Abstract
We previously showed that consumption of protein immediately after exercise in older adults enhances nitrogen balance when energy balance (EB) is maintained. Because daily EB routinely varies, it is important to know whether benefits of postexercise protein consumption also occur with changing EB. Within an experiment, participants consumed an isonitrogenous–isocaloric diet with the timing of a protein (PRO + CHO) or carbohydrate (CHO) beverage immediately after exercise versus earlier in the day. Within hypocaloric and hypercaloric cohorts, 3-day mean nitrogen balance was not different when protein was consumed immediately after exercise, although there was a trend (p = .09) for higher nitrogen balance in the positive EB. However, when data from our three studies were combined, the anabolic effect of postexercise feeding was evident during positive EB but not negative EB. EB is therefore an important consideration in the postexercise anabolic effect of protein feeding.
Key Words: NBAL, Exercise, Wasting
With age, there is a general wasting of proteins in tissue (1). A multitude of factors contribute to the wasting process; however, it is argued that the major contributors to the wasting process are a lack of physical activity or “disuse” as well as inadequate calorie (2) and/or protein intake (3,4). Regardless of the mechanism, wasting is the result of a negative net protein balance, which occurs as a result of decreased protein synthesis, increased protein breakdown, or a combination of both.
Nitrogen balance (NBAL) is dependent on energy balance (EB) and protein intake. Energy restriction leads to a decreased NBAL (5,6) because of increased amino acid oxidation to meet energy needs. However, there is also evidence that increased protein intake is helpful in maintaining NBAL. Specifically, consuming 0.92 gm of protein/kg body weight (bw) has been shown to be more effective than 0.45 gm of protein/kg bw at maintaining NBAL, lean body mass (LBM), muscular strength, and immune function in elderly women over the course of 9 weeks (7). However, even in the presence of adequate protein intake, inadequate energy intake results in lower NBAL than when EB is maintained (8). Also, increasing energy intake will lead to an increased NBAL due to protein sparing effects (9,10). Because protein synthesis is energetically costly (11) and is dependent on ATP availability, elderly individuals who increase their energy intake could reduce their dependence on amino acids for energy purposes.
Exercise can also improve protein balance. Although resistance training has been shown to increase muscle size and strength in older individuals (12), many older individuals are unable to perform resistance training due to prior injury, limited access to equipment, and lack of knowledge regarding proper technique. Whole-body protein synthesis has been shown to increase after a bout of aerobic exercise (3 h at 75% VO2max), with whole-body protein breakdown not differing from values at rest (13). A long-term aerobic exercise program has been shown to increase basal whole-body protein turnover (14), whereas decreasing leucine oxidation at rest (15). Additionally, it has been shown that prolonged aerobic exercise can increase myofiber size in elderly women (16), thus providing evidence of the benefits of aerobic exercise at increasing LBM in elderly individuals. The impact of aerobic exercise on NBAL is also dependent on EB. When individuals maintain EB, aerobic exercise increases NBAL over time (6,15). However, the increases in NBAL observed with habitual aerobic exercise are decreased when individuals are in a hypocaloric state (6).
Combining exercise with proper nutritional strategies has been proposed to be more effective than either intervention alone in stimulating muscle protein synthesis (MPS) (17,18). Ingestion of amino acids with or without carbohydrate caused a shift toward increased protein balance postexercise (17,19,20). However, by consuming amino acids with carbohydrates postexercise, net protein balance was increased to levels greater than if the amino acids or carbohydrate were ingested alone (19). In the elderly, protein combined with carbohydrate (40-g carbohydrate, 20-g whey) led to increases in whole-body protein turnover above those observed with an isoenergetic amount of carbohydrate (60-g carbohydrate; 21).
There is conflicting evidence as to whether timing of protein intake after exercise enhances protein synthesis and thus LBM (22,23). We have addressed this question by using NBAL and have shown that in older individuals in EB, consumption of protein and carbohydrates (chocolate milk) immediately following moderate aerobic exercise at 55% VO2max led to greater NBAL than if consumed earlier in the day (24). Findings from our previous study were interesting given that differences in NBAL were noted with only the timing of protein intake differing between trials. Because many older individuals are habitually in negative EB (25), and increasing energy intake leads to increases in NBAL (9,10), we investigated the impact of the timing of protein intake on NBAL in exercising older individuals in states of hypocaloric and hypercaloric EB. We hypothesized that timing protein intake after exercise in states of hypocaloric and hypercaloric EB would enhance NBAL over values observed when protein is consumed earlier in the day.
METHODS
Study Overview
The study included two separate cohorts, hypocaloric (n = 10) and hypercaloric (n = 6). In the hypocaloric cohort, participants were in 15% negative EB for the duration of the experimental period and participants in the hypercaloric cohort were in 15% positive EB. Each experiment consisted of four periods: preexperimental testing, a 7-day lead-in diet, a 6-day inpatient/experimental period, and post-testing (Figure 1A). Metabolic information collected during pretesting was used for planning the lead-in and experimental diets and to determine the relative intensity of the daily exercise during the experimental period. The 7-day lead-in diet allowed participants to adapt to the level of dietary protein that was provided during the experimental period. All aspects of the study were completed in the same laboratory setting and repeated the design of our previously published study (24). The study protocol was approved by the Colorado State University Institutional Review Board and the Colorado Multiple Institutional Review Board for human research and adhered to the Declaration of Helsinki.
Figure 1.
Study timeline (A) and daily timing of meals, exercise, and consumption of beverages during PRO + CHO and CHO trials (B). The timeline in panel B occurs within the period labeled “Experimental” in panel A. The order of the PRO and CHO trials was randomized.
Participants
Ten healthy sedentary men (n = 2) and women (n = 8) participated in the hypocaloric cohort, and six healthy sedentary men (n = 2) and women (n = 4) participated in the hypercaloric balance cohort as depicted in Table 1. All participants were nonsmokers between the ages of 55 and 75 years and did not take any medications. Participants were required to be lactose tolerant because milk was used as an experimental beverage. Additional exclusion criteria included obesity (body mass index > 30), recent orthopedic injury that would impede the ability to exercise, any condition that affected food digestion or absorption, a thyroid condition (thyroid stimulating hormone <0.05 uU/mL or thyroid stimulating hormone > 5.0 uU/mL), or any current illness or infection.
Table 1.
Participant Characteristics of Both Energy Balance Cohorts
| Hypocaloric Cohort | Hypercaloric Cohort | |||||
| Men (n = 2) | Women (n = 8) | All Participants (n = 10) | Men (n = 2) | Women (n = 4) | All Participants (n = 6) | |
| Age (years) | 67 ± 1 | 63 ± 2 | 64 ± 2 | 63 ± 6 | 66 ± 2 | 65 ± 2 |
| Height (cm) | 183 ± 1 | 165 ± 3* | 169 ± 3 | 178 ± 5 | 160 ± 2* | 166 ± 4 |
| Weight (kg) | 93 ± 1 | 61 ± 2* | 67 ± 4 | 85 ± 3 | 60 ± 3* | 68 ± 6 |
| Body mass index (kg/m2) | 27.4 ± 0.3 | 22.3 ± 0.6* | 23.3 ± 0.8 | 26.9 ± 2.5 | 23.5 ± 1.8 | 24.6 ± 1.5 |
| Body fat (%) | 27.9 ± 1.0 | 34.5 ± 1.7 | 33.2 ± 1.7 | 24.7 ± 1.3 | 37.9 ± 4.2 | 33.5 ± 3.9 |
| VO2max (mL/kg/min) | 32.2 ± 0.5 | 28.5 ± 1.7 | 29.2 ± 1.4 | 29.4 ± 2.1 | 21.1 ± 1.2* | 23.9 ± 2.0* |
Note: *Significant difference between hypocaloric and hypercaloric cohorts.
Pretesting
Over four separate days, participants completed a series of pretesting at the Human Performance Clinical/Research Laboratory at Colorado State University. During the initial visit, participants completed a Balke protocol graded exercise test on a treadmill under the supervision of a cardiologist. Participants were excluded if the graded exercise test indicated a cardiac ischemic or hypertensive response to the exercise. Participants returned to the Human Performance Clinical/Research Laboratory after an overnight fast, and resting metabolic rate (RMR; Parvomedics TrueOne 2400, Sandy, UT) was measured to determine 24-hour resting caloric expenditure. During the test, participants rested in the supine position for 45 min and were instructed to remain still and to refrain from sleeping. The first 15 min of the test was used to achieve the appropriate flow rate and to allow participants to become familiarized with the experimental conditions. The data from the final 30 min of the test were used to predict RMR. Body composition was then determined using dual-energy X-ray absorptiometry (QDR 4500W, Hologic, Inc., Bedford, MA). On the third day of testing, participants completed an incremental exercise test on a cycle ergometer (Monark Excalibur, Groningen, The Netherlands) with indirect calorimetry (Parvomedics TrueOne 2400) to determine maximal oxygen consumption (VO2max). Participants pedaled at 50 watts for the first minute, and watts increased by 20 for women and 30 for men every 2 min thereafter (24). The test was stopped when participants reached volitional exhaustion. After VO2max was determined, 55% of each participant’s VO2max was calculated using the American College of Sports Medicine leg cycle ergometry equation (26).
On a subsequent day, participants returned to the Human Performance Clinical/Research Laboratory for a submaximal steady-state cycling test in which participants cycled at their estimated workload (55% VO2max) for 45 min. Energy expenditure (EE) was determined by averaging the EE from the final 30 min of cycling using indirect calorimetry. The calculated EE from the 30 min of cycling was used to estimate the participant’s exercise EE in kcal/hour for the 1 hour of cycling exercise during the inpatient period.
During pretesting, participants completed an online food preference and food allergy questionnaire from the Clinical and Translational Research Center (CTRC) to ensure that all study diets contained acceptable foods. Participants also recorded all foods and beverages that were consumed over 3 consecutive days (2 weekdays and 1 weekend) in a 3-day diet log. All foods from the 3-day diet records were entered into Nutrition Pro software (Axxya Systems, Stafford, TX) and analyzed to determine habitual (free-living) energy and macronutrient intake.
Lead-in Diet
Following completion of the pretesting, participants completed a controlled 7-day lead-in diet immediately followed by a 6-day inpatient stay at the University of Colorado Denver CTRC. The lead-in diet allowed participants to adapt to a new level of protein intake, so that NBAL measurements made during the inpatient stay would not reflect an acute adaption to a new level of protein intake. During the lead-in diet period, participants arrived every morning at the Colorado State University Nutrition Center for breakfast. Participants ate breakfast at the nutrition center under the supervision of study staff. Food for the remainder of the day was prepared and given to the participants in a cooler. Participants were instructed to eat only the food given to them by the study staff and to eat all of the food given to them. If the participants were unable to eat any of the food provided to them, they brought the food back with them the following day so that it could be weighed. Participants were instructed to continue their typical daily activities throughout the duration of the 7-day lead-in period. The lead-in and inpatient diets followed U.S. Department of Agriculture nutritional guidelines (27) and were constructed using Pronutra software (Viocare, Inc., Princeton, NJ). Participants remained in EB for the duration of the lead-in diet for both the negative and positive EB cohorts. During the negative EB cohort, the lead-in diet (and all inpatient diets) macronutrient breakdown was 15% protein, 30% fat, and 55% carbohydrate expressed as a percentage of total calories. During the positive EB cohort, the lead-in diet (and all inpatient diets) consisted of 1.2-g protein/kg bw, 30% of calories from dietary fat, and the balance as carbohydrate. We used an absolute quantity of protein in the hypercaloric cohort to match the hypocaloric cohort protein intake. For the lead-in diets, total energy intake was calculated using each participant’s RMR, multiplied by an activity factor, which approximated the activity levels of free-living sedentary elderly individuals (28).
Experimental/Inpatient Period
The experimental period involved a 6-day inpatient stay at the University of Colorado Denver CTRC (Figure 1B). Each participant completed 2 consecutive, 3-day trials in a randomized crossover design. The diets for each 3-day trial were reproduced and identical in calorie intake, macronutrients, and foods consumed. The diet plans for Day 1, 2, and 3 were repeated on Day 6, 4, and 5, respectively. The only difference between trials was the timing of intake of the protein beverage. For the negative EB cohort, participants were in a 15% caloric deficit, and similar to the lead-in diet, the percentage of total kilocalories for each macronutrient in the inpatient diet was 55% carbohydrate, 30% fat, and 15% protein. Participants in the positive EB cohort were in positive 15% EB and, similar to the lead-in diet, the inpatient diet consisted of 1.2-gm protein/kg bw, 30% of calories from fat, and the balance as carbohydrate.
Participants were admitted to the CTRC the evening before the 6-day inpatient period and provided dinner as the final meal of their lead-in diet. The study began the following morning with the start of the study diet and 24-hour urine collection. Daily weight measurements were recorded each morning on the same scale after voiding. Day 1 and Day 6 were spent in a whole-room calorimeter, whereas days 2–5 were spent in a patient room on the CTRC. During days 2–5, participants were permitted to leave the CTRC unit twice a day for 30 min. Participants were allowed to eat only the food that was provided to them by study staff and to consume water ad libitum. Every day at 4:30 PM, participants completed 1 hour of cycling exercise (Lode, Groningen, The Netherlands) at 55% of their VO2max. The exercise was intended to simulate a brisk walk, which was well tolerated by all participants. Participants were not permitted to engage in any additional volitional exercise during the day.
Immediately following the daily exercise bout, a postexercise beverage was consumed. The only difference between the two trials was the timing of intake of the two experimental beverages. In one trial (PRO + CHO), a 248-kcal chocolate milk drink that contained 15.3-gm protein, 43.6-gm carbohydrate, and 1.3-gm fat (330-gm skim milk, 4-gm whey protein, and 42-gm chocolate syrup) was consumed immediately postexercise. In the second trial (CHO), a 247-kcal carbohydrate beverage that contained 0.0-gm protein, 63.51-gm carbohydrate, and 0.06-gm fat was consumed immediately postexercise. During the PRO + CHO trial, the CHO beverage was consumed as a snack at 10 AM and during the CHO trial, the PRO + CHO beverage was consumed as a snack at 10 AM.
In order to plan the diets for the inpatient period, total daily EE was estimated. Each participant’s RMR was multiplied by a separate activity factor for calorimeter and noncalorimeter days. The activity factor was lower for calorimeter days because ambulatory activity levels are lower when confined to the calorimeter room (E. L. Melanson, PhD, unpublished data, 2010). Exercise EE was estimated from the steady-state VO2 data (measured during pretesting), and an additional 20% of exercise calories were added in order to account for excessive postexercise oxygen consumption.
Participants entered the calorimeter room at 7:45 AM on Day 1 and Day 6 and exited at 7:15 AM on the following morning. The calorimeter room was 12′ × 12′ and contained a bed, sink, toilet, bicycle ergometer, computer, and television. To prevent air from escaping, all meals were passed through an air lock that could not be simultaneously opened from the inside and outside. Daily EE and substrate oxidation were calculated by the difference in gas content that was entering and exiting the room. EE and respiratory quotient were assessed in 1-minute intervals from oxygen consumption and carbon dioxide production. Gas concentrations were determined from the flow rate and the differences in CO2 and O2 concentrations between entering and exiting air by using a fuel-cell–based dual channel O2 analyzer (FC-2 Oxzilla, Sable Systems, International, Las Vegas, NV) and two infrared CO2 analyzers (CA-10 CO2 analyzers, Sable Systems, International), as previously described (29). The accuracy and precision of the system are tested monthly using propane combustion tests. The average O2 and CO2 recoveries during the course of this study were ≥98.0%. Total EE and substrate oxidation were calculated using oxygen consumption and respiratory quotient (30). Urine N was used to calculate 24-hour protein oxidation, with 1 gm of urine N reflecting 6.25 gm of oxidized protein.
During the inpatient stay, participants wore an accelerometer (Actigraph GT1M, Pensacola, FL), which was removed during exercise, sleep, and showering. EE from activity was estimated from the accelerometer. If activity counts were less than or equal to 1,952/min, the Work–Energy Theorem was used to estimate EE:
The Freedson equation was used to estimate EE for activity counts greater than 1,952/min:
Total daily energy expenditure (TDEE) on calorimeter days was directly measured, and noncalorimeter days were estimated:
Because participants were sedentary, the EE predicted by the accelerometer was used for activity thermogenesis. The measured value for RMR was used, and DIT was estimated to be 10% of TDEE (31). Physical activity level was calculated by dividing TDEE by RMR. EB was determined using calorimeter room EE and energy intake, which was calculated from the diet plans created by the dietitian.
Nitrogen Balance
Twenty-four-hour urine samples were collected continuously beginning with the first void on Day 1 in order to determine urinary nitrogen. Urine was collected in acid, and total volume was measured. Two 10-mL aliquots per 24-hour collection were frozen and stored for later analysis. Nitrogen was analyzed using an Antek 7000 Elemental Nitrogen Analyzer (PAC, Houston, TX). NBAL was calculated as
Nitrogen intake was calculated as (protein intake [gm]/6.25) because 6.25 gm of protein contains on average 1 gm of nitrogen. Nitrogen output is defined as the N excreted in urine and feces plus miscellaneous N losses. Daily miscellaneous N losses were estimated at 5 mg/kg bw, and fecal N losses were estimated at 2 gm (32). Three-day mean NBAL from each trial (PRO + CHO and CHO) was compared with test the hypothesis that timing protein intake after aerobic exercise improves NBAL in older adults.
Post-testing
Approximately 1 week after the inpatient period, participants returned to the Human Performance Clinical/Research Laboratory for a final dual-energy X-ray absorptiometry scan and weight measurement.
Statistical Analysis
To provide further insight into the effect of EB on the anabolic effect of postexercise feeding, we combined data from these two corhorts and our previously published study (24). Although mean data in each EB group (negative, balance, and positive) were in our target range, there was variability within each group leading to real variability. We feel this further analysis was justified because all studies were conducted under identical conditions. The additional insight gained can only be captured in the combined data; however, the data are presented separately so the reader can make that assessment his/herself.
NBAL data, EB data, and participant characteristics were analyzed using student’s paired t-tests. A one-way analysis of variance with a Student Newman–Keuls post hoc analysis was used to examine nutrient intake during the different periods of testing. Differences were considered significant at p < .05. All data are presented as the mean ± SEM unless indicated otherwise.
RESULTS
Macronutrient Intake and EB
There were no differences in macronutrient intake except for the hypercaloric diet in which carbohydrate intake was increased during the inpatient period (Table 2). During the inpatient period in the hypocaloric cohort (Table 2), participants were in negative EB, with a mean daily negative EB of −285 ± 35 kcal during the CHO trial and −291 ± 40 kcal during the PRO + CHO trial (p = .90). During the inpatient period in the hypercaloric cohort, participants had an increased energy intake from free-living conditions that resulted in a positive EB, with a mean daily positive EB of 360 ± 51 kcal for the CHO trial and 354 ± 88 kcal for PRO + CHO trial (p = .98). Mean bw did not change during the inpatient stay in either hypocaloric or hypercaloric cohorts (Figure 2).
Table 2.
Energy Intake, EE, and EB in Hypo- and Hypercaloric Cohorts
| Macronutrient Intake (gm/kg bw) | |||||||||
| Protein | CHO | Fat | |||||||
| Free Living | Lead in | Inpatient | Free Living | Lead in | Inpatient | Free living | Lead in | Inpatient | |
| Hypocaloric | 1.27 ± 0.11 | 1.16 ± 0.04 | 1.07 ± 0.04 | 3.87 ± 0.37 | 4.23 ± 0.15 | 3.91 ± 0.14 | 1.18 ± 0.11 | 1.03 ± 0.04 | 0.95 ± 0.03 |
| Hypercaloric | 1.13 ± 0.09 | 1.23 ± 0.07 | 1.20 ± 0.00 | 3.24 ± 0.23 | 4.53 ± 0.27* | 5.87 ± 0.36*# | 1.13 ± 0.14 | 1.10 ± 0.06 | 1.35 ± 0.07 |
| EB | |||||||||
| Energy Intake (kcal) | PAL | Nonexercise PAL | Mean Energy Balance (kcal) | ||||||
| Free Living | Lead in | Inpatient | CHO | PRO + CHO | CHO | PRO + CHO | CHO | PRO + CHO | |
| Hypocaloric | 2,016 ± 136 | 2,003 ± 122 | 1,860 ± 126 | 1.60 ± 0.02 | 1.61 ± 0.02 | 1.36 ± 0.02 | 1.37 ± 0.03 | −285 ± 35 | −291 ± 40 |
| Hypercaloric | 1,914 ± 180 | 2,160 ± 161 | 2,665 ± 224* | 1.59 ± 0.04 | 1.57 ± 0.04 | 1.37 ± 0.02 | 1.35 ± 0.03 | 360 ± 51 | 354 ± 88 |
Notes: bw = body weight; EB = energy balance; EE = energy expenditure; and PAL = physical activity level.*Significant difference from free-living conditions within the same cohort.#Significant difference from lead-in conditions within the same cohort.
Figure 2.
Mean daily body weight during inpatient period for both negative energy balance (EB; n = 10) and positive EB (n = 6) groups (mean ± SEM).
Nitrogen Balance
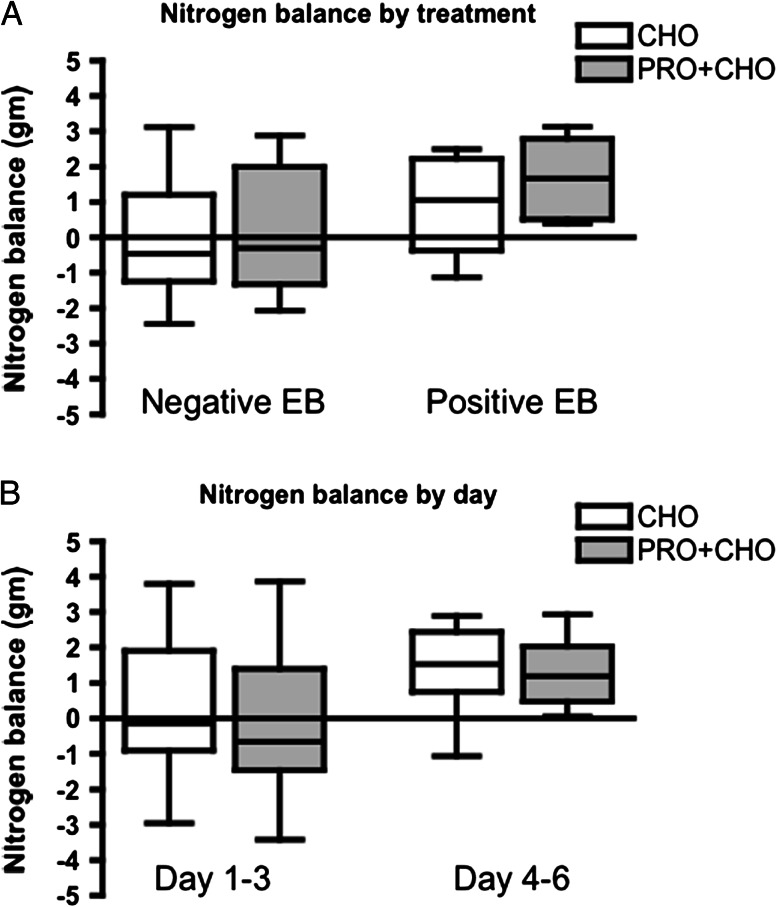
In the hypocaloric cohort, 3-day mean NBAL was not different between CHO and PRO + CHO trials (mean ± SEM, 95% CI for CHO: −0.07 ± 0.52, −1.25 to 1.11; for PRO + CHO: 0.09 ± 0.53, −1.09 to 1.29; p = .28; Figure 3A). In the hypercaloric cohort, 3-day mean NBAL was not different between the CHO and the PRO + CHO trials (mean ± SEM, 95% CI for CHO: 0.97 ± 0.52, 0.36 to 2.30; for PRO + CHO: 1.66 ± 0.43, 0.55 to 2.76), although there was a trend toward significance (p = .09; Figure 3A). In both cohorts, there was no difference in NBAL between CHO and PRO + CHO in days 1–3 and days 4–6 (p = .25 for negative EB and p = .35 for positive EB; Figure 3B).
Figure 3.
Nitrogen balance in negative and positive energy balance (EB) groups. There were no differences between CHO and PRO + CHO groups in either negative EB (p = .28) or positive EB although there was a trend (p = .09; A). There was no order effect of treatment on NBAL (p = .25 for negative EB and p = .35 for positive EB; B).
Combined Data for Negative, Even, and Positive EB Cohorts
When values from all trials together without stratification for EB were compared, mean NBAL was greater for PRO + CHO compared with CHO (CHO: 0.42 ± 0.29 gm N, −0.19 to 1.03 gm N; PRO + CHO: 0.85 ± 0.29 gm N, 0.25 to 1.45 gm N; p < .01; Figure 4A). We then restratified all the combined data so that 6-day mean EB percentage above 0.0 was placed in the positive group and below 0.0 were placed in the negative group. The resultant adjusted model had a greater percent EB in the positive EB group compared with the negative EB group (negative: −13.29 ± 2.41%, −18.85 to −7.74%; positive: 12.85 ± 1.96%, 8.60 to 17.09%; p < .0001; Figure 4B). In the restratified analysis, NBAL was greater in the positive EB groups compared with the negative EB in both the CHO trial (negative: −0.43 ± 0.42 gm N, −1.39 to 0.53 gm N; positive: 0.96 ± 0.34 gm N, 0.25 to 1.69 gm N; p = .009) and the PRO + CHO trial (negative: −0.15 ± 0.47 gm N, −1.24 to 0.94 gm N; positive: 1.49 ± 0.26 gm N, 0.94 to 2.05 gm N; p = .002; Figure 4C). There was no difference in mean NBAL between the CHO and PRO + CHO trial in the negative EB group (CHO: −0.43 ± 0.42 gm N, −1.39 to 0.53 gm N; PRO + CHO: −0.15 ± 0.47 gm N, −1.24 to 0.94 gm N; p = .18; Figure 4D). However, mean NBAL was greater (p = .016) in the PRO + CHO compared with CHO in the positive EB group (CHO: 0.96 ± 0.34 gm N, 0.25 to 1.69 gm N; PRO + CHO: 1.49 ± 0.26 gm N, 0.94 to 2.05 gm N; p = .016; Figure 4D).
Figure 4.
Combined data from all three studies to determine the effect of energy balance (EB) on nitrogen balance. When combining all data without regard to EB, NBAL was greater in PRO + CHO compared with CHO (p < .01; A). Restratified negative EB was lower compared with positive EB (p < .0001; B). When restratified by treatment, positive EB had greater NBAL in both CHO (p = .009) and PRO + CHO groups (p = .002; C). When restratified by EB, there was no increase with PRO + CHO in the negative EB group (p = .18), but there was an increase with PRO + CHO in the positive EB group (p = .016; D).
DISCUSSION
The current studies investigated the effects of the timing of protein intake in relation to a bout of aerobic exercise on NBAL in older individuals with varying energy intakes. Older individuals completed two 3-day isocaloric–isonitrogenous trials with only the timing of protein consumption differing between the two conditions. Contrary to our initial hypotheses, NBAL did not differ in older individuals on either hypocaloric or hypercaloric diets when protein was consumed immediately after moderate aerobic exercise rather than earlier in the day. However, further stratification of positive versus negative EB to account for EB variations within a trial revealed a strong influence of EB on the anabolic effect of postexercise feeding. To our knowledge, this study is unique given its aim to investigate the combined effects of varying EB and the timing of protein intake on NBAL in older individuals.
Energy Balance and Nitrogen Balance
NBAL is dependent on EB such that NBAL is better maintained when caloric intake is adequate and negative NBAL results when energy intake is reduced (6). During caloric restriction, protein breakdown increases the availability of amino acids that are oxidized as energy and thus limits the availability of amino acids for protein synthesis. When caloric intake is inadequate, aerobic exercise can increase NBAL (6). Consistent with this, within the hypocaloric cohort of the current study older individuals were able to maintain NBAL for both the CHO and PRO + CHO trials despite being in negative EB. Because loss of LBM occurs when individuals are in negative NBAL (5), maintaining NBAL while in negative EB could reduce the loss of LBM and thus attenuate the progression of wasting.
Within the negative EB cohort, there was no differences in NBAL between the CHO and PRO + CHO trials, which differs from previous research by Roy and colleagues (33). In that study, participants underwent two 7-day trials in which they increased their training volume and consumed either a mixed-meal beverage or a noncaloric placebo beverage after exercise. Participants were in an exercise-induced negative balance, as calories expended during exercise were not replaced. Similar to our study, diet was replicated so that the only difference between the trials was the timing of consumption of the beverage. Timing nutrient intake immediately after exercise, rather than earlier in the day, resulted in greater NBAL with a trend toward significance (p = .06; 33). However, the participants in the Roy study were young female athletes whereas the present study was in older individuals, exercise was performed every other day rather than every day, and NBAL only determined over the last 24 h as opposed to every day.
Within the restratified negative EB group, there were no differences in NBAL between the PRO + CHO and CHO trials. Although this was consistent with the results from the negative EB cohort, these results differ from previous research by Roy and colleagues (33). In the positive EB group, there was increased protein retention in the PRO + CHO compared with the CHO trials. Thus, our data indicate that timing protein intake after exercise has a greater impact on improving NBAL when older individuals are in positive EB rather than negative EB.
Within the restratified EB groups, mean NBAL was greater in the positive EB groups compared with negative EB for both CHO and CHO + PRO treatments. Previously Howarth and colleagues (34) demonstrated that consuming protein with carbohydrate after aerobic exercise increases NBAL more than an isocaloric amount of only carbohydrate. The differences between EB groups demonstrate that EB alone, a factor often not controlled for, can be increase NBAL (9,10). We therefore recommend consideration of EB when performing short- and long-term studies of protein balance.
Because the NBAL method does not elucidate rates of protein synthesis or breakdown, we were unable to determine how changes in net NBAL occurred. Also, it is not known where protein accrual is taking place. It could be that MPS increased in response to the exercise stimulus (35). However, because skeletal muscle contributes approximately 30% of the whole-body protein turnover rate (36), changes in whole-body protein, outside of skeletal muscle, are also expected to contribute to changes in protein balance. Interestingly, in a recent study in our laboratory, we found in a similar group of older individuals that postexercise feeding of PRO + CHO versus CHO did not result in increased cumulative MPS over a 6-week aerobic exercise-training program (22). There could therefore be differences in whole-body protein retention versus skeletal MPS. NBAL accounts for whole-body protein retention whereas our previous study examined MPS. This relative long-term difference (compared with an acute feeding) in whole-body versus MPS in response to postexercise feeding has still not been adequately addressed. Alternatively, in our previous study (22), EB was not controlled for the 6-week period as the participants were free living and were only encouraged to maintain EB. The current study only demonstrated a positive effect of PRO + CHO compared with CHO in positive EB; therefore, day-to-day variations in EB may change whether protein is accrued or not. These differences illustrate the importance of considering EB when examining skeletal muscle (sarcopenia) or whole-body (wasting) outcomes.
Experimental Diet and Exercise
The study was designed to have practical implications. All study diets followed U.S. Department of Agriculture dietary recommendations for the percentage of total kilocalories for each macronutrient (45–65% carbohydrate, 20–35% fat, and 10–35% protein; 27). The daily exercise completed by participants during the inpatient stay was 1 hour of moderate intensity (55% VO2max) cycling. The exercise intensity and duration were well-tolerated by all participants, and the inpatient physical activity level was consistent with older populations (28). Additionally, exercise at 55% of VO2max simulates a brisk walking pace because older individuals most commonly choose walking as exercise (37).
Chocolate milk was chosen for this study as the protein source because milk contains a high quantity of leucine and consists of both whey and casein proteins (38). Older individuals have a blunted MPS response to anabolic signals compared with younger individuals (39,40). However, increasing the proportion of leucine allows for optimal stimulation of MPS in older individuals (41). Whey protein results in an acute increase in protein synthesis (42), whereas casein protein results in a minor increase in protein synthesis and a significant decrease in protein breakdown (42). Previously, it was shown that consumption of carbohydrate combined with protein after aerobic exercise increases whole-body net protein balance and muscle FSR more than an isocaloric amount of carbohydrate alone (34). The current study indicates that this is true when in positive EB conditions but not necessarily negative EB conditions.
Limitations
Inadequate accounting of protein intake or miscellaneous nitrogen losses can contribute to variability in the NBAL method (43). However, the same foods were consumed during both trials and were closely monitored so any measurement error would occur during both conditions to the same extent. In addition, miscellaneous protein losses were held constant between trials, and we have no reason to believe that would vary. Therefore, any differences in NBAL estimations would be consistent between the two trials. Despite limitations, the NBAL technique can be a useful technique for studying whole-body protein retention in aging populations. The NBAL technique with its noninvasive nature makes it a relatively easy and appropriate method to determine long-term changes in whole-body protein retention, an important outcome when considering wasting, in response to exercise and nutrition interventions in older individuals.
Conclusions and Recommendations
The current study investigated whether having a protein-containing beverage immediately after moderate aerobic exercise rather than earlier in the day can improve NBAL in older individuals with varying EB. The combined results from the current study and our previous study (24) indicate that EB is an important determinant of the anabolic effect of protein feeding and therefore must be considered when using the NBAL method. We demonstrated that nitrogen retention is greater during positive EB than negative EB and that the beneficial effects of a protein-containing beverage on nitrogen retention are present with positive EB but are negligible during negative EB. Future studies should consider the effect of EB on the timing of protein intake after exercise. Further, it may be important to independently assess skeletal muscle and whole-body outcomes because they may not be the same.
FUNDING
This work was funded by the Colorado Agriculture Experimental Station (grant #COL00604), a career development support from NIA K01AG031829-01 (B.F.M.), and the UCD Clinical and Translational Science Award (1UL1 RR025780).
Acknowledgments
The study participants are thanked for their exceptional dedication. Further, we thank Archana Mande for designing the study diets and the University of Colorado Denver staff for their help.
References
- 1.Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56:81–88. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–M380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr. 2008;88:1322–1329. doi: 10.3945/ajcn.2008.26072. [DOI] [PubMed] [Google Scholar]
- 5.Friedlander AL, Braun B, Pollack M, et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am J Physiol. 2005;289:E446–E455. doi: 10.1152/ajpendo.00001.2005. [DOI] [PubMed] [Google Scholar]
- 6.Todd KS, Butterfield GE, Calloway DH. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr. 1984;114:2107–2118. doi: 10.1093/jn/114.11.2107. [DOI] [PubMed] [Google Scholar]
- 7.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. 1995;62:30–39. doi: 10.1093/ajcn/62.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Calloway DH, Spector H. Nitrogen balance as related to caloric and protein intake in active young men. Am J Clin Nutr. 1954;2:405–412. doi: 10.1093/ajcn/2.6.405. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield GE, Calloway DH. Physical activity improves protein utilization in young men. Br J Nutr. 1984;51:171–184. doi: 10.1079/bjn19840021. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbertson DP, Munro HN. A study of the effect of overfeeding on the protein metabolism of man: the protein-saving effect of carbohydrate and fat when superimposed on a diet adequate for maintenance. Biochem J. 1937;31:694–705. doi: 10.1042/bj0310694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312(Pt 1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 13.Devlin JT, Brodsky I, Scrimgeour A, Fuller S, Bier DM. Amino acid metabolism after intense exercise. Am J Physiol. 1990;258:E249–E255. doi: 10.1152/ajpendo.1990.258.2.E249. [DOI] [PubMed] [Google Scholar]
- 14.Lamont LS, Patel DG, Kalhan SC. Leucine kinetics in endurance-trained humans. J Appl Physiol. 1990;69:1–6. doi: 10.1152/jappl.1990.69.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Gaine PC, Viesselman CT, Pikosky MA, Martin WF, Armstrong LE, Pescatello LS, Rodriguez NR. Aerobic exercise training decreases leucine oxidation at rest in healthy adults. J Nutr. 2005;135:1088–1092. doi: 10.1093/jn/135.5.1088. [DOI] [PubMed] [Google Scholar]
- 16.Harber M, Crane J, Dickinson J, Jemiolo B, Raue U, Trappe T, Trappe S. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Reg Integr Comp Physiol. 2009;296 doi: 10.1152/ajpregu.90906.2008. R708. [DOI] [PubMed] [Google Scholar]
- 17.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 18.Miller BF. Human muscle protein synthesis after physical activity and feeding. Exerc Sport Sci Rev. 2007;35:50–55. doi: 10.1097/jes.0b013e31803eac78. [DOI] [PubMed] [Google Scholar]
- 19.Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sport Exerc. 2003;35:449–455. doi: 10.1249/01.MSS.0000053910.63105.45. [DOI] [PubMed] [Google Scholar]
- 20.Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol. 2004;96:674–678. doi: 10.1152/japplphysiol.00333.2003. [DOI] [PubMed] [Google Scholar]
- 21.Murphy C, Miller BF. Protein consumption following aerobic exercise increases whole-body protein turnover in older adults. Appl Physiol Nutr Metab. 2010;35:583–590. doi: 10.1139/H10-047. [DOI] [PubMed] [Google Scholar]
- 22.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. Faseb J. 2011;25:3240–3249. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol. 2001;281:E197–E206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 24.Jordan LY, Melanson EL, Melby CL, Hickey MS, Miller BF. Nitrogen balance in older individuals in energy balance depends on timing of protein intake. J Gerontol A Biol Sci Med Sci. 2010;65:1068–1076. doi: 10.1093/gerona/glq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDowell MA, Briefel RR, Alaimo K, et al. Energy and macronutrient intakes of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988-91. Adv Data. 1994;255:1–24. [PubMed] [Google Scholar]
- 26.Thompson WR, Gordon NF, Pescatello LS. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 27.United States Department of Health and Human Services and United States Department of Agriculture. Dietary Guidelines for Americans, 2005. Washington, DC: U.S. Government Printing Office; 2005. Dietary Guidelines Advisory Committee; pp. 5–12. [Google Scholar]
- 28.Pannemans DL, Westerterp KR. Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr. 1995;73:571–581. doi: 10.1079/bjn19950059. [DOI] [PubMed] [Google Scholar]
- 29.Melanson EL, Ingebrigtsen JP, Bergouignan A, Ohkawara K, Kohrt WM, Lighton JR. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1571–R1579. doi: 10.1152/ajpregu.00055.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 31.D’Alessio DA, Kavle EC, Mozzoli MA, et al. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calloway DH, Odell AC, Margen S. Sweat and miscellaneous nitrogen losses in human balance studies. J Nutr. 1971;101:775–786. doi: 10.1093/jn/101.6.775. [DOI] [PubMed] [Google Scholar]
- 33.Roy BD, Luttmer K, Bosman MJ, Tarnopolsky MA. The influence of post-exercise macronutrient intake on energy balance and protein metabolism in active females participating in endurance training. Int J Sport Nutr Exerc Metab. 2002;12:172–188. doi: 10.1123/ijsnem.12.2.172. [DOI] [PubMed] [Google Scholar]
- 34.Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol. 2009;106:1394–1402. doi: 10.1152/japplphysiol.90333.2008. [DOI] [PubMed] [Google Scholar]
- 35.Sheffield-Moore M, Yeckel CW, Volpi E, et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 36.Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50:107–112. doi: 10.1093/gerona/50a.special_issue.107. [DOI] [PubMed] [Google Scholar]
- 37.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5:65–72. [PubMed] [Google Scholar]
- 38.Jenness R. Comparative aspects of milk proteins. J Dairy Res. 1979;46:197–210. doi: 10.1017/s0022029900017040. [DOI] [PubMed] [Google Scholar]
- 39.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 40.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 42.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopple JD. Uses and limitations of the balance technique. J Parenter Enteral Nutr. 1987;11:79S–85S. doi: 10.1177/014860718701100511. [DOI] [PubMed] [Google Scholar]