Abstract
Background
Although azithromycin promised to be a safe and effective single dose oral treatment for early syphilis, azithromycin treatment failure has been documented and is associated with mutations in the 23S rDNA of corresponding Treponema pallidum strains. The prevalence of strains harboring these mutations varies throughout the US and the world. We examined T. pallidum strains circulating in Seattle, Washington, from 2001–2010 to determine the prevalence of two mutations associated with macrolide resistance, and to determine whether these mutations were associated with certain T. pallidum strain types.
Methods
Subjects were enrolled in a separate ongoing study of cerebrospinal fluid (CSF) abnormalities in patients with syphilis. T. pallidum DNA purified from blood and T. pallidum strains isolated from blood or CSF were analyzed for two 23S rDNA mutations and for the molecular targets used in an enhanced molecular stain typing system.
Results
Nine molecular strain types of T. pallidum were identified in Seattle from 2001–2010. Both macrolide resistance mutations were identified in Seattle strains, and the prevalence of resistant T. pallidum exceeded 80% in 2005 and increased through 2010. Resistance mutations were associated with discrete molecular strain types of T. pallidum.
Conclusions
Macrolide resistant T. pallidum strains are highly prevalent in Seattle, and each mutation is associated with discrete strain types. Macrolides should not be considered for treatment of syphilis in regions where prevalence of the mutations is high. Combining the resistance mutations with molecular strain typing permits a finer analysis of the epidemiology of syphilis in a community.
Introduction
Syphilis represents a serious and continuing threat to human health, especially in the developing world and in populations where human immunodeficiency virus (HIV) infection rates are high. Despite availability of effective treatment for syphilis, the United States and Western Europe are experiencing a resurgence of syphilis, especially among men who have sex with men (MSM). Reported cases of primary and secondary syphilis have doubled in the US over the last decade.1 A dramatic resurgence of syphilis has been documented in China, where reported rates (cases per 100,000 population) have increased 25-fold since the early 1990’s.2 Developing countries also continue to have high burdens of syphilis, and congenital syphilis accounts for a high proportion of prenatal and perinatal morbidity and mortality.
For the past 50 years, the recommended treatment for early syphilis has been a single 2.4 million-unit intramuscular dose of benzathine penicillin G (BPG). The prolonged levels of penicillin G allow for effective action against the slowly replicating agent of syphilis, Treponema pallidum, and BPG resistance has never been documented. Because of penicillin allergy and the discomfort associated with BPG, an equally effective oral alternative has long been sought. Azithromycin, with its long tissue half-life, has been demonstrated to be as effective as BPG in the rabbit model of syphilis and in clinical trials in Uganda and Tanzania, as well as a phase III trial including subjects from Madagascar and North America. 3–5
Clinical failures of azithromycin, characterized by failure of penile ulcers to resolve, new seroconversion, or development of new penile ulcers following azithromycin treatment, were first identified by the San Francisco Public Health Department in eight MSM patients with primary or secondary syphilis between 2002–2003.6 Subsequent analysis of clinical samples obtained from these patients revealed a mutation A2058G (E. coli cognate) in the T. pallidum 23S rRNA gene.7 This same A2058G mutation had been previously identified in the erythromycin-resistant T. pallidum Street 14 strain isolated in 1977.8 In 2009, a second mutation, A2059G, in the 23S rRNA gene of T. pallidum was identified in the Czech Republic in a patient with secondary syphilis who failed treatment with oral spiramycin, another macrolide antibiotic.9
In 2004, we used a PCR-based restriction enzyme digestion method to examine samples from various sites in the United States and Ireland, identifying the A2058G mutation in 88% of samples from Dublin, 22% of samples from San Francisco, 13% of samples from Seattle, and 11% of samples from Baltimore.6 More recent studies have documented a dramatic increase in the frequency of the A2058G mutation at several of these sites, as well as in Shanghai, China where up to 95% of samples tested harbor the A2058G mutation.10 To date, there are no published data on the geographical distribution and prevalence of the A2059G mutation, outside of its initial identification in the Czech Republic.9
While there is a strong association between the A2058G and A2059G mutations and azithromycin treatment failures, controversy and ambiguity surrounds the clinical relevance of these mutations.5 A better understanding of the molecular epidemiology of these resistance mutations will shed light on possible mechanisms for emergence of resistant strains and inform guidelines for treatment of syphilis. The goal of this study was to determine the prevalence of the A2058G and A2059G mutations in T. pallidum samples obtained in Seattle, WA during the past decade, and to examine the correlation of these mutations with molecular strain types of T. pallidum.
Materials and Methods
Sample Collection and Molecular Strain Typing
This study examined 134 samples collected from 129 subjects participating in an ongoing study of cerebrospinal fluid (CSF) abnormalities in patients with syphilis 11–13 conducted at Harborview Medical Center in Seattle, WA. This protocol was approved by the Human Subjects Division of the University of Washington. T. pallidum DNA was isolated as previously described14 from 1) T. pallidum strains isolated from patient blood or CSF by rabbit propagation, or 2) directly from patient blood. We have never found differences in strain type or resistance mutations in different sample sources from an individual subject.14 Strain typing was based on analysis of three DNA target regions: 1) the number of 60bp repeats in the acidic repeat protein (arp) gene, 2) RFLP analysis of sequence differences in the T. pallidum repeat (tpr) subfamily II genes (tprE [tp0313], tprG [tp0317], tprJ [tp0621]), and 3) sequence analysis of a short region of the tp0548 gene (bp131–215), andwas performed as described by Marra et al.14 Strain typing has been previously reported14 for a subset (n=84) of the 134 samples included in this report.
Detection of Macrolide Resistance Mutations
T. pallidum DNA was amplified using a nested polymerase chain reaction (PCR) and tested by restriction enzyme digestion using MboII and BsaI enzymes (New England Biolabs, Beverly, MA) for A2058G and A2059G mutations, respectively, as previously described.7,9 One PCR primer was placed in a unique T. pallidum gene flanking one 23S rRNA allele to ensure that only T. pallidum DNA was amplified for testing. Analysis for the A2058G mutation has previously been reported for a subset of samples (n= 23) collected from 2001–2005.7,15
Statistical Methods
Descriptive statistics are expressed as proportions and percentages. Intercooled Stata version 9.2 for Macintosh (Stata Corporation, College Park, TX) was used for all statistical analyses. Proportions were compared using Chi-square or Fisher’s exact test.
Results
Characteristics of study participants
Subjects with syphilis were enrolled in an ongoing study of CSF abnormalities, as described in Materials and Methods. DNA was extracted from strains isolated from CSF or blood, or directly from blood as previously described.14 From 2001 through 2010, a total of 134 samples containing T. pallidum DNA were obtained from 129 patients (five patients returned during the study with another episode of syphilis). Of these 134 samples, 123 were fully strain typed and included in our typing analysis; 127 and 128 were successfully screened for the A2058G and A2059 mutations, respectively, and were included in our analysis of macrolide resistance mutations. Low concentration of T. pallidum DNA in some samples resulted in PCR amplification failure with some primer sets used for analysis.
Characteristics of study participants are summarized in Table 1. Of 129 patients, 90% had early syphilis (primary 3.1%, secondary 65.9%, early latent 21.7%); stages were defined according the Centers for Disease Control Guidelines.16 Gender, HIV status, MSM status, azithromycin use, and race/ethnicity were self-reported. Most subjects (87%) were Caucasian, 98% were male, 97% self-identified as MSM, and 87% were HIV-infected. This cohort is largely representative of the population with syphilis in Seattle-King County during this period: from 2001–2010, 97.6% of early syphilis was seen in males.17 In 2010, 263 (95%) of 276 Seattle-King County male patients (with known sexual orientation) with early syphilis were MSM. There are two characteristics of our subjects that differ from early syphilis patients in Seattle-King County: 1) A higher proportion of subjects in our study were known to be HIV-infected (86.8%), compared to 56.5% for all Seattle-King County patients with early syphilis (159 HIV+, 82 HIV-, 35 unknown); 2) A higher proportion of our subjects had secondary syphilis (65.9% vs. 49.6% for Seattle-King County). These differences likely reflect the fact that providers were more likely to refer HIV+ patients with high RPR titers for our ongoing study. In the year before entry, 36 patients from 134 syphilis episodes (26.9%) reported taking azithromycin. Azithromycin use was not more common among HIV-infected patients compared to those without HIV (31 of 117 (26.5%) vs. 5 of 17 (29.4%), P = 0.78).
Table 1.
Characteristics of study participants.
| Characteristic | Number (n = 129) |
% |
|---|---|---|
| Gender | ||
| Male | 127 | 98.4 |
| HIV Status | ||
| HIV+ | 112 | 86.8 |
| Azithromycin use in previous 12 months | 35 | 27.1 |
| Men who have sex with men (MSM) | 123 | 96.9* |
| Syphilis Stage | ||
| Primary | 4 | 3.1 |
| Secondary | 85 | 65.9 |
| Early Latent | 28 | 21.7 |
| Late Latent | 12 | 9.3 |
| Race/Ethnicity | ||
| Caucasian (including Hispanic) | 112 | 86.8 |
| African-American | 12 | 9.3 |
| Asian/Pacific Islander | 2 | 1.6 |
| American Indian/Alaska native | 3 | 2.3 |
Denominator is 127 male subjects
Composition and temporal distribution of molecular strain types in Seattle
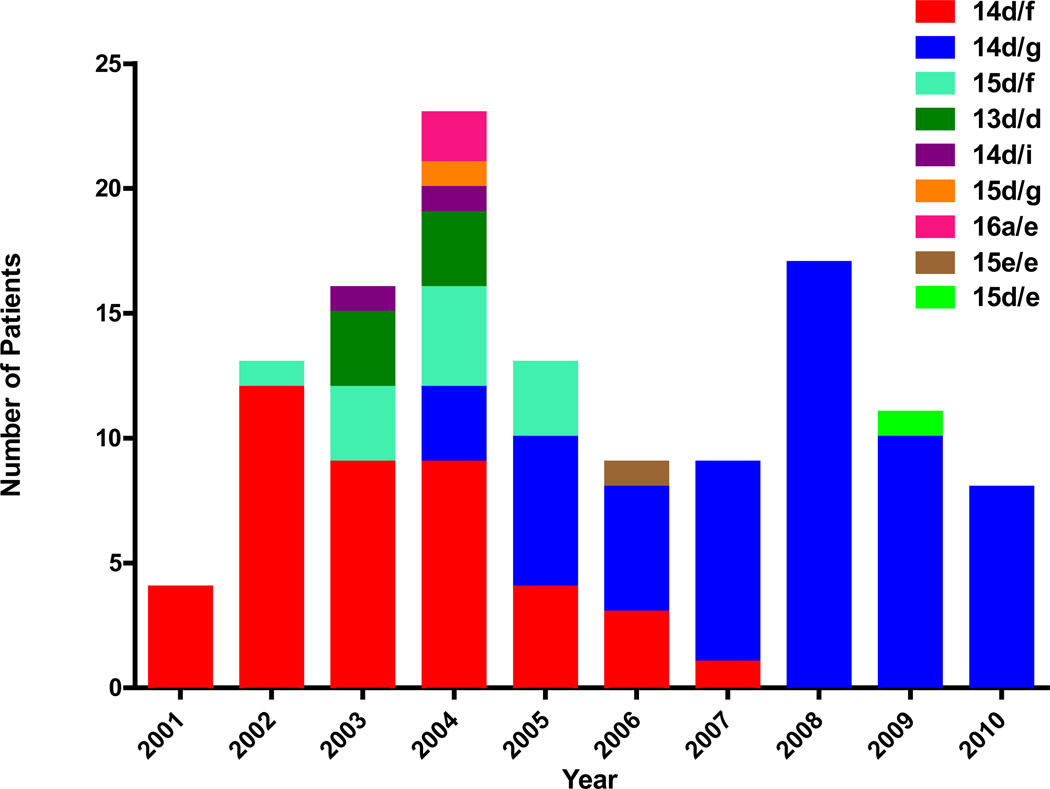
Over the past decade in Seattle, nine molecular strain types were identified among the 123 samples that were typed: 13d/d, 14d/f, 14d/g, 14d/i, 15d/e, 15d/f, 15d/g, 15e/e, and 16a/e. The distribution of these types is shown in Figure 1. Of these nine strain types, the two most frequently occurring types were 14d/f and 14d/g, each of which was present over limited and briefly overlapping time intervals. Type 14d/f predominated from 2001–2004, then remained as a minor strain through 2007. Type 14d/g first appeared in 2004, and predominated from 2005–2010. All other strain types occurred less frequently, and were detected over shorter time periods. The sporadic appearance of types 16a/e and 15e/e in African American subjects (one heterosexual couple and one MSM, respectively) within this largely white MSM subject population, and a trend toward a difference in the distribution of strain types by HIV status (P = 0.05), suggest distinct sexual networks.
Figure 1.
Distribution of Seattle T. pallidum molecular strain types from 2001–2010. The initial predominant strain type 14d/f was replaced by type 14d/g in the middle of the decade. Other strain types were seen transiently during the years.
Increasing prevalence of macrolide resistance mutations among Seattle strains
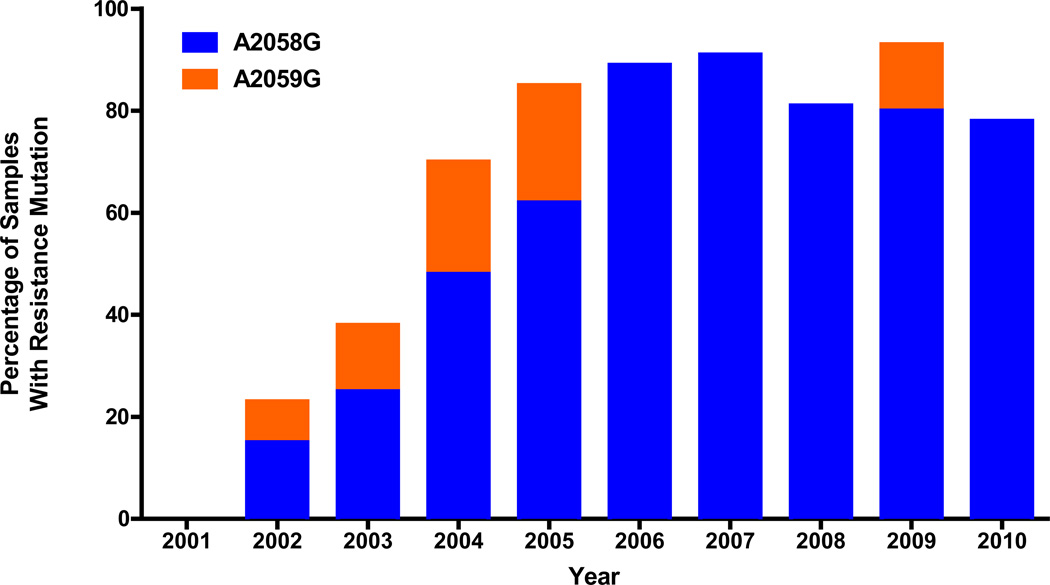
The overall prevalence of samples harboring either the A2058G or A2059G mutation was 92 (72%) of the 128 samples that were successfully screened. Overall, the A2058G mutation was detected in 79 (62%) of the 128 samples, compared to the A2059G mutation, seen in 13 (10%) samples. The proportion of strains that contained either the A2058G or A2059G mutation, by year of collection, is shown in Figure 2. The frequency of the resistance mutations increased from 23.1% (3 of 13) in 2002 to over 90% in both 2007 (10 of 11) and 2009 (14 of 15). This report identifies, for the first time in the United States, the existence of strains harboring the A2059G mutation. Strains with this mutation were seen sporadically in Seattle from 2002–05 and again in 2009.
Figure 2.
The prevalence of Seattle T. pallidum containing 23S rDNA mutations associated with macrolide resistance during the past decade. The frequency of finding macrolide resistance mutations increased from 0% to >80% by 2005, and has remained at high levels since that time. The number of samples analyzed per year averaged 13, and ranged from 4 (2001) to 23 (2004).
Association of macrolide resistance mutations with strain types
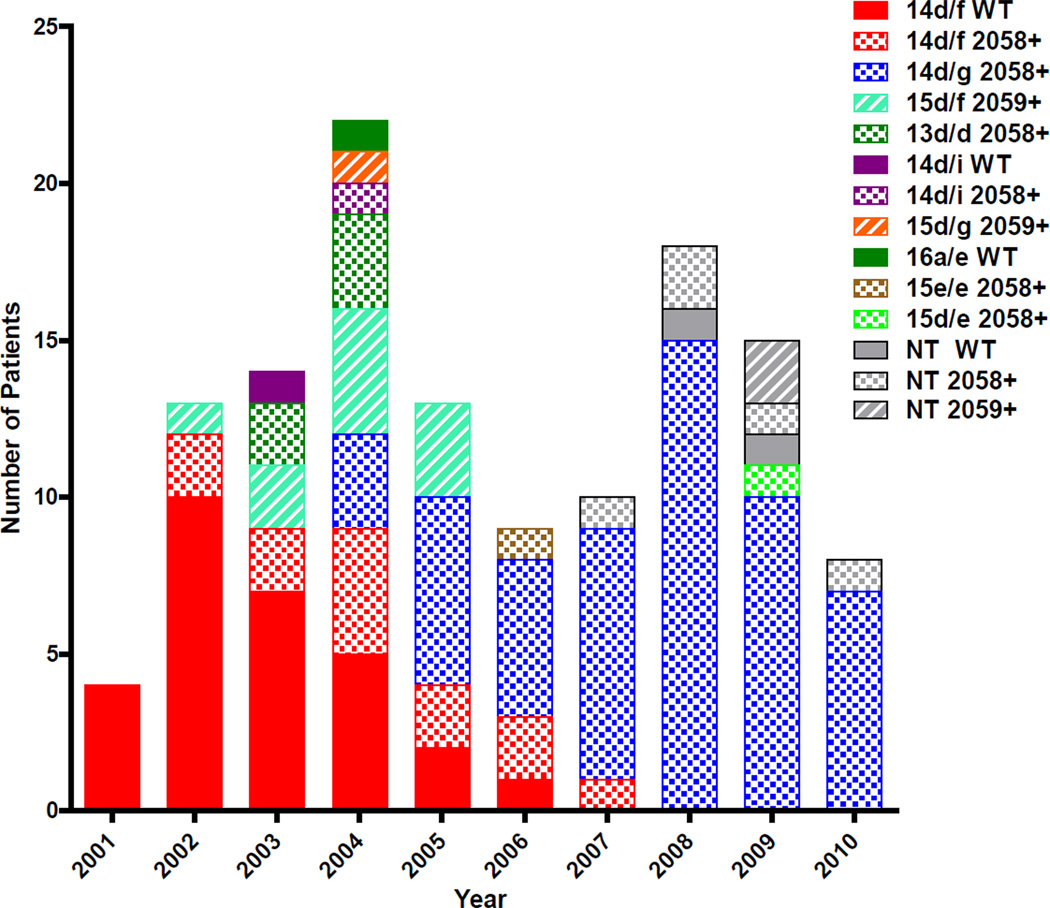
The A2058G and A2059G mutations were present in distinct and unique sets of molecular strain types, as shown in Figure 3. A2058G was detected in six strain types including the two predominant types: 14d/f and 14d/g, as well as 13d/d 14d/i, 15d/e, and 15e/e. The 14d/f strains were initially all wild type (WT) in 2001, but the proportion of WT 14d/f strains diminished until 2005 when the A2058G mutant became predominant in this type. Type 14d/f was one of two strain types that moved from all WT to all A2058G during the study; the other such strain was 14d/i, which shifted from all WT in 2003 to all A2058G in 2004. In contrast, other strain types were 100% mutant (either A2058G or A2059G) from the time they first appeared in our patients; these include 14d/g, 13d/d, 15d/e, 15d/f, 15d/g, and 15e/e. Once any strain type became 100% mutant, a WT variant of the same strain type never subsequently appeared. The A2059G mutation was detected in only two strain types: 15d/f and 15d/g, and all such samples contained the mutation (Figure 3). No strain type harbored both the A2058G and A2059G mutations; the segregation by strain type was absolute. Only one strain type, 16a/e mentioned above, was never associated with either mutation.
Figure 3.
Association of T. pallidum molecular strain type with 23S rDNA mutations in Seattle samples. Strain types are designated by color and 23S rDNA mutations are designated by pattern (WT= solid; A2058G= checked; A2059G= striped). Nine samples (2007–09) were not able to be fully typed (grey) from blood samples although macrolide resistance mutations could be determined.
We examined the association between the presence of a resistance mutation and azithromycin use in the previous year. The prevalence of resistance mutations was higher among patients who had taken azithromycin in the previous 12 months compared to those who had not. Specifically, either the A2058G or A2059G mutation was detected in 32 of 36 subjects (88.9%) who reported taking azithromycin in the previous 12 months, compared to 60 of 98 (61.2%) who did not. The relative risk of having either mutation was 1.45 (95% CI 1.19–1.77; P = 0.002) for subjects who reported taking azithromycin in the previous year, compared to those who did not. The reason for macrolide use in our subjects was not recorded, but azithromycin has never been recommended for syphilis treatment in Seattle-King County, so its use was likely to be for other indications.
Discussion
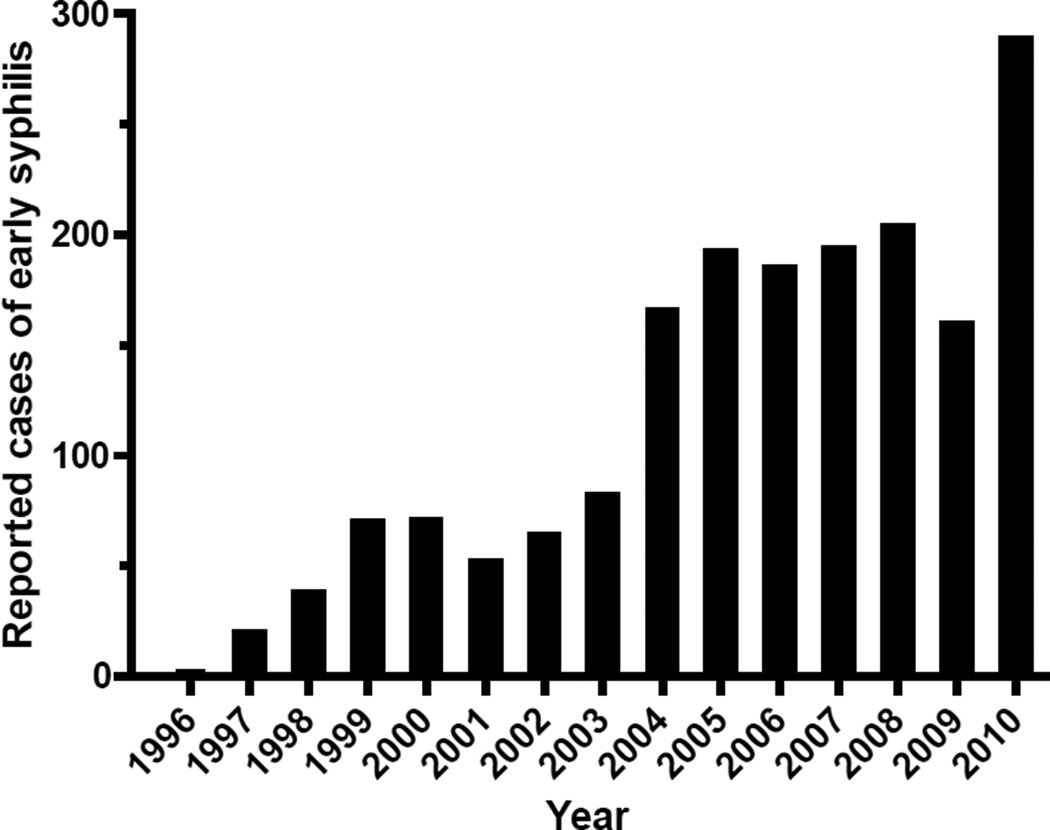
In Seattle/King County, increasing numbers of early syphilis cases have been reported since 1996, with a 145-fold increase from 2 cases in 1996 to 289 cases in 2010 (Figure 4). In 2010, 92% of all men with early syphilis (263 of 285) were self-identified MSM, and only 4 cases of early syphilis were seen in women. In MSM with early syphilis, 59% (155 of 263) were known to be HIV-infected.17 The introduction of a highly discriminating strain typing system for syphilis permits us to more closely examine the molecular epidemiology of syphilis outbreaks, and to identify associations between resistance mutations and individual strain types. Examination of total numbers of cases, as in Figure 4, simply shows a rapidly enlarging outbreak. However, examination of the molecular strain types comprising this outbreak from 2001–2010 reveals the disappearance of one predominant strain type, with replacement by another. Sporadic appearances of other strain types may reveal heterosexual networks, or perhaps MSM cases whose infections were acquired elsewhere. A broader sampling of syphilis cases would be needed to investigate the full picture of syphilis-related sexual networks in Seattle; cases included in this study represent only those examined in our ongoing study. Segregation by strain type has also allowed us to look beyond the general increase in prevalence of resistance mutations to identify associations between resistance mutations and strain types.
Figure 4.
Reported cases of early syphilis in Seattle/King County, Washington. Source: Seattle King County Public Health17
The rapid increase in the proportion of T. pallidum strains harboring the A2058G mutation during the past decade, the identification of the A2059G mutation in Seattle, and the current near universality of resistance mutations in strains currently circulating in Seattle clearly demonstrate that the use of azithromycin or other macrolides for treatment of syphilis is inappropriate in such settings. This situation is likely not unique to Seattle, as prevalence of the A2058G mutation has been documented as >50% in smaller studies in Dublin (88%), Shanghai (100%), San Francisco (77%), and London (67%) in recent years.6,7,10,18,19 Strains containing these mutations, at lower prevalence, have also been reported in Canada20 and the Czech Republic.9 Importantly, these rates likely underestimate the overall prevalence of macrolide resistance in circulating T. pallidum strains, as most of these studies tested for only the A2058G mutation.
For most parts of the world, there are no data regarding the prevalence or geographic distribution of macrolide resistant strains of T. pallidum. Three large randomized trials conducted in Uganda, Tanzania, and Madagascar have demonstrated equivalent or superior efficacy of azithromycin vs. BPG for treatment of early syphilis.3–5 These studies have led to the suggestion that macrolide resistance is either absent or rare in Africa, and that azithromycin may be an acceptable alternative, or even primary, treatment for syphilis. There are several issues to consider. First, the three large African clinical trials drew most of their participants from heterosexual populations, whereas most of the published data on macrolide resistance have come from MSM populations. The apparent preponderance of macrolide-resistant strains in MSM in the published literature likely reflects an ascertainment bias, as most of these studies were conducted in regions where syphilis is currently primarily seen in MSM. In Shanghai, however, where syphilis is seen in both MSM and heterosexual populations, there have been many documented azithromycin clinical failures21 and the overall prevalence of macrolide resistance mutations rivals that observed in predominately MSM U.S. populations. 10 Thus, macrolide resistant strains are not seen only in MSM.
Second, the trials in Uganda, Tanzania, and Madagascar took place from 1994–1998, 2000–2003, and 2000–2007 respectively. During most of this time period, the prevalence of macrolide resistant strains was also low in other cities that now have very high prevalence, generating concern that subsequent rapid emergence of macrolide resistant strain types may occur, or may have already occurred, in Africa. Molecular analysis of samples from a subset of subjects in the Madagascar study revealed no evidence of the A2058G mutation, consistent with the observed clinical efficacy of azithromycin in that setting.22 Subsequent unpublished screening of these same samples for the A2059G mutation revealed one sample containing this mutation. Importantly, this sample was obtained from one of the four azithromycin treatment failures in that study (personal communication, Edward W. Hook and Frieda Behets); samples from the three remaining azithromycin treatment failures were not available for molecular testing. This finding demonstrates that macrolide resistant strains of T. pallidum do exist in African nations, albeit at low levels in this limited sample, and there have been no published follow-up studies to examine prevalence of resistant strains in Africa in recent years. Widespread surveillance for such mutations is needed to determine their geographical distribution and prevalence throughout Africa so that we know whether the window of opportunity for convenient oral treatment of syphilis with azithromycin is closing, or has already closed, in Africa. Clearly it is important to consider the consequences of widespread use of azithromycin as a primary therapy in populations where mutation rates are high. Additionally, given the association we observed between recent azithromycin use and T. pallidum strains containing 23S rRNA mutations, widespread use of azithromycin has the potential to drive emergence of resistance mutations in currently susceptible strains.
This study made use of a recently refined strain typing system and molecular assay in order to detect the A2058G and A2059G mutations and determine their segregation by molecular strain type. Our results demonstrate that the two mutations occur in multiple, but separate, strain types. This raises the question of how these resistant strains originated. One possible mechanism is that strains with pre-existing mutations were introduced into Seattle sexual networks where they might have spread even in the absence of macrolide pressure. Alternatively, the mutations may have emerged de novo within T. pallidum strains in response to selective pressures generated by widespread macrolide use. The pharmacokinetics of persisting low levels of azithromycin in tissue for weeks following treatment is conducive to selection for individual bacteria with mutations. In previous studies 6,15 and in this report, the risk of being infected with a strain containing a mutation was increased in persons with exposure to macrolides in the previous 12 months. This suggests that selective pressure is involved and that these mutations may develop de novo. The declining relative risk, from 2.2 for the period 2001–200515 to 1.45 for 2001–2010 (in this study) likely reflects recent high rates of transmission of already-resistant strains even without macrolide selection, rather than continued de novo mutation in individual bacteria. Lastly, a mechanism for the origin of multiple T. pallidum strain types containing the A2058G and A2059G mutations is that the mutation may be transmitted between strain types via horizontal transfer of DNA. As transformation has never been described for T. pallidum, this mechanism is unlikely. Overall, the observation that the predominant strain type 14d/f and another strain type 14d/i were initially seen as wild type, then quickly shifted to resistant over the course of a few years suggests that mutation occurred in T. pallidum within the Seattle MSM community. In contrast, the A2059G-containing strain type 15d/f and several other minor types contained 23S rDNA mutations when they were initially detected in Seattle; these may have been introduced to the community with pre-existing mutation.
The increasingly high prevalence of the A2058G and A2059G mutations cautions against the use of azithromycin and other macrolides for treatment of syphilis in Seattle and in other areas where the prevalence of these mutations is high. The ease of administration and high efficacy in sensitive strains provide significant temptations to use azithromycin as a primary therapy in areas where the burden of syphilis is high, the use of injectable drugs is problematic, and the prevalence of resistance mutations is thought to be low. This approach has been suggested to treat female sex workers in Indonesia23 and to control a resurgence of yaws (caused by the closely related Treponema pallidum subspecies pertenue) in Vanuatu.24 However, such policies should be implemented only after baseline surveillance for resistance mutations is conducted. It should be recognized that introduction of azithromycin as a primary treatment may carry significant risk for the rapid development of, and selection for, macrolide resistant strains of T. pallidum. Clearly, if azithromycin is used as a primary treatment, the importance of ongoing surveillance for known and new macrolide resistance mutations within the treated population will be paramount.
SUMMARY.
A study of persons with syphilis in Seattle, Washington, from 2001–2010 identifies the presence of two mutations associated with macrolide resistance, each associated with different molecular strain types of Treponema pallidum. The prevalence of strains with these mutations has been >80% since 2005.
Acknowledgments
Sources of Support: This study was supported by NIH grants AI063940, AI42143, and AI094122 to SAL and by NS034235 to CMM.
Footnotes
Conflict of Interest: SAL, BCG, LCT, and CM are conducting research sponsored by Cempra Pharmaceuticals. For the remaining authors, no conflict was declared.
REFERENCES
- 1.Centers for Disease Control. STD Surveillance Report. Morbidity & Mortality Weekly Report. 2011 [Google Scholar]
- 2.Tucker JD, Yin YP, Wang B, Chen XS, Cohen MS. An expanding syphilis epidemic in China: epidemiology, behavioural risk and control strategies with a focus on low-tier female sex workers and men who have sex with men. Sex Transm Infect. 2011;87(Suppl 2):ii16–ii18. doi: 10.1136/sti.2010.048314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiddugavu MG, Kiwanuka N, Wawer MJ, et al. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda. Sex Transm Dis. 2005;32:1–6. doi: 10.1097/01.olq.0000148297.48590.d8. [DOI] [PubMed] [Google Scholar]
- 4.Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 5.Hook EWI, Behets F, Van Damme K, et al. A Phase III Equivalence Trial of Azithromycin versus Benzathine Penicillin for Treatment of Early Syphilis. J. Infect. Dis. 2010;201:1729–1735. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SJ, Engelman J, Kent CK, Lukehart SA, Godornes C, Klausner JD. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin Infect Dis. 2006;42:337–345. doi: 10.1086/498899. [DOI] [PubMed] [Google Scholar]
- 7.Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 8.Stamm LV, Bergen HL. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob Agents Chemother. 2000;44:806–807. doi: 10.1128/aac.44.3.806-807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matejkova P, Flasarova M, Zakoucka H, et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp pallidum. J Med Microbiol. 2009;58:832–836. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 10.Martin IE, Gu W, Yang Y, Tsang RS. Macrolide reistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin Infect Dis. 2009;49:515–521. doi: 10.1086/600878. [DOI] [PubMed] [Google Scholar]
- 11.Marra CM, Maxwell CL, Tantalo L, et al. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis. 2004;38:1001–1006. doi: 10.1086/382532. [DOI] [PubMed] [Google Scholar]
- 12.Marra CM, Maxwell CL, Tantalo LC, Sahi SK, Lukehart SA. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clin Infect Dis. 2008;47:893–899. doi: 10.1086/591534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marra CM, Maxwell CL, Smith SL, et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004;189:369–376. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 14.Marra CM, Sahi SK, Tantalo L, et al. Enhanced molecular typing of Treponema pallidum: Geographical distribution of strain types and association with neurosyphilis. J. Infect. Dis. 2010;202:1380–1388. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marra CM, Colina AP, Godornes C, et al. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J Infect Dis. 2006;194:1771–1773. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control. Sexually Transmitted Diseases Treatment Guidelines, 2010. Morbidity & Mortality Weekly Report. 2010;59:26–38. [PubMed] [Google Scholar]
- 17.Public Health Seattle King County. [Accessed June 26, 2012];2010 King County Sexually Transmitted Diseases Epidemiology Report. 2012 http://www.kingcounty.gov/healthservices/health/communicable/std/statistics.aspx 2010.
- 18.Katz KA, Klausner JD. Azithromycin resistance in Treponema pallidum. Curr Opin Infect Dis. 2008;21:83–91. doi: 10.1097/QCO.0b013e3282f44772. [DOI] [PubMed] [Google Scholar]
- 19.Tipple C, McClure MO, Taylor GP. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex Transm Infect. 2011;87:486–488. doi: 10.1136/sextrans-2011-050082. [DOI] [PubMed] [Google Scholar]
- 20.Martin IE, Tsang RS, Sutherland K, et al. Molecular characterization of syphilis in patients in Canada: azithromycin resistance and detection of Treponema pallidum DNA in whole-blood samples versus ulcerative swabs. J Clin Microbiol. 2009;47:1668–1673. doi: 10.1128/JCM.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P, Li K, Lu H, et al. Azithromycin treatment failure among primary and secondary syphilis patients in Shanghai. Sex Transm Dis. 2010;37:726–729. doi: 10.1097/OLQ.0b013e3181e2c753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Damme K, Behets F, Ravelomanana N, et al. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex Transm Dis. 2009;36:775–776. doi: 10.1097/OLQ.0b013e3181bd11dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majid N, Bollen L, Morineau G, et al. Syphilis among female sex workers in Indonesia: need and opportunity for intervention. Sex Transm Infect. 2010;86:377–383. doi: 10.1136/sti.2009.041269. [DOI] [PubMed] [Google Scholar]
- 24.Fegan D, Glennon MJ, Thami Y, Pakoa G. Resurgence of yaws in Tanna, Vanuatu: time for a new approach? Trop Doct. 2010;40:68–69. doi: 10.1258/td.2009.090249. [DOI] [PubMed] [Google Scholar]