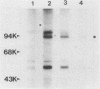
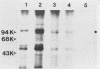
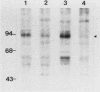
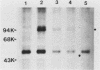
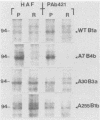
Abstract
Cell growth control appears to be drastically altered as a consequence of transformation. Because the cell surface appears to have a role in modulating cell growth and simian virus 40 (SV40)-transformed cells express large T antigen (T-Ag) in the plasma membrane, we investigated whether surface T-Ag expression varies according to cell growth rate. Different growth states were obtained by various combinations of seeding density, serum concentration, and temperature, and cell cycle distributions were determined by flow microcytofluorometry. Actively dividing SV40-transformed mouse cell cultures were consistently found to express higher levels of surface T-Ag and T-Ag/p53 complex than cultures in which cells were mostly resting. In addition, the T-Ag/p53 complex disappeared from the surface of tsA7-transformed cells cultured under restrictive conditions known to induce complete growth arrest (39.5 degrees C), although the surface complex did not disappear from other tsA transformants able to keep cycling at 39.5 degrees C. These results suggest that surface SV40 T-Ag or surface T-Ag/p53 complex, or both, are involved in determining the growth characteristics of SV40-transformed cells.
Full text
PDF
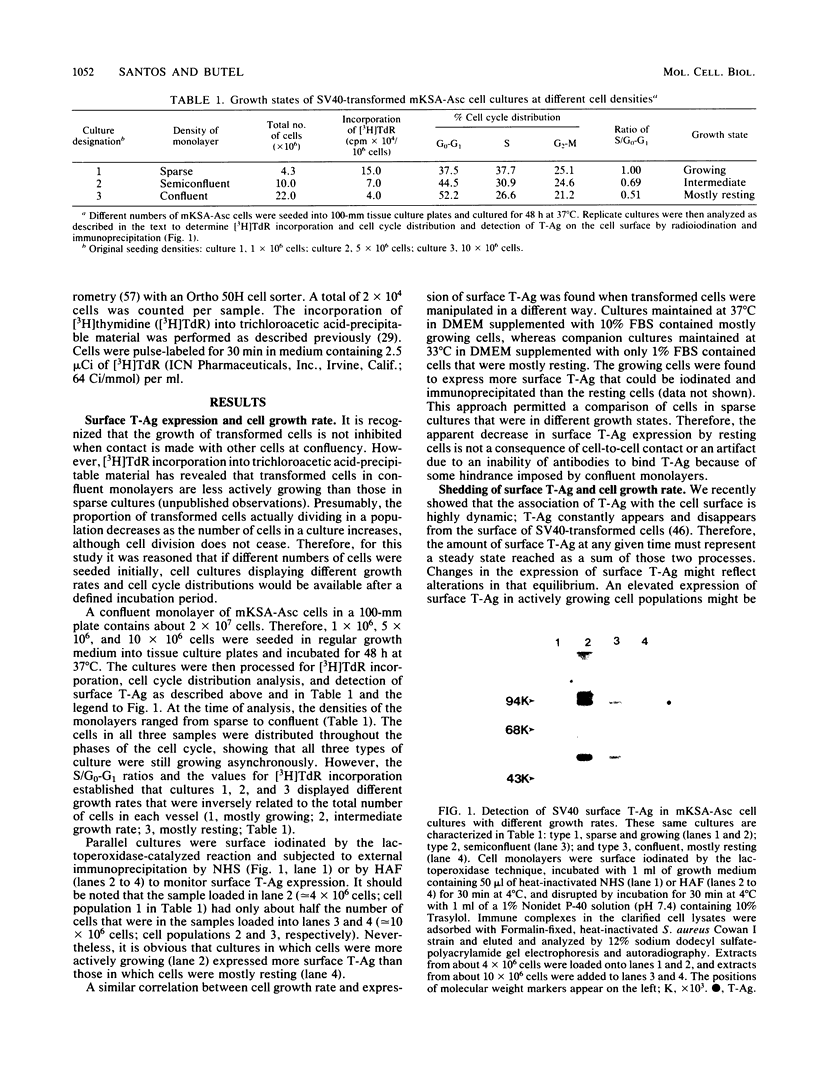
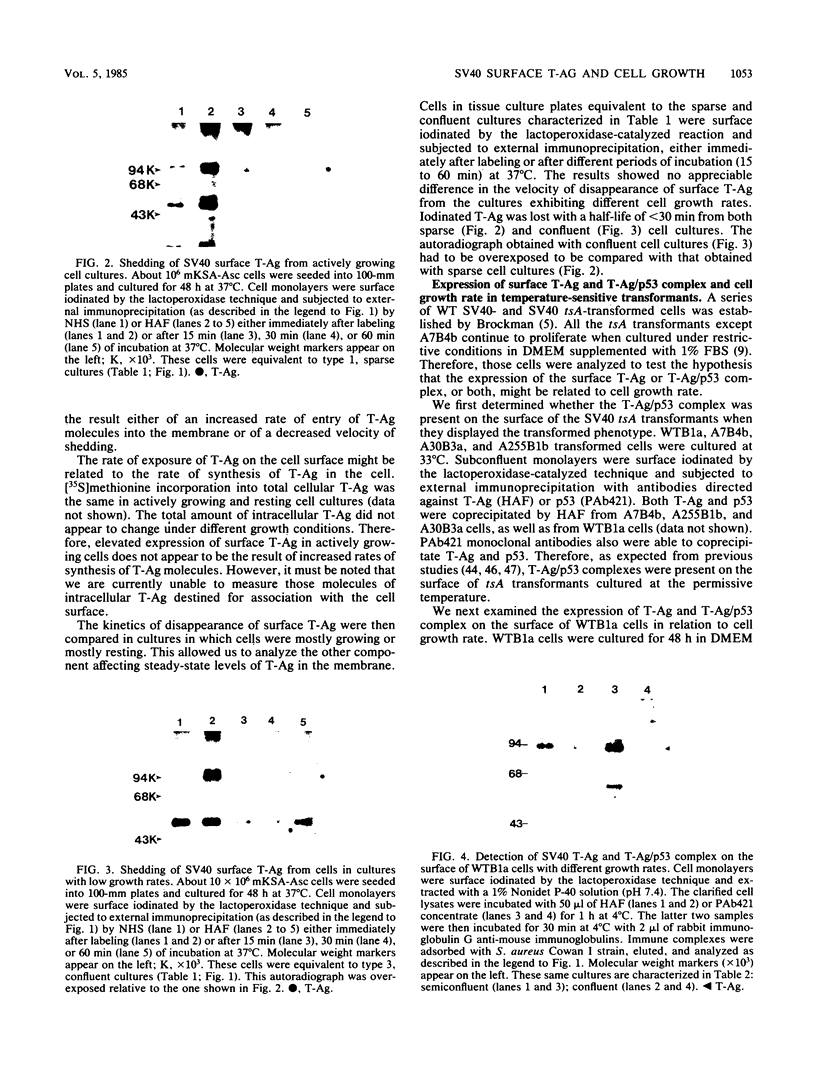
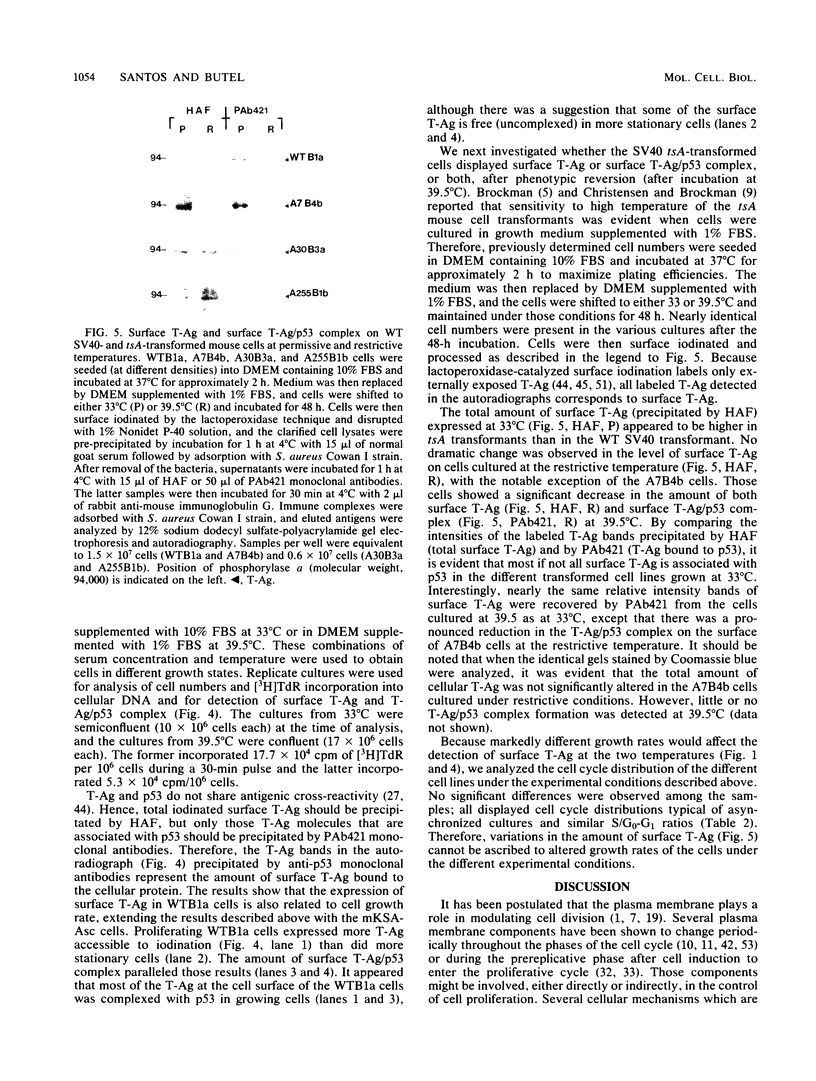



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baserga R. Growth in size and cell DNA replication. Exp Cell Res. 1984 Mar;151(1):1–5. doi: 10.1007/978-3-642-67986-5_1. [DOI] [PubMed] [Google Scholar]
- Ben-Dori R., Resnitzki D., Kimchi A. Reduction in p53 synthesis during differentiation of Friend-erythroleukemia cells. Correlation with the commitment to terminal cell division. FEBS Lett. 1983 Oct 17;162(2):384–389. doi: 10.1016/0014-5793(83)80792-3. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Warmsley A. M., Pasternak C. A. Phospholipid synthesis and degradation during the life-cycle of P815Y mast cells synchronized with excess of thymidine. Biochem J. 1970 Sep;119(3):489–492. doi: 10.1042/bj1190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. Surface changes in transformed cells detected by lectins. Fed Proc. 1973 Jan;32(1):91–101. [PubMed] [Google Scholar]
- Butel J. S., Soule H. R. Role of the simian virus 40 gene A product in regulation of DNA synthesis in transformed cells. J Virol. 1978 Jun;26(3):584–594. doi: 10.1128/jvi.26.3.584-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. B., Brockman W. W. Effects of large and small T antigens on DNA synthesis and cell division in simian virus 40-transformed BALB/c 3T3 cells. J Virol. 1982 Nov;44(2):574–585. doi: 10.1128/jvi.44.2.574-585.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Quantitative studies of antigen expression in cultured murine lymphoma cells. II. Cell-surface antigens in synchronized cultures. J Natl Cancer Inst. 1972 Dec;49(6):1607–1611. doi: 10.1093/jnci/49.6.1607. [DOI] [PubMed] [Google Scholar]
- Cikes M., Klein G. Quantitative studies of antigen expression in cultured murine lymphoma cells. I. Cell-surface antigens in "Asynchronous" cultures. J Natl Cancer Inst. 1972 Dec;49(6):1599–1606. doi: 10.1093/jnci/49.6.1599. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Hanke K., Henning R. Simian virus 40 T-antigen-related cell surface antigen: serological demonstration on simian virus 40-transformed monolayer cells in situ. J Virol. 1980 Aug;35(2):505–518. doi: 10.1128/jvi.35.2.505-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Domains of simian virus 40 large T-antigen exposed on the cell surface. Virology. 1982 Oct 15;122(1):56–70. doi: 10.1016/0042-6822(82)90377-4. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Jay G., DeLeo A. B., Khoury G., Old L. J. p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1695–1699. doi: 10.1073/pnas.78.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Sheppard J. R., Burger M. M. Cyclic membrane changes in animal cells: transformed cells permanently display a surface architecture detected in normal cells only during mitosis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):244–247. doi: 10.1073/pnas.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti N., Jonak G. J., Soprano K. J., Floros J., Kaczmarek L., Weissman S., Reddy V. B., Tilghman S. M., Baserga R. Characterization and biological activity of cloned simian virus 40 DNA fragments. J Biol Chem. 1981 Jun 25;256(12):6469–6474. [PubMed] [Google Scholar]
- Graham J. M., Sumner M. C., Curtis D. H., Pasternak C. A. Sequence of events in plasma membrane assembly during the cell cycle. Nature. 1973 Nov 30;246(5431):291–295. doi: 10.1038/246291a0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Henning R., Lange-Mutschler J., Deppert W. SV40-transformed cells express SV40 T antigen-related antigens on the cell surface. Virology. 1981 Jan 30;108(2):325–337. doi: 10.1016/0042-6822(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Antigenic relationship of SV40 early proteins to purified large T polypeptide. Virology. 1979 Sep;97(2):295–306. doi: 10.1016/0042-6822(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Effect of nuclear localization of large tumor antigen on growth potential of SV40-transformed cells. Virology. 1981 Apr 15;110(1):147–158. doi: 10.1016/0042-6822(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Intracellular transport of SV40 large tumor antigen: a mutation which abolishes migration to the nucleus does not prevent association with the cell surface. Virology. 1982 May;119(1):169–184. doi: 10.1016/0042-6822(82)90074-5. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Wong C., Butel J. S. Differential ability of a T-antigen transport-defective mutant of simian virus 40 to transform primary and established rodent cells. Mol Cell Biol. 1985 May;5(5):1043–1050. doi: 10.1128/mcb.5.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Solis R. O., Durham J. P. Purification of plasma membranes from mouse parotid gland and membrane reorganization in response to isoproterenol. Biochim Biophys Acta. 1983 Apr 6;729(2):237–248. doi: 10.1016/0005-2736(83)90490-x. [DOI] [PubMed] [Google Scholar]
- Luborsky S. W., Chandrasekaran K. Subcellular distribution of simian virus 40 T antigen species in various cell lines: the 56K protein. Int J Cancer. 1980 Apr 15;25(4):517–527. doi: 10.1002/ijc.2910250414. [DOI] [PubMed] [Google Scholar]
- López R., Galanti N. The effect of isoproterenol upon the chemical composition of plasma membranes in the mouse parotid gland. Differentiation. 1976 Jun 4;5(2-3):155–160. doi: 10.1111/j.1432-0436.1976.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., McCormick F. Lymphocyte stimulation: concanavalin A induces the expression of a 53K protein. Cell Biol Int Rep. 1980 Jul;4(7):663–667. doi: 10.1016/0309-1651(80)90205-2. [DOI] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Warmsley A. M., Thomas D. B. Structural alterations in the surface membrane during the cell cycle. J Cell Biol. 1971 Aug;50(2):562–564. doi: 10.1083/jcb.50.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Antigenic structure of simian virus 40 large tumor antigen and association with cellular protein p53 on the surfaces of simian virus 40-infected and -transformed cells. J Virol. 1984 Aug;51(2):376–383. doi: 10.1128/jvi.51.2.376-383.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Association of SV40 large tumor antigen and cellular proteins on the surface of SV40-transformed mouse cells. Virology. 1982 Jul 15;120(1):1–17. doi: 10.1016/0042-6822(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Detection of a complex of SV40 large tumor antigen and 53K cellular protein on the surface of SV40-transformed mouse cells. J Cell Biochem. 1982;19(2):127–144. doi: 10.1002/jcb.240190204. [DOI] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Dynamic nature of the association of large tumor antigen and p53 cellular protein with the surfaces of simian virus 40-transformed cells. J Virol. 1984 Jan;49(1):50–56. doi: 10.1128/jvi.49.1.50-56.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Galanti N., Jonak G. J., McKercher S., Pipas J. M., Peden K. W., Baserga R. Mutational analysis of simian virus 40 T antigen: stimulation of cellular DNA synthesis and activation of rRNA genes by mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):214–219. doi: 10.1128/mcb.3.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Butel J. S. Subcellular Localization of simian virus 40 large tumor antigen. J Virol. 1979 May;30(2):523–532. doi: 10.1128/jvi.30.2.523-532.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Lanford R. E., Butel J. S. Antigenic and immunogenic characteristics of nuclear and membrane-associated simian virus 40 tumor antigen. J Virol. 1980 Feb;33(2):887–901. doi: 10.1128/jvi.33.2.887-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Lanford R. E., Butel J. S. Detection of simian virus 40 surface-associated large tumor antigen by enzyme-catalyzed radioiodination. Int J Cancer. 1982 Mar 15;29(3):337–344. doi: 10.1002/ijc.2910290318. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Sumner M. C., Collin R. C., Pasternak C. A. Synthesis and expression of surface antigens during the cell cycle. Tissue Antigens. 1973;3(6):477–484. doi: 10.1111/j.1399-0039.1973.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Avila L., Gaudray P., Cuzin F. Two polyoma virus gene functions involved in the expression of the transformed phenotype in FR 3T3 rat cells. II. The presence of the 56K middle-T protein in the cell membrane is not sufficient for maintenance. Virology. 1981 Oct 30;114(2):501–506. doi: 10.1016/0042-6822(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Vindelov L. L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch B Cell Pathol. 1977 Aug 10;24(3):227–242. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Banks-Schlegel S. P., Pastan I. H. Immunocytochemical localization in normal and transformed human cells in tissue culture using a monoclonal antibody to the src protein of the Harvey strain of murine sarcoma virus. Exp Cell Res. 1983 Nov;149(1):141–149. doi: 10.1016/0014-4827(83)90387-7. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Transplantation immunity to simian virus 40-transformed cells in tumor-bearing mice. I. Development of cellular immunity to simian virus 40 tumor-specific transplantation antigens during tumorigenesis by transplanted cells. J Natl Cancer Inst. 1973 Jan;50(1):137–147. doi: 10.1093/jnci/50.1.137. [DOI] [PubMed] [Google Scholar]