C, with differentiating before lignifying (E) and lignifying vigorously in the secondary wall (F). Stage 1 contained fibers under thickening of the secondary wall; stage 2 consisted of fibers after secondary wall thickening. R, Ray parenchyma; V, vessel. Bars = 200 µm (A–D) and 50 µm (E and F).
ATP-Dependent Uptake of Coniferin by Microsomal Fractions of Several Trees
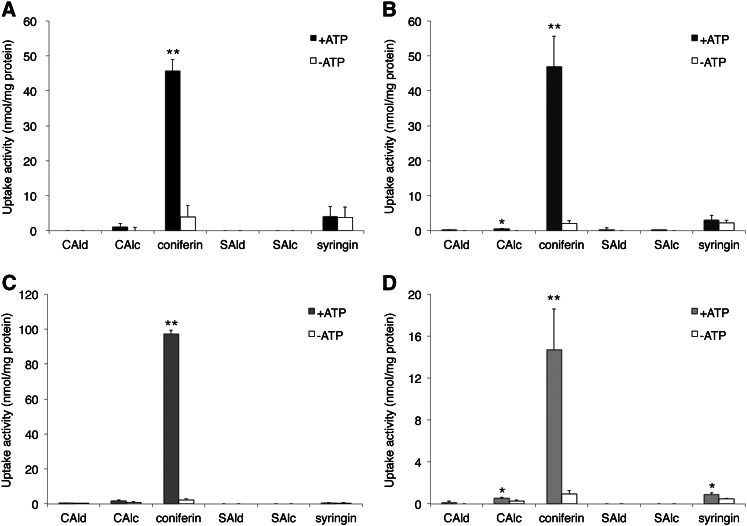
The transport assay method was adapted from that described for alkaloid transport (Otani et al., 2005). Figure 2 shows the uptake of various putative lignin precursors (Supplemental Fig. S3) into the membrane vesicles from differentiating xylem of two angiosperms and two gymnosperms. Strong uptake activity was observed for coniferin in the four tree species, but only in the presence of magnesium (Mg)/ATP. ATP-dependent transport activity was detected for coniferyl alcohol and coniferyl aldehyde (Fig. 2; Supplemental Fig. S4), but even the maximum uptake of these compounds was only 4% of coniferin. Almost no uptake activity was observed for sinapyl alcohol or sinapaldehyde in any tree species tested in this study. We harvested differentiating xylem tissues once per year, and repeated the experiments over 3 years to obtain similar ATP-dependent coniferin uptake (Supplemental Fig. S5). For more detailed biochemical investigations, we used mainly hybrid poplar in the following experiments because of the availability of sufficient plant materials.
Figure 2.
Uptake of various putative lignin precursors into membrane vesicles. Membrane vesicles were incubated with 50 μm of each compound in the presence or absence of 5 mm Mg/ATP for 20 min. Uptake activities of lignin precursors in membrane vesicles were obtained from differentiating xylem of hybrid poplar (A), wild poplar (B), Japanese cypress (C), and pine (D). CAld, Coniferyl aldehyde; CAlc, coniferyl alcohol; SAld, sinapaldehyde; SAlc, sinapyl alcohol. Data are means ± sd of three replicates. *P < 0.05, **P < 0.01 compared with/without ATP by Student’s t test.
Characterization of the Coniferin Uptake Mechanism of Hybrid Poplar
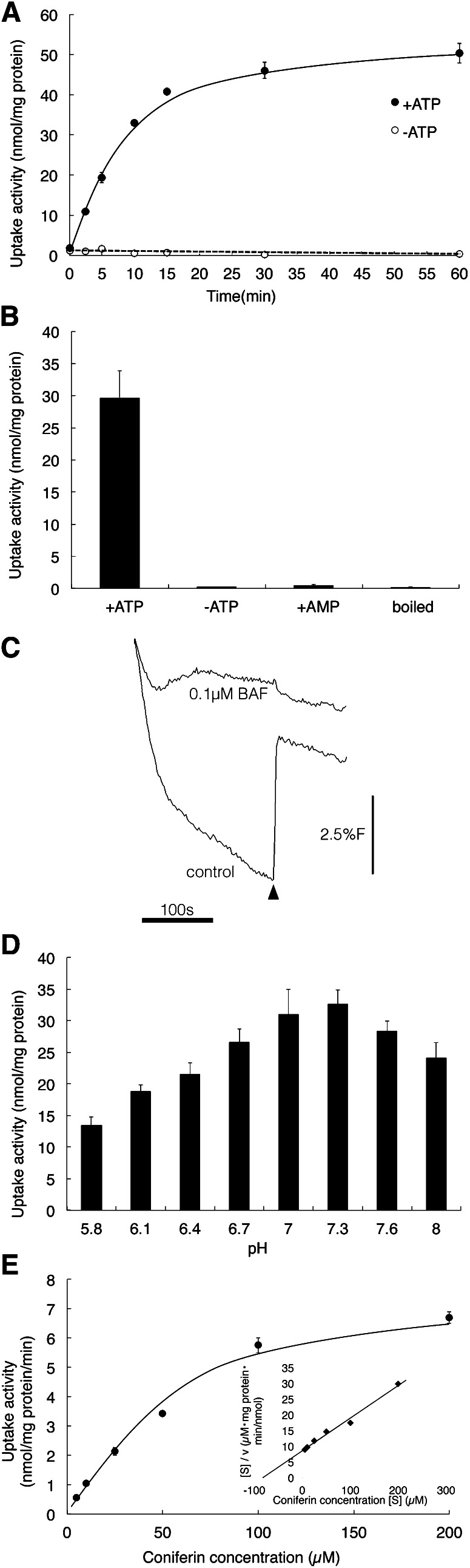
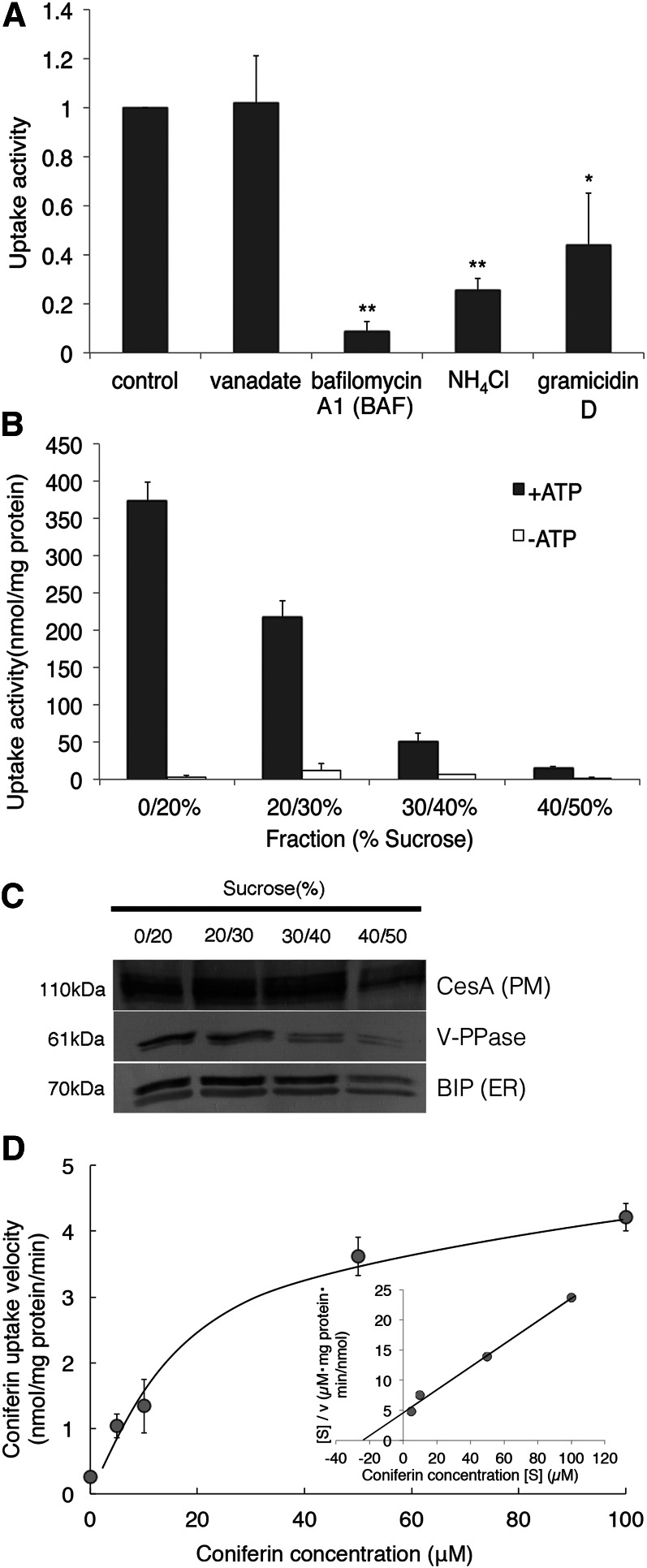
Figure 3A shows the time course of coniferin uptake by membrane vesicles prepared from differentiating xylem of a hybrid poplar in the presence and absence of Mg/ATP. Rapid uptake (within 15 min) was detected in the presence of Mg/ATP; no uptake was seen in the absence of Mg/ATP. Figure 3B shows the negative controls of coniferin transport in hybrid poplar membrane vesicles. Transport was not detected in the absence of ATP or in the presence of AMP instead of ATP, suggesting that ATP hydrolysis is necessary for transport. Transport activity was also lacking when a heat-denatured microsomal fraction was incubated with coniferin and Mg/ATP. Vesicle formation was confirmed by the generation of an H+ gradient (acid inside), as shown by fluorescence quenching of acridine orange (Ward and Sze, 1992a, 1992b; Fig. 3C). The addition of ATP to membrane fractions generated an H+ gradient and NH4Cl erased the H+ gradient of the fractions, indicating that membrane vesicles were formed. The optimum pH of the ATP-dependent coniferin transport is shown in Figure 3D. Transport activity rose as pH increased from 5.8, peaked at 7.3, the cytosolic pH of plant cells, and then decreased. The coniferin transport activity of membrane vesicles of hybrid poplar exhibited Michaelis-Menten-type saturation kinetics, and a Hanes-Woolf plot indicated that the apparent Km value of coniferin transport in hybrid poplar membrane was 60 to 80 µm (Fig. 3E). This coniferin transport Km value was in the range of other Km values known for vacuole transporters functioning as secondary transport for other organic substances (Supplemental Table S1).
Figure 3.
A, Time course of coniferin uptake in hybrid poplar membrane vesicles. Membrane vesicles were incubated with 50 μm coniferin in the presence (black circles) or absence (white circles) of 5 mm Mg/ATP. B, Negative control of coniferin uptake. Membrane vesicles of hybrid poplar were incubated with 50 μm coniferin for 10 min with 5 mm Mg/ATP (+ATP), without Mg/ATP (–ATP), or with 5 mm Mg/AMP (+AMP). “Boiled” refers to a microsomal fraction heat denatured for 15 min. C, Quenching of acridine orange fluorescence depending on H+ gradient. ATP (1 mm) was added with a 2-mL solution containing 10 mm MES-Tris (pH 8.0), 0.4 m glycerol, 25 mm KCl, 4 mm MgSO4, 5 µm acridine orange, membrane fractions (120 µg of protein), and with/without 0.1 µm bafilomycin A1 (BAF). At the indicated time (arrowhead), 1 mm NH4Cl was added. D, Effect of pH on ATP-dependent coniferin uptake. Reaction mixtures were as described in “Materials and Methods,” except that 100 mm MES-KOH buffer was used from pH 5.8 to 6.7. E, Coniferin uptake shows saturation kinetics. Membrane fractions of hybrid poplar were incubated in the presence of 5 mm Mg/ATP and each concentration of coniferin. The inset shows Hanes-Woolf plots. Coniferin uptake was determined after 10 min of incubation. Data are means ± sd of three replicates.
Inhibition of Coniferin Transport by Several Inhibitors or Flavonoid Glucosides
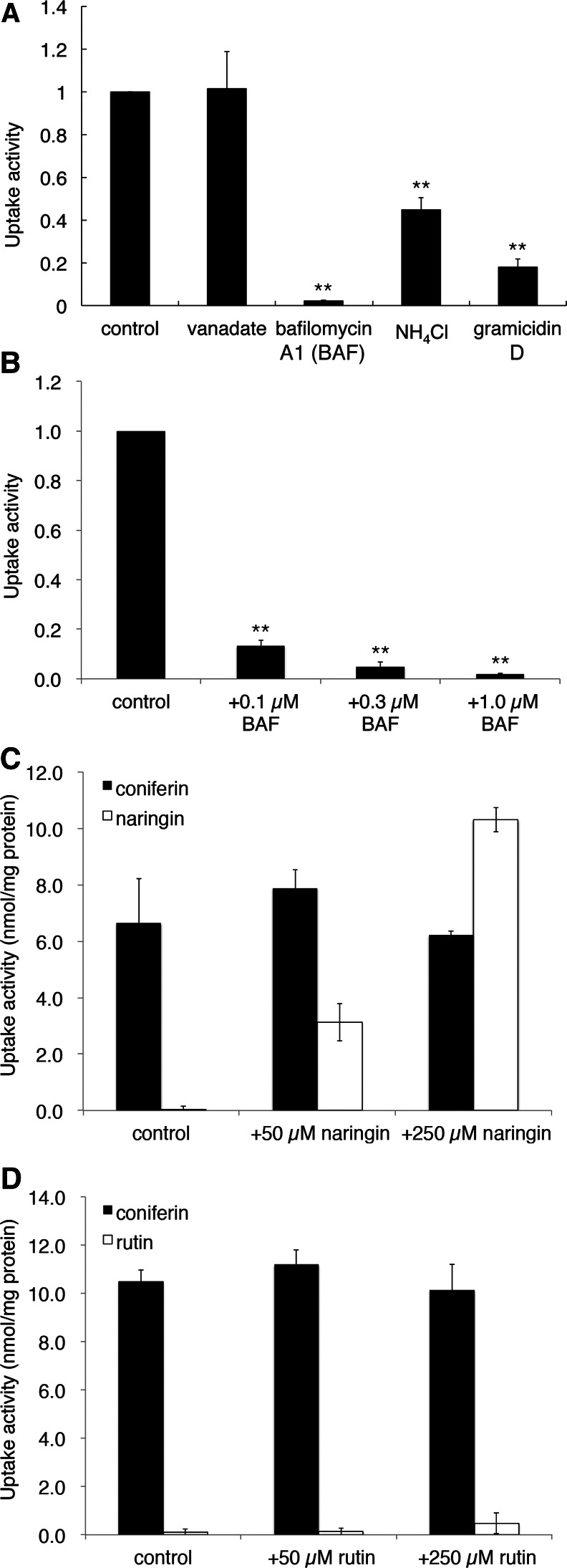
To characterize coniferin transport in further detail, we carried out various inhibitor experiments. As shown in Figure 4A, bafilomycin A1, a specific vacuolar proton-ATPase (V-ATPase) inhibitor, strongly inhibited coniferin uptake by the membrane vesicles of hybrid poplar. The acidification of membrane vesicles by the addition of ATP was monitored with the acridine orange quenching method. Bafilomycin A1 apparently inhibited both the formation of the pH gradient (Fig. 3C) and the coniferin transport of membrane vesicles (Fig. 4B). Similarly, abolishing the H+ gradient across the membrane by the addition of NH4Cl or the ionophore gramicidin D also strongly suppressed coniferin transport activity. In contrast, vanadate, a common inhibitor of ABC transporters, had no effect on coniferin transport (Fig. 4A).
Figure 4.
Effects of various inhibitors or flavonoid glucosides on coniferin transport in hybrid poplar. A, Microsomal fractions were incubated with 50 μm coniferin and 5 mm Mg/ATP, to which vanadate (1 mm), bafilomycin A1 (BAF; 1 µm), NH4Cl (10 mm), or gramicidin D (50 µm) was added. Coniferin transport was determined after 10 min of incubation. B, Membrane fractions were incubated with 50 μm coniferin and 5 mm Mg/ATP, to which BAF was added at the indicated concentrations. C and D, Coniferin transport was not inhibited by phenol glucosides. Membrane vesicles were incubated with 50 µm coniferin and 5 mm Mg/ATP in the presence of 0 (control), 50, or 250 µm of the indicated flavonoid glucosides, and coniferin (black bars) or flavonoid (white bars) glucoside content in the vesicles was determined. Although naringin itself is transported by the vesicles in a dose-dependent manner, no effect on coniferin transport was observed (C). Data are means ± sd of three replicates. **P < 0.01 compared with the control by Student’s t test.
To evaluate transport specificity, we conducted cis-inhibition experiments in which the influence of phenol glucosides (Supplemental Fig. S3) on coniferin transport was examined. Figure 4C shows the effect of naringin, a naringenin glucoside, on coniferin transport. This flavanone glucoside was clearly transported by the poplar membrane vesicles in a dose-dependent manner, but it did not suppress coniferin transport. Another flavonoid glucoside, rutin, also did not influence coniferin transport, although rutin seemed to be a less favorable substrate than naringin (Fig. 4D). Flavonoid glucosides may inhibit the transport of similar compounds without being transported (Marinova et al., 2007), but these data suggest that the transport activity we observed was not via a broadly specific transporter responsible for coniferin transport.
Membrane Localization of a Putative Coniferin Transporter
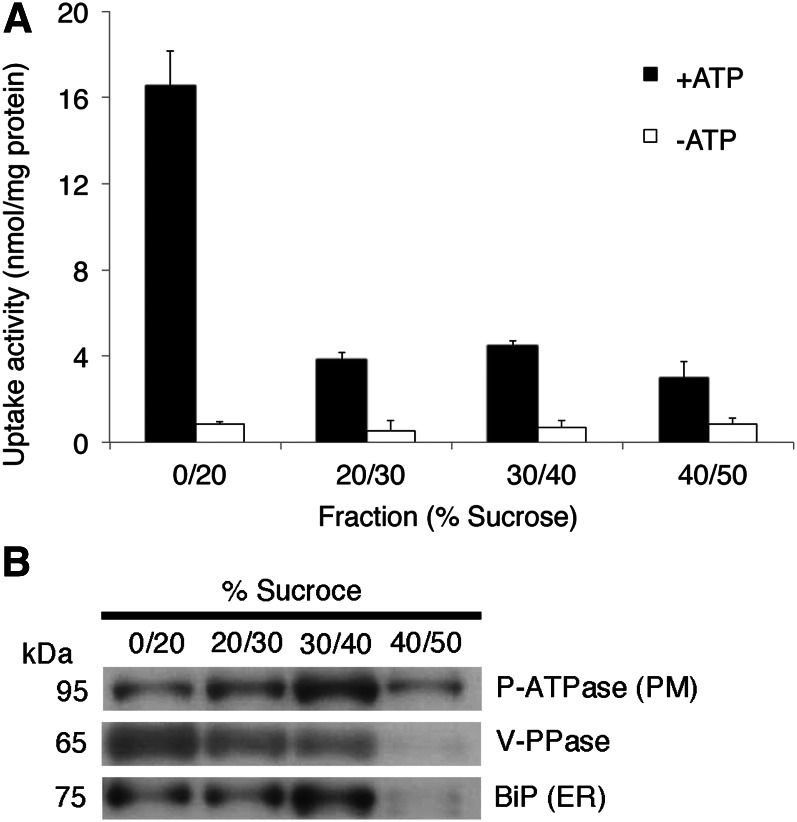
To investigate the origin of microsome-involved coniferin uptake, we purified microsomes by fractionation on a discontinuous Suc density gradient. The enrichment of tonoplast and endomembrane in the 0% to 20% fraction was suggested by immunodetection of vacuolar pyrophosphatase (V-PPase), a tonoplast and endomembrane marker, whereas enrichment of plasma membrane in the 40% to 50% Suc fraction was suggested by immunodetection of H+-ATPase (Fig. 5B). The tonoplast- and endomembrane-rich fraction (Suc 0%–20% fraction) showed clear coniferin uptake, while other fractions did not show uptake activity even in the presence of ATP (Fig. 5A).
Figure 5.
Discontinuous Suc gradient fractionation of hybrid poplar microsomes and transport assay. A, Coniferin uptake activity of each membrane fraction collected from the interface between the indicated Suc concentrations. Fractions were incubated with 50 μm coniferin in the presence or absence of 5 mm Mg/ATP. Data are means ± sd of three replicates. B, Plasma membrane H+-ATPase, V-PPase, and BiP were immunodetected to confirm the purity of plasma membrane vesicles, tonoplast and endomembrane vesicles, and endoplasmic reticulum (ER) membrane vesicles, respectively.
Coniferin Transport Mechanism in a Gymnosperm
To elucidate the mechanism of coniferin transport in a gymnosperm, we carried out various inhibitor experiments using membrane vesicles of Japanese cypress. As shown in Figure 6A, bafilomycin A1 markedly inhibited coniferin uptake, and the H+ gradient erasers also suppressed coniferin transport activity. In contrast, vanadate had no effect on coniferin transport. Furthermore, we carried out a transport assay with fractioned microsomes from Japanese cypress (Fig. 6B). The tonoplast- and endomembrane-rich fraction mainly contributed to coniferin uptake activity. The coniferin transport activity of membrane vesicles of Japanese cypress also exhibited Michaelis-Menten-type saturation kinetics, and the apparent Km value was 24 to 26 µm (Fig. 6D; Supplemental Table S1). These results were similar to coniferin transport in hybrid poplar membrane vesicles (Figs. 4 and 5).
Figure 6.
Characterization of ATP-dependent coniferin uptake in Japanese cypress. A, Microsomal fractions were incubated with 50 μm coniferin and 5 mm Mg/ATP, to which vanadate (1 mm), bafilomycin A1 (BAF; 1 µm), NH4Cl (10 mm), or gramicidin D (50 µm) was added. Data are means ± sd of three replicates. *P < 0.05, **P < 0.01 compared with the control by Student’s t test. B and C, Discontinuous Suc gradient fractionation of Japanese cypress microsomes and transport assay. B, Coniferin uptake activity of each membrane fraction collected from the interface between the indicated Suc concentrations. Fractions were incubated with 50 μm coniferin in the presence or absence of 5 mm Mg/ATP. Data are means ± sd of three replicates. C, Cellulose synthase A (CesA), V-PPase, and BiP were immunodetected to confirm the purity of plasma membrane vesicles, tonoplast and endomembrane vesicles, and endoplasmic reticulum (ER) membrane vesicles, respectively. D, Coniferin uptake into membrane fractions shows saturation kinetics. Membrane fractions were incubated in the presence of 5 mm Mg/ATP and each concentration of coniferin. The inset shows Hanes-Woolf plots. Data are means ± sd of three replicates.
Coniferin Distribution in Differentiating Xylem
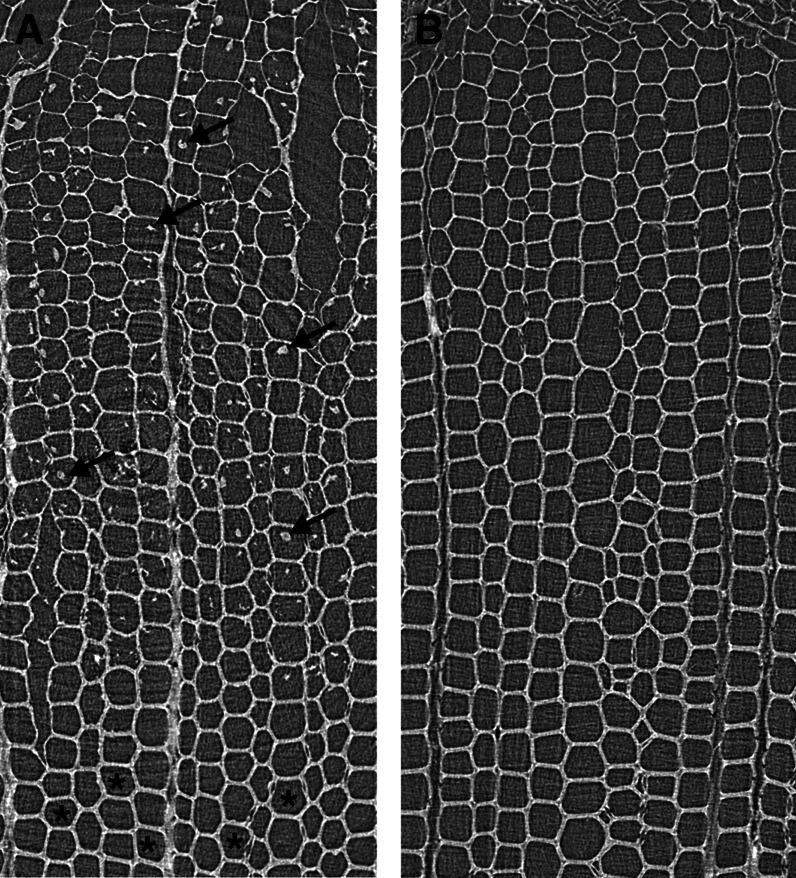
X-ray computed tomography confirmed coniferin distribution in differentiating xylem of Japanese cypress (Fig. 7). Residues were observed in the lumina of tracheids of a freeze-dried specimen (Fig. 7A). The previous study (Morikawa et al., 2010) confirmed that coniferin was contained in the residues. In the freeze-dried specimen, coniferin was observed in differentiating xylem cells; however, it disappeared at the stage of forming the inner secondary wall (S3) layer, where the lignification of secondary walls occurred vigorously. Coniferin was not observed in the specimen prepared by washing with water followed by dehydration with ethanol (Fig. 7B).
Figure 7.
X-ray computed tomography images of differentiating xylem of Japanese cypress. A, Freeze-dried specimen. B, Washed specimen. In the freeze-dried specimen (A), coniferin was observed in differentiating xylem cells (arrows); however, coniferin disappeared at the stage of forming the S3 layer (asterisks), where lignification of secondary walls occurred vigorously. Coniferin was not observed in the specimen prepared by washing (B). The previous study (Morikawa et al., 2010) confirmed that coniferin was contained in the residues.
DISCUSSION
To investigate the transport mechanism of lignin precursors in actively lignifying xylem is essential to understand the mechanism of lignification in secondary xylem that contains the greatest amount of lignin in plants. The Wiesner and Mäule reactions showed that the differentiating xylem we collected from hybrid poplar lignifies vigorously (Fig. 1).
The transport assay revealed that membrane vesicles prepared from differentiating xylem of both hybrid poplar (approximately 35 years old) and poplar (approximately 15 years old) have strong coniferin transport activity (Fig. 2, A and B) that is dependent on the presence of ATP and occurs in a time-dependent manner (Fig. 3). Similarly, strong active transport of coniferin in membrane vesicles was observed in two gymnosperms, Japanese cypress and pine (Fig. 2, C and D). The optimum pH of coniferin transport is in the physiological range of cytosolic pH at around 7.3 (Fig. 3D). This result supports that coniferin transport occurs not only in vitro but also in vivo, although this could be reflected by the pH dependency of V-ATPase activity. The Km values of coniferin transport are similar to those of the characterized secondary transporters of various secondary metabolites (Supplemental Table S1; Klein et al., 1996; Frangne et al., 2002; Dean et al., 2005; Marinova et al., 2007). It is noteworthy that the ATP-dependent transport activity of aglycone forms of monolignols, such as coniferyl alcohol and sinapyl alcohol, is considerably lower (less than 4% of coniferin transport; Fig. 2; Supplemental Fig. S4). Similar data were obtained for gymnosperms as well (Fig. 2; Supplemental Fig. S4, C and D). For syringin, another glucoside of monolignol, however, ATP dependency was not observed in poplar, likely because syringyl lignin formation occurs during a much later phase (Fig. 1); therefore, no clear transport activity was observed with the membrane vesicles we used in this study.
Inhibition experiments strongly indicate that the major coniferin transport activity depends on an H+ gradient, suggesting that a secondary transport mechanism is involved and the involvement of ABC transporters is unlikely (Fig. 4A), because no inhibition was observed by vanadate, which is a common inhibitor of ABC transporters. Our data also show that such H+-dependent transport is not restricted to angiosperms but occurs in the gymnosperm Japanese cypress. This is contradictory to data from Arabidopsis (Miao and Liu, 2010), in which similar experimental procedures were employed. One possible reason for this discrepancy may be the different plant materials used: Arabidopsis leaves, which are primarily mesophyll cells that do not lignify the cell wall, versus the differentiating xylem of tree species, in which the majority of cells are actively forming lignin.
ABC transporters compose a large gene family with more than 120 members, some of which may have broad specificity (Yazaki et al., 2009). It should be noted that endogenous and exogenous compounds can be transported by different mechanisms; for example, the flavone glucoside saponarin can be transported via H+-antiport in barley (Hordeum vulgare) as its main endogenous flavonoid, while the same compound is transported as an exogenous substance with the characteristics typical of an ABC transporter in tobacco (Nicotiana tabacum; Frangne et al., 2002). It is possible that an ABC transporter in leaf mesophyll cells recognizes coniferyl alcohol for detoxification in Arabidopsis; thus, the physiological meaning may differ from the transport we observed in tree xylem tissues. To evaluate the specificity of coniferin transport, we carried out a cis-inhibition experiment (Fig. 4; Supplemental Fig. S6, A and B). The possibility that a Suc transporter would be involved in coniferin transport (Chandran et al., 2003; Sivitz et al., 2007) was tested, but coniferin transport was barely influenced in the presence of Suc (Supplemental Fig. S6C). Results suggest that the transport activity is not broad.
Many studies have suggested that coniferin is involved in lignification while it is stored in vacuoles of differentiating xylem (Whetten et al., 1998; Samuels et al., 2002; Morikawa et al., 2010). Our data suggest that coniferin transport activity is localized to the tonoplast and endomembrane (Fig. 5) and, accordingly, is responsible for accumulation in the vacuole of differentiating xylem, which is common to angiosperms and gymnosperms (Figs. 5 and 6).
Coniferin is localized in the lumen of differentiating tracheids of Japanese cypress (Morikawa et al., 2010), while coniferin β-glucosidase is localized in the secondary cell wall in lodgepole pine (Pinus contorta; Samuels et al., 2002). Moreover, autoradiography studies indicate that coniferin is incorporated into lignin, suggesting it to be a candidate lignin precursor (Terashima and Fukushima, 1988; Tsuji and Fukushima, 2004). Because a large number of polyphenol glycosides are studied as “dead-end products” that accumulate in vacuoles in various plant species, monolignol glucosides are thought to be storage materials (Whetten et al., 1998; Boerjan et al., 2003). However, in the development of xylem tissues of trees, the behavior of coniferin changes dramatically; coniferin observed in the lumina of differentiating tracheids during middle secondary wall layer formation disappears at the stage of S3 layer formation, during which the lignification of secondary walls actively occurs (Fig. 7). These data strongly support that the transport event we demonstrated in this study is not for end product accumulation but is positively involved in the lignification of secondary walls.
However, it is not clear how coniferin stored in vacuoles is moved to the cell wall. V-ATPase is not localized solely to mature vacuoles but also to small vesicles, Golgi, and the trans-Golgi network (TGN; Herman et al., 1994; Schumacher and Krebs, 2010). Our data show that coniferin transporter is enriched in the light membrane fraction by Suc density gradient fractionation (Fig. 5), although there is no strong enrichment of marker enzymes for each membrane population (Fig. 5; Supplemental Fig. S7). This light membrane fraction may include endosomes/TGN that are trafficking to and from the plasma membrane. Coniferin might be loaded into the small vesicles, or in the Golgi and TGN, and then delivered to the outside via secretory vesicles, as in hypothesis 4 (Supplemental Fig. S2). This model could serve as an alternative and fast route to deliver monolignols to the wall. Still, the involvement of ABC transporters has been reported for monolignol transport into the cell wall (Samuels et al., 2002; Ehlting et al., 2005; Kaneda et al., 2008; Alejandro et al., 2012). Plants may have different transport mechanisms whose responsibility for monolignol transport may differ among tissues or plant species. Further studies are necessary to reach an understanding of the transport mechanism of lignin precursors into the secondary cell wall, which contains a large quantity of lignin.
MATERIALS AND METHODS
Chemicals
Chemicals used in this study were purchased from Nakalai Tesque or Wako Pure Chemicals. Coniferin and syringin were provided by Dr. N. Terashima of Nagoya University.
Plant Materials
An approximately 35-year-old hybrid poplar (Populus sieboldii × Populus grandidentata), approximately 15-year-old poplar (Populus sieboldii), approximately 20-year-old Japanese cypress (Chamaecyparis obtusa), and approximately 20-year-old pine (Pinus densiflora) were felled during the growing season in Japan (middle of June to early July). After longitudinal slitting, logs were quickly debarked by hand, and the pale yellow differentiating xylem was scraped off with razor blades at the site. Differentiating xylem tissues were aliquoted, immediately frozen in liquid nitrogen, and stored at −80°C until required.
Histochemical Analyses
Cross sections (30 µm thick) of differentiating xylem from the hybrid poplar were obtained before and after scraping. For Wiesner staining, sections were incubated with 2% (w/v) phloroglucinol in 95% (v/v) ethanol for 1 min, followed by the addition of 6 n HCl to the sections. For Mäule staining, sections were treated with 1% (w/v) KMnO4 solution for 5 min. After a brief wash in distilled water, sections were incubated in 2 n HCl for 5 min and then washed with distilled water. Sections were mounted onto microscope slides and then treated with concentrated NH3 solution.
Preparation of Microsomal Fractions
All procedures were performed on ice or at 4°C. Microsomal fractions were prepared from scraped xylem according to the method of Otani et al. (2005) with some modifications. Frozen xylem tissue (approximately 10 g) from each tree sample was washed with 20 mL of homogenizing buffer (10% [v/v] glycerol, 0.5% [w/v] polyvinylpolypyrrolidone, 5 mm EDTA, and 0.1 m HEPES-KOH adjusted to pH 8.0). Prior to use, 150 mm KCl, 3.3 mm dithiothreitol, and 1 mm phenylmethylsulfonyl fluoride were added to the buffer, and the mixture was stirred for 1 min. The xylem tissue was homogenized with a mortar and pestle for 10 min in 2 mL g−1 ice-cold homogenizing buffer. The homogenate was filtered through Miracloth (Merck) and centrifuged at 3,600g for 10 min in a 50-mL conical tube; the supernatant was collected and centrifuged at 123,000g for 30 min. The obtained microsomal pellet was resuspended with resuspension buffer (10% [v/v] glycerol, 1 mm EDTA, and 10 mm HEPES-KOH adjusted to pH 7.6) and centrifuged at 123,000g for 30 min. The obtained microsomal pellet was resuspended in 500 µL of resuspension buffer in a standard assay and stored at −80°C. The average protein content of this microsomal fraction was approximately 4 mg mL−1.
Measurement of Coniferin Transport
Uptake of coniferin by membrane vesicles in microsomal fractions was measured at 25°C for 10 min in 200 μL of reaction mixture containing 100 mm HEPES-KOH (pH 7.5), 5 mm Mg/ATP, 50 μm coniferin, and microsomal fractions for 40 μg of protein, unless otherwise stated. Fifty micromolar coniferin is on the order of a physiological concentration in differentiating xylem of hybrid poplar (T. Tsuyama, unpublished data). Inhibitors were added to the above mixture to a final volume of 200 μL. After incubation, 80 μL of the reaction mixture was loaded on a Sephadex G-50 spin column and centrifuged at 2,000 rpm at 2 min. Methanol (60 μL) was added to an equal volume of filtrate, and the mixture was subjected to HPLC analysis. Ammonium chloride and vanadate were dissolved in water, whereas bafilomycin A1 and gramicidin D were dissolved in dimethyl sulfoxide (DMSO). In the control, DMSO was added to the reaction mixture to a final concentration of 0.1%. DMSO at this concentration did not affect coniferin uptake. Data are given as technical replications.
Measurement of the Acidification of Membrane Vesicles
The acidification of membrane vesicles by ATP addition was monitored with acridine orange quenching in fluorescence spectrophotometer F-2500 (Hitachi) according to the method by Ward and Sze (1992a, 1992b).
Fractionation of Membrane Vesicles
For Suc density gradient fractionation of the hybrid poplar sample, the microsomal fraction (2.2 mL) was layered onto a discontinuous gradient containing 20% to 50% (w/v) Suc in centrifugation buffer (1 mm EDTA and 10 mm Tris-HCl adjusted to pH 7.6) in an 11.5-mL centrifugation tube. The gradient was then centrifuged at 100,000g for 2 h (SW40Ti; Beckman), and each fraction recovered from interfaces was resuspended with resuspension buffer instead of water. For the sample of Japanese cypress, these procedures were conducted with one-fifth scale.
Protein Gel Blotting
For immunoblotting, hybrid poplar membranes (4.8 µg per lane) were denatured in denaturation buffer for 10 min at 50°C, subjected to 7% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane. After blocking, the membrane was incubated with primary antibodies, followed by incubation with secondary horseradish peroxidase-conjugated anti-rabbit IgG antibody using standard procedures. The band was visualized by chemiluminescence. Antibodies used for immunodetection were raised against plasma membrane H+-ATPase, V-PPase from Arabidopsis (Arabidopsis thaliana), and endoplasmic reticulum luminal BiP (for the sources of antibodies, see “Acknowledgements”).
For the Japanese cypress sample, membranes (13.9 µg per lane) were subjected to SDS-PAGE as above. The transferred membrane was blocked and incubated with primary antibodies followed by incubation with secondary alkaline phosphatase-conjugated anti-rabbit IgG antibody (MP Biomedicals). Primary antibodies used were raised against V-ATPase, endoplasmic reticulum luminal BiP, and cellulose synthase A.
Synchrotron X-Ray Computed Tomography
Specimens of Japanese cypress used in the x-ray computed tomography experiment were collected from the same tree used for transport assays. Cellular distribution of coniferin in this tree was confirmed by Raman microscopy (Morikawa et al., 2010). Small blocks of 5 mm (radial) × 5 mm (longitudinal) × 1 mm (tangential) containing inner phloem, cambial zone, differentiating xylem, and mature xylem were collected from the wood discs. These blocks were stored at −30°C and then freeze dried. As a control, blocks were washed with water, dehydrated through an ethanol series, substituted by t-butyl alcohol, and then freeze dried. Small sticks (about 0.7 mm in diameter and 5 mm in length) containing differentiating xylem were collected from dried blocks and subjected to the measurement of synchrotron radiation x-ray microcomputed tomography as described (Mizuno et al., 2010).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Lignin biosynthetic pathway and compartmentation of lignin precursors and their biosynthesis enzymes.
Supplemental Figure S2. Hypotheses of transport mechanisms involved in the deposition of lignin precursors to the cell wall.
Supplemental Figure S3. Chemical structures of lignin precursors and flavonoid glucosides used in this study.
Supplemental Figure S4. Uptake of various aglycone precursors into membrane vesicles.
Supplemental Figure S5. Biological replicates of coniferin uptake into membrane vesicles.
Supplemental Figure S6. Effects of various compounds on coniferin transport in hybrid poplar.
Supplemental Figure S7. Enzyme activities in Suc gradient fractions of hybrid poplar.
Supplemental Table S1. Km values of coniferin uptake compared with other known vacuolar secondary transporters.
Acknowledgments
We thank Dr. S. Inoue (Akita Jujo Chemicals) for providing hybrid poplar trees; Dr. N. Terashima (Nagoya University) for providing coniferin and syringin; Dr. M. Boutry (Université Catholique de Louvain) for providing anti-H+-ATPase antibodies; Dr. N. Koizumi (Nara Institute of Science and Technology) for providing anti-BiP antibodies; Dr. M.H. Sato (Kyoto University) for anti-V-PPase antibodies; and Dr. M. Maeshima (Nagoya University) for anti-V-ATPase and anti-BiP antibodies.
Glossary
- ABC
ATP-binding cassette
- H+
proton
- Mg
magnesium
- V-PPase
vacuolar pyrophosphatase
- V-ATPase
vacuolar proton-ATPase
- TGN
trans-Golgi network
- DMSO
dimethyl sulfoxide
- S3
= inner secondary wall
References
- Alejandro S, Lee Y, Tohge T, Sudre D, Osorio S, Park J, Bovet L, Lee Y, Geldner N, Fernie AR, et al (2012) AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr Biol 22: 1207–1212 [DOI] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Boudet AM, Lapierre C, Grima-Pettenatp J. (1995) Biochemistry and molecular biology of lignification. New Phytol 129: 203–236 [DOI] [PubMed] [Google Scholar]
- Chandran D, Reinders A, Ward JM. (2003) Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J Biol Chem 278: 44320–44325 [DOI] [PubMed] [Google Scholar]
- Dean JV, Mohammed LA, Fitzpatrick T. (2005) The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221: 287–296 [DOI] [PubMed] [Google Scholar]
- Ehlting J, Mattheus N, Aeschliman DS, Li E, Hamberger B, Cullis IF, Zhuang J, Kaneda M, Mansfield SD, Samuels L, et al. (2005) Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J 42: 618–640 [DOI] [PubMed] [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenböck G, Martinoia E, Klein M. (2002) Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles: energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol 128: 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EM, Li X, Su RT, Larsen P, Hsu H, Sze H. (1994) Vacuolar-type H+-ATPase are associated with the endoplasmic reticulum and provacuoles of root tip cells. Plant Physiol 106: 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T (1985) Biosynthesis of lignin. In T Higuchi, ed, Biosynthesis and Biodegradation of Wood Components. Academic Press, New York, pp 141–160 [Google Scholar]
- Kaneda M, Rensing KH, Wong JCT, Banno B, Mansfield SD, Samuels AL. (2008) Tracking monolignols during wood development in lodgepole pine. Plant Physiol 147: 1750–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Weissenböck G, Dufaud A, Gaillard C, Kreuz K, Martinoia E. (1996) Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem 271: 29666–29671 [DOI] [PubMed] [Google Scholar]
- Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, Debeaujon I, Klein M. (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19: 2023–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YC, Liu CJ. (2010) ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc Natl Acad Sci USA 107: 22728–22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S, Torizu R, Sugiyama J. (2010) Wood identification of a wooden mask using synchrotron X-ray microtomography. J Archaeol Sci 37: 2842–2845 [Google Scholar]
- Morikawa Y, Yoshinaga A, Kamitakahara H, Wada M, Takabe K. (2010) Cellular distribution of coniferin in differentiating xylem of Chamaecyparis obtusa as revealed by Raman microscopy. Holzforschung 64: 61–67 [Google Scholar]
- Neish AC (1968) Monomeric intermediates in the biosynthesis of lignin. In K Freudenberg, AC Neish, eds, Constitution and Biosynthesis of Lignin. Springer-Verlag, New York, pp 3–43 [Google Scholar]
- Otani M, Shitan N, Sakai K, Martinoia E, Sato F, Yazaki K. (2005) Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica. Plant Physiol 138: 1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps JD. (1968) Radioautographic investigations using lignin precursors. Protoplasma 65: 181–205 [Google Scholar]
- Samuels AL, Rensing KH, Douglas CJ, Mansfield SD, Dharmawardhana DP, Ellis BE. (2002) Cellular machinery of wood production: differentiation of secondary xylem in Pinus contorta var. latifolia. Planta 216: 72–82 [DOI] [PubMed] [Google Scholar]
- Schumacher K, Krebs M. (2010) The V-ATPase: small cargo, large effects. Curr Opin Plant Biol 13: 724–730 [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CPL, Perroux JM, Ward JM. (2007) Arabidopsis sucrose transporter AtSUC9: high-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol 143: 188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Fujita M, Harada H, Saiki H. (1985) Autoradiographic investigations of lignification in the cell walls of cryptomeria (Cryptomeria japonica D. Don). Mokuzai Gakkaishi 31: 613–619 [Google Scholar]
- Takabe K, Takeuchi M, Sato T, Ito M, Fujita M. (2001) Immunocytochemical localization of enzymes involved in lignification of the cell wall. J Plant Res 114: 509–515 [Google Scholar]
- Terashima N, Fukushima K. (1988) Heterogeneity in formation of lignin. XI. An autoradiographic study of the heterogeneous formation and structure of pine lignin. Wood Sci Technol 22: 259–270 [Google Scholar]
- Tsuji Y, Fukushima K. (2004) Behavior of monolignol glucosides in angiosperms. J Agric Food Chem 52: 7651–7659 [DOI] [PubMed] [Google Scholar]
- Ward JM, Sze H. (1992a) Subunit composition and organization of the vacuolar H+-ATPase from oat roots. Plant Physiol 99: 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Sze H. (1992b) Proton transport activity of the purified vacuolar H+-ATPase from oats. Plant Physiol 99: 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten R, Sederoff R. (1995) Lignin biosynthesis. Plant Cell 7: 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten RW, MacKay JJ, Sederoff RR. (1998) Recent advances in understanding lignin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 49: 585–609 [DOI] [PubMed] [Google Scholar]
- Wooding FB. (1968) Radioautographic and chemical studies of incorporation into sycamore vascular tissue walls. J Cell Sci 3: 71–80 [DOI] [PubMed] [Google Scholar]
- Yazaki K, Shitan N, Sugiyama A, Takanashi K. (2009) Cell and molecular biology of ATP-binding cassette proteins in plants. Int Rev Cell Mol Biol 276: 263–299 [DOI] [PubMed] [Google Scholar]