Summary
Glutamate is the most abundant excitatory neurotransmitter in the brain, and distinct classes of glutamate receptors coordinate synaptic transmission and spike generation upon various levels of neuronal activity. However, the mechanisms remain unclear. Here, we found that loss of synaptic AMPA receptors increased kainate receptor activity in cerebellar granule cells, without changing NMDA receptors. The augmentation of kainate receptor-mediated currents in the absence of AMPA receptor activity is required for spike generation, and is mediated by the increased expression of the GluK5 high-affinity kainate receptor subunit. Increase in GluK5 expression is sufficient to enhance kainate receptor activity by modulating receptor channel properties, but not localization. Furthermore, we demonstrate that the combined loss of the AMPA receptor auxiliary TARPγ-2 subunit and the GluK5 subunit leads to early mouse lethality. Our findings reveal novel mechanisms, mediated by distinct classes of postsynaptic glutamate receptors, for the homeostatic maintenance of the neuronal activity.
Introduction
Glutamate is the primary excitatory neurotransmitter in the vertebrate brain. Upon its release from presynaptic terminals, glutamate interacts with three distinct classes (the AMPA-, kainate-, and NMDA-type) of ionotropic glutamate receptors (iGluR), leading to the depolarization of the postsynaptic membrane and the generation of spikes in the postsynaptic neuron. The precise control and regulation of synaptic strength and spike generation is critical for normal brain function.
Homeostatic synaptic plasticity is a mechanism to maintain synaptic activity at a level appropriate to the neuron or neural circuit. Both pre- and post-synaptic homeostatic mechanisms have been proposed. The inhibition of postsynaptic glutamate receptor activity has been shown to alter glutamate quantal content and release from presynaptic terminals, and several presynaptic molecules have been implicated in activity-dependent changes in glutamate release in the fly neuromuscular junction (Davis, 2006). As an example of a postsynaptic mechanism, AMPA receptor (AMPAR) activity is increased upon the blockade of action potentials with tetrodotoxin (TTX) treatment in cultured neurons. As mechanisms, changes in AMPAR subunit composition, the involvement of signaling molecules, and transcriptional activity have been reported (Burrone and Murthy, 2003; Lee, 2012; Man, 2011; Nelson and Turrigiano, 2008; Shepherd and Bear, 2011; Vitureira et al., 2011). Therefore, both pre- and postsynaptic mechanisms implicate the AMPAR as an important mediator of homeostatic synaptic plasticity. It remains unclear, however, how the different classes of iGluRs, contribute to the physiological regulation of synaptic activity.
Among the three classes of iGluRs, the kainate receptor (KAR) has been the least-studied. The KAR is composed of three types of subunits: the low-affinity subunits (GluK1/2/3), high-affinity subunits (GluK4/5), and Neto1/2 auxiliary subunits (Contractor et al., 2000; Fernandes et al., 2009; Kumar et al., 2011; Mulle et al., 1998; Pinheiro et al., 2007; Straub et al., 2011a; Tang et al., 2011; Tomita and Castillo, 2012; Zhang et al., 2009). The KARs mediate both synaptic transmission and plasticity (Contractor et al., 2011; Jane et al., 2009; Lerma, 2006; Nicoll and Schmitz, 2005; Pinheiro and Mulle, 2008; Traynelis et al., 2010). Although the KAR-mediated excitatory postsynaptic current (EPSC) is typically of small amplitudes, its distinctly slow kinetics can induce significant charge transfer and contributes to spike generation by temporal summation (Cunningham et al., 2006; Frerking and Ohliger-Frerking, 2002; Sachidhanandam et al., 2009). Despite detailed knowledge of the KAR, it remains unclear whether the regulation of KARs is important for synaptic homeostasis.
In the current study, we have identified a novel postsynaptic mechanism controlling spike generation. We show that the loss of synaptic AMPAR activity increases KAR-mediated synaptic transmission without changing NMDARs at the cerebellar mossy fiber–granule cell (MF–GC) synapses. At this synapse, the upregulation of KAR activity concurrent with AMPAR inhibition is required for spike generation. The loss of both the AMPAR auxiliary subunit TARPγ-2 and KAR subunits (GluK2 or GluK5) causes mouse lethality, while mice lacking only TARPγ-2 or the KAR subunits are viable. Furthermore, suppression of neuronal activity by TTX increases KAR activity in wild-type neurons, but not in neurons lacking AMPARs. We show that the upregulation of postsynaptic KAR activity is mediated by the increased expression of the high-affinity GluK5 KAR subunit, which alters the KAR channel properties, but not the synaptic localization. From these data, we conclude that the maintenance of homeostatic neuronal activity in cerebellar granule cells is accomplished by distinct classes of iGluRs.
Results
Homeostatic control of spike generation by upregulation of KAR activity in the absence of AMPAR activity
Spike generation in the postsynaptic neuron is controlled by a summation of the activities of all three classes of iGluRs. To examine how each class of iGluR contributes to spike generation, we evaluated activity at cerebellar mossy fiber – granule cell (MF–GC) synapses, where all three classes of iGluR are highly expressed (Hollmann and Heinemann, 1994). To distinguish excitatory transmission from inhibitory GABAergic transmission, the GABAA receptor antagonist picrotoxin (100 μM) was included in all experiments unless specified otherwise.
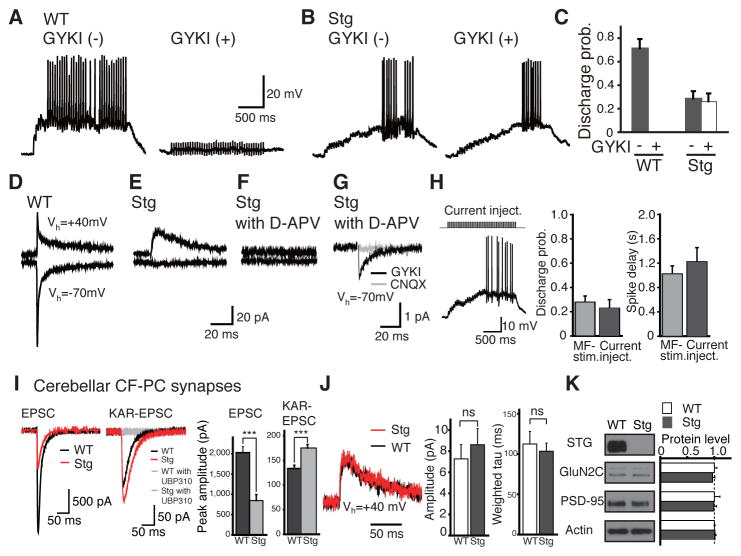
We compared the relative contribution of AMPARs and KARs to spike generation in GC neurons using a repetitive 20 Hz stimulation applied to the MFs, which is in a range of the stimulation frequency observed in vivo (Arenz et al., 2008). We showed that the minimum stimulation of MFs generated spikes in cerebellar GCs in the presence of the NMDAR antagonist D-APV (50 μM) (Figure 1A). The application of a preferential AMPAR antagonist, GYKI53655 (30 μM), completely blocked spike generation in cerebellar GCs, suggesting that the AMPAR mediates this activity in the GCs (Figure 1A, C).
Figure 1. KAR activity is upregulated in the absence of AMPAR activity.
(A, B) Representative MF-evoked EPSP traces in response to 20 Hz stimulation trains in GCs from wild type (WT; A) and stargazer (Stg; B) cerebellar slices before (left; −) and after (right; +) 30 μM GYKI53655 bath application. (C) Summary graph of discharge probability. See also Figure S1. (D–F) EPSCs elicited by MF stimulation in cerebellar GCs from WT (D) and Stg (E, F) mice at holding potentials of +40 mV (top panels) and −70 mV (bottom panels). Each trace is a single-sweep record. 50 μM D-APV was added to block NMDAR-mediated EPSCs. (G) Averaged trace of nearly 100 single-sweeps of MF-evoked EPSCs in the presence of GYKI53655 (30 μM) in a GC from a stargazer mouse at a holding potential of −70 mV. MF stimulation was repeated at 0.1 Hz. (H) Spikes generated by directly injecting a train of depolarizing current steps in a GC from a stargazer cerebellar slice. The top panel shows the current injection protocol (0.75 pA for 20 ms, 20 Hz, 40 pulses). Summary graphs of discharge probability and spike delay from MF stimulation (n = 10) and current injection (n = 5). (I) Representative climbing fiber (CF)-evoked EPSC traces in Purkinje cells (PC) from wild type (WT) and stargazer (Stg) cerebellar slices. KAR-mediated EPSCs were isolated by blocking AMPAR-EPSCs with 100 μM GYKI53655 and all responses were confirmed to be blocked by KAR antagonist, 10 μM UBP310. Summary graph of EPSC amplitude (wt, n = 7; Stg, n = 6). (J) NMDAR-EPSCs at cerebellar MF–GC synapses from WT and Stg upon single stimulation (Vh = +40 mV). Scaled and superimposed traces are shown in the upper panel. No significant differences were detected in either peak amplitudes (left) or decay kinetics (right) between WT and Stg mice (n = 6–8). (K) Protein expression in total cerebellar lysate from WT and Stg mice (n = 3 for each). No significant difference was detected between genotypes. Data are shown as mean ± SEM.
To examine the contribution of the other iGluRs to spike generation in GC neurons, we prepared acute cerebellar slices from stargazer mice, which show no AMPAR activity due to disruptions in the AMPAR auxiliary subunit, TARPγ-2/stargazin (Hashimoto et al., 1999; Letts et al., 1998). In these mice, we also observed spikes in GC neurons following MF stimulation, and this activity was not blocked by GYKI53655 (30 μM) (Figure 1B, C). Similar results were observed in the absence of picrotoxin (Figure S1). These results suggest that the potentiation of a channel insensitive to 30 μM GYKI53655, presumably KARs, provides a homeostatic compensatory mechanism to maintain synaptic activity in the AMPAR-deficient stargazer mice.
To further examine transmission at the MF-GC synapse, we measured excitatory post-synaptic currents (EPSCs) in acute cerebellar slices. The specific loss of AMPAR-EPSCs in stargazer (Vh = −70 mV) was confirmed (Figure 1D, E) (Hashimoto et al., 1999). Upon blockade of NMDARs with D-APV (50 μM), no obvious EPSCs were observed using voltage-clamp mode (Vh = −70 mV and +40 mV) with either Cs+-based (Figure 1F) or K+-based intracellular solutions (data not shown; see details in Methods section). However, by averaging nearly 100 traces of evoked EPSCs, we observed “tiny” EPSCs that had very small amplitudes and slow decay kinetics (2.53 ± 0.73 pA, 15.6 ± 1.5 ms, n = 4). The decay kinetics of these tiny EPSCs were 4.6 times slower than those of AMPAR-mediated EPSCs at the KAR-lacking synapse in GluK2 KAR single knockout mice (65.6 ± 4.3 pA, 3.4 ± 0.4 ms, n = 12), and could be blocked by CNQX (100 μM), but not by GYKI53655 (30μM; Figure 1G). To examine whether these tiny EPSCs are sufficient for spike generation, we directly injected repetitive 20 Hz currents (0.75 pA, 20 ms) into the GCs, which represent a charge transfer similar to that of the endogenous tiny currents observed (0.0157 ± 0.0045 pC; Figure 1H). With this protocol, we observed that the direct current injections mimicked both the discharge probability and the spike latency of action potentials elicited in the GCs by MF stimulation (Figure 1H). These results suggest that these tiny, but slow, EPSCs are sufficient for spike generation in cerebellar GCs. In addition, we also found the upregulation of KAR in the reduction of AMPAR in Purkinje cells (PCs). At the cerebellar climbing fiber-PC synapses from stargazer, the amplitude of AMPAR-EPSCs was reduced as published previously (Hashimoto et al, 1999: Menuz et al, 2008). In contrast, the amplitude of KAR-EPSC was significantly increased compared to wildtype littermates (Figure 1I).
The loss of AMPAR activity in the stargazer mice may cause global changes in synapses such as, for example, a delay in synaptic development. To examine this possibility, we measured the activity and subunit composition of NMDARs in acute cerebellar slices. We found that the amplitudes and kinetics of NMDAR-mediated EPSCs in cerebellar GCs showed no obvious differences between the wild type and stargazer mice (Figure 1J). Furthermore, the protein expression of GluN2C and PSD-95, which increase during synaptic development (Akazawa et al., 1994; Monyer et al., 1994; Watanabe et al., 1992), were not altered in the stargazer mouse cerebellum compared to wildtype controls (Figure 1K). These results indicate that the 30 μM GYKI53655-resistant channel activity in the AMPAR-deficient stargazer is not due to global changes in synaptic properties. Rather, this activity appears to be due to specific changes in the iGluR composition contributing to spike generation.
The GluK2 KAR is required for viability and spike generation in the absence of the AMPAR auxiliary TARPγ-2 subunit
The slow decaying EPSCs insensitive to GYKI53655 (30 μM) indicate that KARs may be upregulated in the absence of AMPAR activity. To directly examine the contribution of KARs to spike generation, we took a genetic approach using the GluK2 knockout (KO) mouse, in which the most abundant KAR isoform in cerebellar GCs, GluK2, is deleted (Bahn et al., 1994), as well as the GluK2/stargazer double knockout (DKO) mouse. In cerebellar slices prepared from GluK2/stargazer DKOs, no GC spikes were elicited by repetitive 20 Hz MF stimulation (Figure 2A, C, D). In contrast, similar to our findings in wild-type mice (Figure 1H), direct current injection into cerebellar GCs did generate spikes in the GluK2/stargazer DKOs, indicating that the machinery for the spike generation in the postsynaptic GCs is intact (Fig 2B, E). For the GC spike generation elicited by MF stimulation, both the discharge probability and the spike delay were reduced in the stargazer mice, and spikes were absent entirely in the GluK2/stargazer DKOs, while no significant differences were detected from the GluK2 single KO (Figure 2A, C, D). These results indicate that both KARs and AMPARs can contribute to spike generation, and GluK2 KARs play essential roles in spike generation in the absence of AMPAR activity at cerebellar MF–GC synapses. Furthermore, spike generation unaltered in the GluK2 single KO (Figure 2) is consistent with no KAR contribution in spike generation in wild-type mice (Figure 1A).
Figure 2. GluK2 KAR subunits play an essential role in spike generation and mouse survival in the absence of AMPAR auxiliary TARPγ-2 subunit.
(A) Representative MF-evoked EPSP traces in response to 20 Hz 40 MF stimulation trains in GCs from WT, Stg, GluK2/Stg DKO, and GluK2 KO cerebellar slices. In stargazer slices, some spikes were observed, whereas no spikes were observed in slices from GluK2/Stg DKOs. (B) Spikes generated by direct current injection to the same GC as in (A). Summary graphs of discharge probability (C), spike delays (D), and the number of spikes in B (E) from the different genotypes (n = 8–10). (F, G) GluK2/Stg DKOs showed severe neurologic phenotypes, and all mice died by P30. (H) Nissl staining of sagittal sections from GluK2 KO and GluK2/Stg DKO brains. No obvious differences in brain gross anatomy were detected. D-APV (50 μM) was added in the external solution in A–E. Data are shown as mean ± SEM. ***P < 0.005.
The stargazer and the GluK2 single KO can survive to at least one year of age (Mulle et al., 1998; Noebels et al., 1990). In contrast, the GluK2/stargazer DKOs, generated by crossing pairs of homozygous GluK2 KO (GluK2−/−) and heterozygous stargazer (γ-2+/stg), are born at Mendelian ratios then die by postnatal day (P)30 with severe deficits in locomotion (Figure 2F, G) but no obvious changes in cerebellar gross anatomy (Figure 2H). This result indicates that TARPγ-2/stargazin and GluK2 play redundant roles in overall survival, and that GluK2-containing KARs can maintain viability in the absence of TARPγ-2/stargazin and, presumably, AMPAR activity. As mammals can survive without a cerebellum (Lemon and Edgley, 2010), the lethality of the GluK2/stargazer DKOs likely arises not only from the dysfunction of cerebellar GCs, but also from the disrupted function of other neurons that express GluK2 and TARPγ-2/stargazin.
KAR activity is upregulated by homeostatic mechanisms
We have shown that KAR activity is potentiated in the stargazer GCs (Figure 1). This KAR potentiation could be due to compensatory or homeostatic regulation. To distinguish these possibilities, we explored a relationship between homeostatic plasticity and KAR upregulation in the stargazer GCs.
We first examined changes in postsynaptic KAR activity by measuring glutamate-evoked currents using combinations of various antagonists. Glutamate (300 μM) applied together with the NMDAR antagonist D-APV (100 μM), which presumably activates both AMPARs and KARs, elicited a two-fold larger current in cerebellar GCs from wild-type, compared to stargazer, mice (Figure 3A). On the other hand, application of GYKI53655 (30 μM) completely inhibited the glutamate-evoked currents in cerebellar GCs from wild-type, but not stargazer, mice (Figure 3A). These residual GYKI53655-resistant currents in the stargazer GCs were completely blocked by 100 μM CNQX (Figure 3A). These results indicate that KAR activity is potentiated in the postsynaptic GCs of the AMPAR-deficient stargazer cerebellum.
Figure 3. Loss of AMPAR activity and suppression of neuronal activity potentiate KAR activity in the postsynaptic cells.

(A) Glutamate-evoked currents were recorded in GCs from WT and Stg cerebellar slices (Vh =−70 mV). The summary graph (n = 5 each) shows that the KAR activity that was resistant to GYKI53655 (30 μM) was potentiated in Stg mice. (B) Glutamate-evoked KAR currents were measured in cultured GCs from WT and Stg mice, with or without TTX treatment, in the presence of 30 μM GYKI53655 (Vh = −70 mV). The summary graph of steady-state values (n = 5 each) shows that the inhibition of action potentials with TTX or the loss of AMPAR activity enhanced KAR activity in the postsynaptic neurons, and those enhancements occluded each other. Data are shown as mean ± SEM.
We next examined whether KAR activity in the postsynaptic GCs is potentiated in homeostatic plasticity induced by treating primary cultures of wildtype cerebellar GCs with TTX (2μM), which blocks action potentials (Burrone and Murthy, 2003; Nelson and Turrigiano, 2008). We measured glutamate-evoked currents (Vh =−70 mV) using the same experimental conditions as in Figure 3A. We found that TTX treatment enhanced glutamate-evoked KAR activity in GCs from wild-type mice (Figure 3B). In contrast, the glutamate-evoked KAR activity in stargazer GCs was not altered in the presence of TTX, indicating that TTX-induced KAR activity is occluded by the loss of AMPAR activity in stargazer GCs (Figure 3B). These results suggest that KAR upregulation observed in the stargazer GCs and in the TTX-induced homeostatic plasticity share a common mechanism.
Homeostatic KAR regulation is mediated by increasing the expression of the GluK5 KAR subunit, which cannot form a homomeric channel
The potentiation of postsynaptic KAR activity could be mediated by an increase of KAR expression. To test this possibility, we examined protein levels in total lysate and postsynaptic density (PSD) fractions from stargazer and wildtype cerebellum. We observed a specific increase in the levels of GluK5, but not GluK2 or Neto2, protein in both the cerebellum (Figure 4A) and cultured cerebellar GCs (Figure 4B) from stargazer compared to wildtype controls. Furthermore, we compared mRNA expression levels in the cerebellum of wild-type and stargazer mice using quantitative (q)PCR. We observed a significant increase in the mRNA levels for GluK2, GluK5, and Neto2 in the stargazer cerebellum compared to wildtypes, with no changes in the mRNA levels of the NMDAR subunit GluN1 (Figure 4C). Importantly, we also observed that TTX treatment increased the protein expression of GluK5, but not GluK2 and other synaptic markers, in both the total cell lysate and in the Triton-insoluble (PSD-enriched) fractions from cultured cerebellar GCs (Figure 4D).
Figure 4. Potentiation of KAR activity is through increased expression of the GluK5 high-affinity subunit.
(A–C) Protein expression in total cerebellar lysate and in the PSD fraction (n = 3–4, A), in total lysate of cultured GCs and in the Triton X-100 insoluble fraction (n = 4, B), and mRNA expression measured by quantitative RT-PCR (n = 3, C) in cerebella from WT and Stg mice. Protein expression of GluK5 is specifically increased in Stg cerebella and cultured GCs. (D) Protein expression in total lysate and in the Triton X-100 insoluble fraction from cultured WT GCs with or without TTX (2 μM) treatment (n = 4 each). GluK5 protein expression was increased specifically. (E) Glutamate-evoked KAR currents were measured in cultured GCs from WT and Stg mice, with or without GluK5-overexpression, in the presence of 30 μM GYKI53655 (Vh =−70 mV). The summary graph of steady-state values (n = 5 each) shows that GluK5 expression enhanced KAR activity in WT GCs, but not in Stg GCs. All data are shown as means ± SEMs. *P < 0.05, ***P < 0.005.
These data indicate that both the suppression of neuronal activity and the loss of the AMPAR auxiliary TARPγ-2 subunit increase GluK5 protein expression in the PSD. However, it has been shown that the GluK5 subunit cannot form a homomeric channel, and must form heteromers with the GluK1–3 subunits for proper function (Barberis et al., 2008; Christensen et al., 2004; Fernandes et al., 2009; Kumar et al., 2011; Nasu-Nishimura et al., 2006; Ruiz et al., 2005). Therefore, we asked whether the increase in GluK5 expression alone is sufficient for the increased KAR activity observed in stargazer neurons. To answer this question, GluK5 was transfected into cultured GCs and glutamate-evoked KAR currents were measured. We found that GluK5 overexpression enhanced glutamate-evoked KAR currents in cultured GCs from wild-type mice, while having no effect on glutamate-evoked KAR currents in GCs from stargazer mice, which were already elevated (Figure 4E), suggesting that the up-regulation of GluK5 in neurons treated with TTX and in AMPAR-deficient stargazer GCs is sufficient to increase KAR activity. These results identified a mechanism of KAR upregulation, i.e., increase in GluK5 expression, shared in the stargazer GCs and in the TTX-induced homeostatic plasticity.
The GluK5 KAR subunit is required for upregulated KAR activity in cerebellar GCs
To determine whether the GluK5 KAR subunit is required for synaptic homeostasis, we measured MF-elicited spike generation from GluK5 KOs and GluK5/stargazer DKOs. We observed that spike generation was similar between the GluK5 KO and wild-type mice (Figure 5A). However, spikes were completely abolished in the GluK5/stargazer DKO (Figure 5A, B). Direct current injection into GCs induced spikes in all three genotypes to a similar extent, suggesting that there were no differences in the postsynaptic machinery for spike generation between these groups (Figure 5C, D). Similar to the GluK2/stargazer DKOs (Figure 2F, G), the GluK5/stargazer DKOs all died by P30, with severe deficits in locomotion (Figure 5E, F). No obvious differences in cerebellar gross anatomy were observed between the GluK5 KOs and the GluK5/stargazer DKOs (Figure 5G). Like the GluK2/stargazer DKOs, this lethality is likely a result from the disrupted functions of many kinds of synapses, not just those in the GCs.
Figure 5. GluK5 is required for spike generation and mouse survival in the absence of AMPAR auxiliary TARPγ-2 subunit.
(A) Representative MF-evoked EPSP traces in response to 40 MF stimulation trains at 20 Hz in GCs from acute cerebellar slices from the WT, GluK5 KO, and GluK5/Stg DKO mice. No spikes were observed in the GluK5/Stg DKOs. (B) Summary graph of discharge probability from the different genotypes. (C) Spikes genersated by direct current injection to the same cell as in A. (D) Summary graph of the numbers of spikes in C from the different genotypes (n = 8–9). (E, F) GluK5/Stg DKOs show severe neurologic phenotypes, and all mice die by P30, similar to the GluK2/Stg DKOs. (G) Nissl staining of sagittal sections from GluK5 KO and GluK5/Stg DKO brains shows no gross morphological abnormalities. All data are shown as means ± SEMs. ***P < 0.005
Loss of the GluK5 subunit does not alter the synaptic localization of the KAR complex
The lack of KAR activity in the GluK5/stargazer DKOs (Figure 5A) may be due to changes either in the KAR channel properties or in the number of KARs. Indeed, loss of the high-affinity KAR subunits GluK4/5 have been shown to reduce the synaptic localization of KARs at the hippocampal MF–CA3 synapses to half of wildtype levels (Fernandes et al., 2009). To evaluate KAR localization, we compared protein levels in total protein lysate and PSD fractions from GluK5 KO to those from wild type (Figure 6A, B) and from the GluK5/stargazer DKO cerebellum to those from stargazer mice (Figure 6C, D). We did not detect any obvious differences in the protein levels of GluK2, Neto2, GluN1, or PSD-95 in the cerebella of the different genotypes. These results suggest that KARs comprised of GluK2/Neto2 localize to the synapse in wildtype, GluK5 KO, and GluK5/stargazer DKO mice, although we did not detect KAR-mediated transmission in the wild-type cerebellum (Figure 1A)
Figure 6. GluK5 did not change the synaptic localization of KARs in cerebellum.
Protein expression in the PSD fraction (A, C) and total lysate (B, D) from the WT compared to the GluK5 single KO (left, n = 4) and from Stg compared to the GluK5/Stg DKO (right, n = 2). No significant changes in protein levels were detected. (E, F) Postembedding immunogold showing preferential labeling for GluK2/3 and Neto2 at cerebellar MF–GC synapses, but not at the attachment plaques (AP) formed between the dendritic digits of the GCs (d). Quantitative analysis of tangential (G, H) and vertical (I, J) distributions of immunogold showing that both GluK2/3 and Neto2 are almost limited to the postsynaptic site of the cerebellar MF–GC synapses. Filled and blank arrowheads indicate the edges of PSD of the synapse and APs, respectively. Numbers of analyzed immunogold are 59 (G, I) and 52 (H, J). (K-M) Postembedding immunogold showing that GluK2/3 is predominantly localized to the PSD of MF–CA3 pyramidal cell spine (Sp) synapses in the CA3 strata lucidum. Quantitative analysis of the vertical (L) and tangential (M) distribution of immunogold (n = 48) showing that GluK2/3 is almost limited to the postsynaptic site Arrowheads indicate the edges of the PSD. Scale bars, 200 nm (E, F, K).
To further confirm the synaptic localization of KARs in the wild-type cerebellum, we performed immuno-electron microscopy and found that both GluK2/3 and Neto2 predominantly localized to the cerebellar MF–GC synapses, but not evident in the attachment plaques formed between the digits of GC dendrites (Figure 6E, F). Quantitative analysis further revealed that immunogold labeling for GluK2/3 and Neto2 was limited almost exclusively to the synaptic membrane (Figure 6G, H) on the postsynaptic side of the MF–GC synapse (Figure 6I, J). Likewise, we found that GluK2/3 localized mainly to the PSD of the hippocampal MF–CA3 pyramidal cell synapse (Figure 6K–M).
Modulation of KAR glutamate affinity by GluK5 is required for synaptic KAR responses
We showed that the upregulation of the GluK5 subunit is required for spikes in the absence of AMPAR activity, but that the synaptic localization of the KAR complex is not affected by the presence or absence of GluK5. Therefore, we next determined whether the presence of GluK5 alters KAR channel properties that impact synaptic transmission. GluK5 is shown to modulate both the agonist affinity and the decay kinetics of KAR in heterologous cells (Barberis et al., 2008; Straub et al., 2011b).
To examine which type of modulation contributes to the GluK5-mediated KAR upregulation in the absence of AMPAR activity, we first compared the EPSP summation in the MF-GC synapse of stargazer and GluK5/stargazer DKOs (Figure 7A). Second we compared summation of glutamate-evoked responses of KAR expressed in HEK cells using outside-out patches and Piezo electric device (Figure 7B). Five consequent stimuli (20 Hz) of the MF pathway elicited KAR-EPSPs and a significant summation in the presence of D-APV (50 μM; Figure 7A, black line in each trace). In contrast, KAR-EPSPs were not detected in cerebellar slices from GluK5/stargazer DKOs, in which the KARs are presumably composed of GluK2/Neto2 without GluK5 (Figure 7A, C).
Figure 7. GluK5 regulates KAR activity by increasing the affinity to glutamate.
(A) Superimposed traces of MF-evoked KAR-mediated EPSPs from stargazer (Black) and GluK5/stargazer DKO (Gray) in response to 5 pulses at 20 Hz stimulation with D-APV (50 μM). (B) Superimposed traces of outside-out patched membranes from HEK cells expressing GluK2/GluK5/Neto2 (Red) and GluK2/Neto2 (Green) in response to pulses of glutamate (300 μM, 1 ms duration, 5 pulses at 20 Hz) driven by a Piezo-electric device in current-clamp mode. (C) Averaged ratios for the fifth peak potentials normalized to the first peak amplitudes. (D) Scaled and superimposed traces of MF-evoked KAR-mediated EPSCs and outside-out patched membranes from HEK cells expressing GluK2/GluK5/Neto2 in response to glutamate pulses (300 μM, 1 ms duration). Vh = −70 mV. (E) Dose-response curves of peak amplitudes for GluK2/GluK5/Neto2 (black, n = 8) and GluK2/Neto2 (green, n = 6) receptors expressed in HEK cells to glutamate pulses administered using a Piezo-electric device (1 ms duration). The left panel shows the representative traces recorded with 300 μM glutamate stimulation normalized to IGlu[50mM]. (F) Dose-response curves of KAR-mediated steady-state currents in cerebellar GCs from stargazer and GluK5/stargazer double knockout (GluK5 DKO). (G) Scaled and superimposed traces of MF-evoked KAR-mediated EPSPs (Black) and recombinant KARs activated by glutamate (Color) as (A, B). (H) Fractional peak amplitudes normalized to the first peak in response to glutamate pulses (5 pulses at 20 Hz) of the different durations with 300 μM glutamate (H) and the different glutamate concentrations for 1 ms duration (I) indicated. Dashed curves in H, I show values from MF-evoked EPSPs from stargazer mice in the presence of D-APV (50 μM; n = 6). (J) Scaled and superimposed traces of the first peak from KAR-EPSP and GluK2/GluK5/Neto2 KAR expressed heterologously and stimulated with glutamate (300 or 1000 μM) for 1 ms under current-clamp configuration. Inset shows summary graph for the 10–90% rise time. (K, L) Scaled and superimposed traces from native and recombinant AMPARs using the same stimulation condition (300 μM glutamate, 1 ms duration, 5 pulses at 20 Hz). Black, representative MF-evoked AMPAR-mediated EPSP in response to 5 pulses at 20 Hz stimulation in GluK2 knockout with D-APV (50 μM). Red, representative trace of GluA2/GluA4/Stg expressed in a HEK cell membrane in response to glutamate pulses (300 μM, 1 ms duration, 5 pulses at 20 Hz) in current-clamp mode (left) and voltage-clamp mode (Vh = −70 mV; right). Data are shown as means ± SEMs.
The kinetics of KARs containing GluK2/GluK5/Neto2 following a 1 ms application of 300 μM glutamate in outside-out patches from transfected HEK cells mimicked the kinetics of the KAR-EPSPs and EPSCs observed in acute slices (Figure 7A–D), although difference in membrane properties and composition of endogenous channels likely exist between the two systems. Namely, we detected glutamate-evoked currents and summation in outside-out patches from HEK cells expressing GluK2 and Neto2 without GluK5 (Figure 7B, C). Since the presence of GluK5 robustly enhanced the peak amplitude of recombinant and native KARs, we conclude that the modulation of the KAR amplitudes by GluK5 contributes to the upregulation of KAR activity in stargazer mice.
Next, we evaluated the contribution of GluK5-mediated changes in agonist affinity to synaptic transmission by measuring the glutamate dose-response curve for GluK2/Neto2-containing KARs in outside-out patches from transfected HEK cells. To determine the channel affinity for glutamate, we measured the peak amplitudes evoked by 1 ms application of different concentrations of glutamate. We found that the presence of GluK5 shifted the glutamate dose-response curve of GluK2/Neto2 KAR to the left (Figure 7E). In addition, to evaluate roles of GluK5 in modulating glutamate affinity of KARs in neurons, we measured glutamate dose-response curve of KAR-mediated steady state currents by whole-cell recording of GCs on acute cerebellar slices. We found that KARs in stargazer mice show smaller EC50 than KARs in GluK5/stargazer DKO, indicating that GluK5 shifts glutamate affinity higher in cerebellar GCs (Figure 7F). These results support that GluK5-dependent modulation of agonist affinity is the type of modulation involved in upregulating KAR activity in the absence of AMPAR activity.
The application of 300 μM glutamate for 1 ms is a relatively low amount of glutamate compared to the concentration of glutamate at the synapse previously estimated using primary hippocampal neurons and competitive inhibitors (Clements et al., 1992; Lester et al., 1990). Therefore, we further evaluated the effects of glutamate concentration and KAR composition upon KAR-mediated EPSP/Cs in the stargazer mice. As described above, we found that recombinant GluK2/Neto2 KARs with GluK5 mimicked KAR-EPSPs (Figure 7A–D, G), whereas KAR missing any one component did not (Figure 7G). Furthermore, only the 1 ms application of 300 μM glutamate mimicked KAR-EPSPs in GluK2/GluK5/Neto2-containing membranes, whereas applications of a longer duration or a lower/higher concentration of glutamate did not (Figure 7H, I). We observed that the higher concentrations of glutamate increased the rise kinetics of KARs, and that 300 μM, but not 1 mM, glutamate mimicked the rise kinetics of native KARs (Figure 7J). Furthermore, the 1 ms application of 300 μM glutamate on outside-out HEK cell patches expressing recombinant AMPAR, GluA2/GluA4/stargazin, which recapitulate the native AMPAR composition of cerebellar GCs (Hashimoto et al., 1999; Hollmann and Heinemann, 1994), mimicked the AMPAR-EPSP/Cs detected in the cerebellar GCs from GluK2 KO slices in the presence of D-APV (50 μM) (Figure 7K, L). These results suggest that the concentration (300 μM) and duration (1 ms) of glutamate application are reasonable parameters for the active concentration and duration of glutamate exposure to postsynaptic glutamate receptors at the cerebellar MF–GC synapses in stargazer mice, if other factors are not involved.
From these studies, we conclude that KARs comprised of GluK2/Neto2, without GluK5, localize to synapses in wild-type mice, but do not respond sufficiently to endogenous glutamate in physiological conditions due to their low affinity for glutamate. With the loss of AMPAR activity in the stargazer mice, the incorporation of the GluK5 subunit into the KAR complexes significantly increases its affinity for glutamate, allowing the receptor to respond to endogenous glutamate sufficiently to generate spikes and maintain synaptic activity.
Discussion
In the current work, we show that two distinct classes of iGluRs, the AMPARs and the KARs, control synaptic transmission and spike generation via a novel mechanism to regulate postsynaptic strength at the cerebellar MF-GC synapse.
KARs share essential roles as postsynaptic depolarizers with AMPARs in vivo
Our analysis of genetically disrupted mice shows that both the TARPγ-2-containing AMPAR and the GluK2/5-containing KAR contribute to spike generation and overall mouse survival. We also found that GluK2/Neto2 KAR subunits localize to the PSD in cerebellar glomeruli. Although it is well known that NMDAR activity impacts synaptic plasticity and that AMPAR activity can determine synaptic strength, the function of KARs has remained unclear. Our studies have indicated that tiny, nearly undetectable, KAR-EPSCs are sufficient to generate spikes due to the large charge transfer from their sustained decay kinetics in cerebellar granule cells (Castillo et al., 1997; Straub et al., 2011a; Vignes and Collingridge, 1997). Since the primary role of synapses is to transmit spikes from presynaptic to postsynaptic neurons, it is important to measure the efficiency of spike generation across the synapse. As the KAR-mediated EPSCs we observed are very small, it is important to examine synapses in the absence of AMPAR- and NMDAR-EPSCs to examine the contribution of the KAR activity to spike generation.
KARs play distinct homeostatic roles in regulating postsynaptic strength
Our study is the first study to identify a mechanism by which distinct classes of iGluRs contribute to the homeostatic control of synaptic transmission. In Hebbian-type plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), NMDAR activity induces changes in AMPAR activity (Collingridge et al., 2004; Kerchner and Nicoll, 2008; Kessels and Malinow, 2009; Malenka and Bear, 2004; Shepherd and Huganir, 2007). On the other hand, we find that a reduction in AMPAR function increases KAR activity, thus maintaining spike generation via the up-regulation of KAR activity in cerebellar granule cells. This type of homeostatic control of spike generation was unexpected, given that we observed that the function of the AMPARs and the KARs appeared at least partially redundant.
In the current report, the loss of AMPAR activity and neuronal activity induced KAR upregulation at the cerebellar MF-GC glomeruli-type synapses. In addition, the reduction in AMPAR activity potentiated KAR activity at the CF-PC synapses. These results suggest similar type of KAR upregulation at other types of synapses. At the cerebellar MF-GC synapses, the high affinity GluK5 subunit was incorporated into a KAR complex of low-affinity GluK2 subunit and auxiliary Neto2 subunit at synapses. Therefore, similar type of KAR upregulation could be occurred at synapses, where low-affinity subunit (GluK1-3) and auxiliary subunit (Neto1/2) express. Indeed, though KARs are known to be highly expressed in glomeruli-type synapses, weak expression of KARs is also observed at other types of synapses (Bahn et al., 1994; Monaghan and Cotman, 1982). On the other hand, at the CF-PC synapses, reduction in AMPAR activity increased KAR activity. KAR-EPSCs have been observed in interneurons, and in different brain areas (Bureau et al., 2000; Cossart et al., 1998; DeVries and Schwartz, 1999; Frerking et al., 1998; Li and Rogawski, 1998; Li et al., 1999; Wondolowski and Frerking, 2009; Wu et al., 2005). These synapses might show this reactive plasticity. More examples of KAR regulation at different synapses should be explored to examine the generality of the mechanism in the future.
KAR-EPSCs switch to AMPAR-EPSCs during development and in response to LTP at thalamocortical synapses (Kidd and Isaac, 1999). At hippocampal synapses, KAR sensitivity is also reduced during development and LTP (Lauri et al., 2006). Both studies showed that the increase in AMPAR activity reduces KAR activity, which was in the opposite direction to our findings of the increase in KAR activity upon reduction in AMPAR activity. Perhaps, KAR and AMPAR control their activity each other bi-directionally.
Compensatory regulation vs homeostatic plasticity
We observed upregulation of KAR activity both in the stargazer GCs lacking AMPAR activity and TTX-treated wild-type GCs. TTX is widely used to induce homeostatic plasticity. However, TTX suppresses global network activity and makes difficult to identify downstream signaling. Furthermore, analysis of miniature EPSCs in primary cultured neurons, which is often used to study homeostatic plasticity, does not allow high-resolution analysis in the intact tissue, for examples, evaluation of general presynaptic properties and each iGluR activity. On the other hands, knockout mice may show compensatory regulation, but are a great tool to identify signaling cascade by selective disruption of genes. Therefore, combinations of TTX-treated wild-type neurons and knockout mice will provide complement information for mechanisms of homeostatic plasticity.
We here used STG mice as a tool to suppress AMPAR activity specifically instead of global suppression of network activity using TTX. However, as mentioned above, a disadvantage to use STG mice is a possibility of compensatory regulation. To examine this possibility, we performed TTX treatment for wild-type neurons to induce homeostatic effects (Figure 3). Briefly, we used TTX treated wild-type neurons to show that KAR upregulation in the stargazer mice is due to homeostatic regulation, but not compensatory regulation. We also showed occlusion experiments, i.e., both KAR activity and GluK5 expression are upregulated both in STG neurons and TTX-treated wild-type neurons, but TTX did not further enhance KAR activity and GluK5 expression in STG neurons (Figure 3 and 4D, E). These results suggest that KAR is upregulated in the stargazer mice by a mechanism shared with homeostatic plasticity induced by TTX, i.e., increase in GluK5 expression.
Activity-dependent switch in KAR subunit composition
Low-affinity KAR subunits (GluK1-3) can form homomeric ion channels, whereas the high-affinity subunit (GluK4/5) requires GluK1-3 for ion channel activity. Here, we found that both the suppression of network activity by TTX and a loss of AMPAR activity increased GluK5 expression in cultured GCs and in the mouse cerebellum. In addition, GluK5 overexpression was sufficient to increase KAR activity at the cell surface as measured by glutamate-evoked KAR currents in wild-type neurons. On the other hand, GluK5 expression did not further enhance KAR activity in stargazer neurons, which lack AMPAR activity. This result indicates that, though GluK5 cannot form homomeric channels, GluK5 expression in stargazer neurons is sufficient to increase KAR activity and to switch KARs from the low-affinity to high-affinity type. Future measurements of neural circuit activity will be required to fully reveal the contribution of GluK5 expression to homeostatic plasticity in the brain.
Towards reconstituting synaptic transmission
Amongst the three classes of iGluRs, the KARs show unique channel properties, including slow decay kinetics and lower glutamate affinity (Castillo et al., 1997; Straub et al., 2011a; Tang et al., 2011; Vignes and Collingridge, 1997). By determining conditions that mimicked KAR-EPSP/Cs and AMPAR-EPSCs, we estimated the glutamate concentration available to postsynaptic glutamate receptors to be approximately 300 μM for a duration of 1 ms in cerebellar GCs of stargazer mice. The estimated concentration of 300 μM is relatively low compared to that in previously published reports using primary cultured neurons and competitive inhibitors (1100 μM, 1.2 ms) (Clements et al., 1992; Lester et al., 1990). However, the availability of glutamate to the postsynaptic receptors represents an equilibration between the diffusion of glutamate released from presynaptic terminals and glutamate removal from synaptic clefts. Both of these factors may be vulnerable to disruption by alterations in synaptic structure and the expression of proteins, such as glutamate transporters (Bergles et al., 1999; Conti and Weinberg, 1999; Jonas and Spruston, 1994). Therefore, the threefold difference between our estimate of glutamate concentrations in the cerebellar glomerulus synapses from acute cerebellar slices and those in previous studies using hippocampal synapses from primary cultured neurons might be accounted for simply by the different types of neurons or synapses involved. In addition, since our estimation is based on comparisons of the kinetics of recombinant and native receptors, there might be difference in some factors between two distinct systems, for example, membrane capacitance. If this is the case, the estimation should be re-evaluated by considering other factors. However, our data represent the first successful example of mimicking both AMPAR- and KAR-EPSCs by recombinant receptors expressed in HEK cells. Future studies that compare different types of neurons and synapses are needed to explore this variety in active neurotransmitter concentrations and to identify factors shaping transmission at individual synapses.
Experimental Procedures
Antibodies
The following antibodies were used: rabbit polyclonal antibodies to GluA2/3, GluK2/3, GluK5 (Millipore), Neto1 (Straub et al., 2011a), and Neto2 (Zhang et al., 2009); mouse monoclonal antibodies to GluN1 (BD Biosciences), PSD-95 (ABR), and actin (Millipore); guinea pig polyclonal antibodies to GluA2 (Yamazaki et al., 2010), GluA4 (Nagy et al., 2004), PSD-95 (Fukaya and Watanabe, 2000); and goat polyclonal antibodies to VGluT1 (Miyazaki et al., 2003). The rabbit polyclonal antibody to GluK2/3 used for immunohistochemistry in the present study was raised against the 31 C-terminal amino acids of mouse GluK2.
Animals
We obtained the stargazer and GluK5 knockout mice from the Jackson Laboratory. Animal handling and use followed protocols approved by the Institutional Animal Care and Use Committee at Yale University and were in accordance with the National Institutes of Health guidelines.
Synaptic physiology
Heterozygous male and female mice were mated to obtain homozygous stargazer mice. Sagittal cerebellar slices with a thickness of 200 μm were prepared from mice (P21–30). Patch-clamp recordings from GCs that were identified visually in cerebellar slices were performed as described previously (Hashimoto et al., 1999; Sumioka et al., 2010). The resistance of patch pipettes was 5–10 MΩ when filled with intracellular solution. For voltage-clamp mode, the intracellular solution was composed of (in mM): 130 cesium methanesulfonate, 5 HEPES, 5 Mg-ATP, 0.2 Na-GTP, 20 TEA-Cl, and 5 EGTA (pH 7.3, adjusted with CsOH). For current-clamp mode, the intracellular solution was composed of (in mM): 125 potassium gluconate, 20 KCl, 5 HEPES, 5 Mg-ATP, 0.2 Na-GTP, and 10 EGTA (pH 7.3, adjusted with KOH). The composition of the standard bathing solution was (in mM): 125 NaCl, 2.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 25 glucose; this solution was bubbled continuously with a mixture of 95% O2 and 5% CO2. All chemicals were obtained from Tocris Cookson or Sigma. Stimulation and online data acquisition were performed using the Clampex program (version 10.2, Axon Instruments). Signals were filtered at 3 kHz and digitized at 20 kHz. Presynaptic MFs were stimulated by placing a micropipette in the GC layer and stimulating with 5–15 μA (A365 stimulus isolator; World Precision Instruments). In the range of stimulation, we observed input-output relationship as digital manner, i.e., output detected was not dependent on stimulus intensity. The stimulation intensity was adjusted just above the sharp threshold for activation of the synaptic response with a holding potential at +40 mV. All recordings were performed at room temperature.
Physiology with Piezo electric device
HEK293 cells were were maintained in humidified 95% H2O/5% CO2. GluK2, Neto2, and stargazin were cloned into pCAGGS vectors containing IRES-GFP or mCherry, and transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. A stable cell line expressing GluK5 was used in the experiments concerning the GluK5 subunit. Neto2 was transfected in tenfold excess relative to GluK2, and GluA4i, GluA2i(R), and stargazin were transfected at a ratio of 1:3:10. Expression was confirmed by western blotting. Recordings from outside-out patches were performed at room temperature at a holding potential of −70 mV for voltage clamping. The external solution contained 140 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 2.7 mM MgCl2, 1.3 mM MgSO4, 10 mM glucose, and 10 mM HEPES (pH 7.4). Patch pipettes (open tip resistance, 2–3 MΩ) were filled with a solution containing 130 mM cesium methanesulfonate, 20 mM TEA-Cl, 5 mM Mg-ATP, 0.2 mM Na-GTP, 5 mM EGTA, and 5 mM HEPES (pH 7.3, adjusted with CsOH). Glutamate was applied in extracellular solution with theta glass pipettes mounted on a piezoelectric bimorph. Agonist-evoked currents were analog low-pass filtered at 3 kHz, sampled at 25 kHz, and analyzed with Igor software5 (Zhang et al., 2009).
Recording glutamate-evoked currents from cerebellar GCs in culture and in slice
Cerebellar GC cultures were prepared from postnatal day seven mice and were transfected at DIV 5 as described previously (Zhang et al., 2009). TTX was added on DIV 4. Whole-cell recordings from GCs (DIV10–12) were performed in the same external and internal solutions as used for the outside-out patch recordings. All recordings were performed at room temperature. In recordings from GCs in both culture and slice, to measure AMPAR/KAR-mediated agonist-evoked currents, TTX (1 μM), D-APV (100 μM), and picrotoxin (100 μM) were added to the external solution. In glutamate dose-response experiments for slices (Figure 7F), D-APV (100 μM) and MK801 (100 μM) were pre-incubated and blockade of NMDAR activity was confirmed, followed by measuring glutamate-evoked currents. Glutamate was applied in extracellular solution close to the cell soma with a gravity perfusion system (ValueLink 8.2, Automate Scientific). The current was analog low-pass filtered at 1 kHz and digitally sampled at 25 kHz.
Immunohistochemistry
For postembedding immunogold electron microscopy (EM), microslicer slices (400 μm) were cryoprotected with 30% glycerol in PB, and frozen rapidly with liquid propane in the EM CPC unit (Leica Microsystems). Frozen sections were immersed in 0.5% uranyl acetate in methanol at −90°C in the AFS freeze-substitution unit (Leica Microsystems), infiltrated at −45°C with Lowicryl HM-20 resin (Chemische Werke Lowi, Waldkraiburg, Germany), and polymerized with UV light. Ultrathin sections on nickel grids were etched with saturated sodium-ethanolate solution for 1–5 s, and then treated with following solutions: blocking solution containing 2% normal goat serum (Nichirei, Tokyo, Japan) in the incubation solution (0.03% Triton-X100 in Tris-buffered saline, pH 7.4; TTBS) for 20 min, primary antibodies (20 μg/ml for each) diluted with the incubation solution overnight, and colloidal gold (10 nm)-conjugated anti-rabbit or anti-guinea pig IgG (1:100, British BioCell International, Cardiff, UK) in the blocking solution for 2 h. Finally, the grids were washed in TTBS for 30 min, fixed with 2% glutaraldehyde in PBS for 15 min and 1% OsO4 for 20 min, and stained with 2% uranyl acetate for 5 min and Reynold’s lead citrate solution for 1 min. Photographs were taken with an H-7100 electron microscope (Hitachi, Tokyo, Japan). For quantitative analysis, postsynaptic membrane-associated immunogold particles, defined as those less than 35 nm away from the cell membrane, were counted on scanned electron micrographs and analyzed using MetaMorph software (Molecular Devices, Downingtown, PA).
Statistical analysis
All data are given as the mean ± standard error of the mean (SEM). Statistical significance between means was calculated using the unpaired Student’s t test. In all figures, the error bars indicate ± SEM.
Supplementary Material
Acknowledgments
The authors thank the members of the Tomita lab and Dr. James Howe (Yale Pharmacology) for helpful discussions. We thank Mr. Gerard Somers for maintaining the mouse colonies, Mr. Kwang S. Kim for preliminary studies, Dr. S.F. Heinemann (Salk Institute) for generating the GluK2 mice, Deltagen and Jackson for generating and maintaining GluK5 mice, and Dr. Hyoungseok Ju and Dr. Janghoo Lim for their valuable comments on the qPCR and real-time PCR equipment. S.T. is supported by NIH/NIMH R01 MH085080 and MH077939. M.W. is supported by Grants-in-Aid for Scientific Research (19100005) provided by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. C.S. is supported by a Boehringer-Ingelheim Fonds PhD fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Arenz A, Silver RA, Schaefer AT, Margrie TW. The contribution of single synapses to sensory representation in vivo. Science. 2008;321:977–980. doi: 10.1126/science.1158391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A, Sachidhanandam S, Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Bureau I, Dieudonne S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci U S A. 2000;97:6838–6843. doi: 10.1073/pnas.97.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci. 2000;20:5899–5905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JK, Paternain AV, Selak S, Ahring PK, Lerma J. A mosaic of functional kainate receptors in hippocampal interneurons. J Neurosci. 2004;24:8986–8993. doi: 10.1523/JNEUROSCI.2156-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends Neurosci. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O’Gorman S, Heinemann SF. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci U S A. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16:5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci. 2002;22:7434–7443. doi: 10.1523/JNEUROSCI.22-17-07434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Watanabe M. Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: a study in the adult mouse brain. J Comp Neurol. 2000;426:572–586. [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Jonas P, Spruston N. Mechanisms shaping glutamate-mediated excitatory postsynaptic currents in the CNS. Curr Opin Neurobiol. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Kumar J, Schuck P, Mayer ML. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71:319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JT, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Lee HK. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci. 2012;5:17. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Edgley SA. Life without a cerebellum. Brain. 2010;133:652–654. doi: 10.1093/brain/awq030. [DOI] [PubMed] [Google Scholar]
- Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Li H, Rogawski MA. GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology. 1998;37:1279–1286. doi: 10.1016/s0028-3908(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Man HY. GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity? Curr Opin Neurobiol. 2011;21:291–298. doi: 10.1016/j.conb.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Fukaya M, Shimizu H, Watanabe M. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci. 2003;17:2563–2572. doi: 10.1046/j.1460-9568.2003.02698.x. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 1982;252:91–100. doi: 10.1016/0006-8993(82)90981-7. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu-Nishimura Y, Hurtado D, Braud S, Tang TT, Isaac JT, Roche KW. Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J Neurosci. 2006;26:7014–7021. doi: 10.1523/JNEUROSCI.0573-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990;7:129–135. doi: 10.1016/0920-1211(90)90098-g. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci. 2005;25:11710–11718. doi: 10.1523/JNEUROSCI.4041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH, Mulle C. Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J Neurosci. 2009;29:5000–5008. doi: 10.1523/JNEUROSCI.5807-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The Cell Biology of Synaptic Plasticity: AMPA Receptor Trafficking. Annu Rev Cell Dev Biol. 2007 doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci. 2011a;14:866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci. 2011b;31:8078–8082. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Neto1 Is an Auxiliary Subunit of Native Synaptic Kainate Receptors. J Neurosci. 2011;31:10009–10018. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Castillo PE. Neto1 and Neto2: auxiliary subunits that determine key properties of native kainate receptors. J Physiol. 2012;590:2217–2223. doi: 10.1113/jphysiol.2011.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, Sibley D. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Vitureira N, Letellier M, Goda Y. Homeostatic synaptic plasticity: from single synapses to neural circuits. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Wondolowski J, Frerking M. Subunit-dependent postsynaptic expression of kainate receptors on hippocampal interneurons in area CA1. J Neurosci. 2009;29:563–574. doi: 10.1523/JNEUROSCI.4788-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Zhao MG, Toyoda H, Ko SW, Zhuo M. Kainate receptor-mediated synaptic transmission in the adult anterior cingulate cortex. J Neurophysiol. 2005;94:1805–1813. doi: 10.1152/jn.00091.2005. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fukaya M, Hashimoto K, Yamasaki M, Tsujita M, Itakura M, Abe M, Natsume R, Takahashi M, Kano M, et al. TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum. Eur J Neurosci. 2010;31:2204–2220. doi: 10.1111/j.1460-9568.2010.07254.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.