Abstract
Epigenetic mechanisms, such as histone modifications, play an active role in the differentiation and lineage commitment of mesenchymal stem cells. In the present study, epigenetic states and differentiation profiles of two odontogenic neural crest-derived intermediate progenitor populations were compared: dental pulp (DP) and dental follicle (DF). ChIP on chip assays revealed substantial H3K27me3-mediated repression of odontoblast lineage genes DSPP and dentin matrix protein 1 (DMP1) in DF cells, but not in DP cells. Mineralization inductive conditions caused steep increases of mineralization and patterning gene expression levels in DP cells when compared to DF cells. In contrast, mineralization induction resulted in a highly dynamic histone modification response in DF cells, while there was only a subdued effect in DP cells. Both DF and DP progenitors featured H3K4me3-active marks on the promoters of early mineralization genes RUNX2, MSX2, and DLX5, while OSX, IBSP, and BGLAP promoters were enriched for H3K9me3 or H3K27me3. Compared to DF cells, DP cells expressed higher levels of three pluripotency-associated genes, OCT4, NANOG, and SOX2. Finally, gene ontology comparison of bivalent marks unique for DP and DF cells highlighted cell–cell attachment genes in DP cells and neurogenesis genes in DF cells. In conclusion, the present study indicates that the DF intermediate odontogenic neural crest lineage is distinguished from its DP counterpart by epigenetic repression of DSPP and DMP1 genes and through dynamic histone enrichment responses to mineralization induction. Findings presented here highlight the crucial role of epigenetic regulatory mechanisms in the terminal differentiation of odontogenic neural crest lineages.
Introduction
Teeth are complex organs that develop from at least three different progenitor populations, the ectodermal enamel organ and two neural crest-derived ectomesenchymal populations, the dental papilla and the dental follicle (DF). The ectomesenchymal populations contain intermediate progenitor populations derived from the odontogenic neural crest and share similar phenotypical characteristics and gene expression patterns [1,2]. For example, DF cells and dental papilla cells both have extensive proliferation ability, express similar surface antigens, and are capable of forming hard tissue in vivo and in vitro [3]. Upon further development, dental papilla progenitors differentiate into dental pulp (DP) and odontoblasts, which in turn secrete tooth dentin, while DF progenitors migrate extensively and eventually form periodontal ligament (PDL), alveolar bone (AB), and root cementum (CEM) [4–8]. The terminal differentiation of these intermediate progenitors into DP, odontoblasts, tooth dentin, and cells and tissues of the periodontal apparatus is controlled by both genetic and epigenetic factors [9].
A number of genetic factors have been associated with specific events in pulp and periodontal differentiation. The terminal differentiation of preodontoblasts into secretory odontoblasts is stimulated by growth factors, for example, GDF11, FGF1, TGFβ-1, and TGFβ-3 [10,11] and matrix molecules such as the dentin matrix protein 1 (DMP1) [12]. Both DMP1 and DSPP are matrix molecules detected in odontoblasts and osteoblasts, but only DSPP is considered somewhat of a marker for odontoblasts [13–15]. Within the periodontal progeny, the differentiation of DF cells into committed periodontal progenitors PDL, AB, and CEM is accomplished by the growth factors GDF 5–7, while BMP2, DLX3, NR4A3, KLF9, and TSC22D3, which promote osteogenic differentiation of DF precursor cells, presumably into AB osteoblasts and cementoblasts [9,16–19].
While many genetic factors involved in odontogenic differentiation have been well characterized, little is known about the epigenetic factors that affect odontogenic lineage differentiation. In general, epigenetic mechanisms involved in development and differentiation include methylation of cytosines on DNA, covalent modifications of histone tails, and noncoding RNA-mediated gene regulation [20–23]. Gene expression changes during development are accompanied or caused by epigenetic mechanisms [24–28], and terminal differentiation of cells is controlled by dynamic activation and repression of developmental genes by epigenetic mechanisms [29,30]. In mesenchymal stem cells (MSCs), post-translational histone modifications play an active role in the determination of differentiation capacity [31]. For example, the repressive combination of trimethylated H3K4 and H3K27 on adipocyte lineage-specification promoters is lost during adipogenic differentiation [32].
The trimethylated forms of H3K4, H3K9, and H3K27 are the most commonly studied histone modifications and significantly contribute to the stemness of ES and MSCs [33,34] apart from being predictive of gene expression states [35–37]. In periodontal progenitors, DF intermediate progenitors have higher global levels of the active H3K4me3 mark, while the lineage committed PDL, AB, and CEM progenitor cells are enriched for the heterochromatic H3K9me3 mark [9]. Moreover, osteogenic differentiation of DF cells leads to a switch from the H3K4me3 mark to the H3K9me3 mark [9], further highlighting the role of epigenetic marks in the differentiation of DF progenitors into terminal periodontal lineages.
In the present study, we have characterized histone methylation profiles and dynamics in odontogenic progenitors. To compare histone dynamics during differentiation, DP and DF cells were subjected to mineralization conditions as a differentiation-inducing environment. Gene ontology (GO) analyses of bivalent marks were performed to identify unique subsets of poised genes in DF and DP progenitors. Together, this analysis provides the first comprehensive epigenetic signature characterization of odontogenic intermediate progenitors and of regulatory mechanisms that take place during their differentiation.
Materials and Methods
Isolation of human dental mesenchymal progenitors
Healthy human teeth (patients ranging from 12 to 15 years) were extracted for orthodontic reasons in accordance with the human subjects protocol approved by UIC's Institutional Review Board and the Office for the Protection Research Subjects. DF, DP, AB, and PDL were dissected from developing tooth organs, and mesenchymal progenitors were isolated from dental tissues after digestion with collagenase/dispase as described previously [9]. All experiments were performed with cells in early passages (P3–P6).
Osteogenic differentiation of DF and DP cells
DF and DP progenitors were seeded at a density of 26,000 cells/cm2 and cultured for 24 h before treatment. Osteogenic differentiation was induced with the osteogenic induction medium (complete media supplemented with 50 μg/mL ascorbic acid 2-phosphate, 10 mM β-glycerophosphate, and 10 nM dexamethasone). The induction medium was changed on alternate days. Cells before induction were used as the reference group (day 0), while control and osteoinduced cells were processed after 7 or 14 days of induction.
Alkaline phosphatase assay, alizarin red staining, and light microscopy
To determine the alkaline phosphatase (ALP) activity, control and treated cells were fixed with cold methanol, and then stained with the NBT/BCIP solution for 12 min. Mineral deposits in these cultures were detected after fixing with cold methanol and staining with a 1% alizarin red S (ARS) solution [9]. Phase-contrast images of control and treated progenitors were obtained using a Leica inverted microscope (W. Nuhsbaum, Inc.).
Chip on chip analysis
Sample preparation
DF and DP progenitors grown on 150-mm plates were crosslinked with 1.1% formaldehyde for 10 min at room temperature. After stopping the reaction with 125 mM glycine, cells were harvested, and pooled cells were then aliquoted, flash frozen, and stored at −80°C. ChIP assays were performed as previously described [38] with minor modifications. Briefly, nuclei were prepared from 1×108 cells and resuspended in the lysis buffer. After incubation on ice, nuclear lysates were sonicated to a size of 300 bp–1 kb in a cup horn sonicator (Q Sonica). The sheared chromatin was centrifuged at 12,000 rpm for 10 min and incubated overnight with 100 μL of DynaI beads (Invitrogen) prebound with 10 μg of antibody against each histone modification: histone H3, histone H3 tri methyl K4, histone H3 tri methyl K9, and histone H3 tri methyl K27 (Abcam). An input fraction corresponding to 5% of the starting chromatin was kept aside for background normalization. Subsequently, beads were separated on a magnetic stand and washed five times with the RIPA buffer and once with 1XTE. Bound protein-DNA complexes were eluted from the beads by incubating with the elution buffer at 65°C, followed by crosslink-reversal overnight at 65°C. DNA was purified by Proteinase K digestion, phenol-chloroform extraction, and ethanol precipitation. The resulting pellet was resuspended in 50 μL of 10 mM Tris pH 8.0. Whole-genome amplification and array hybridization procedure are detailed in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/scd). ChIP experiments were performed as triplicates.
Raw data processing and gene annotation
Datasets corresponding to histone enrichment values in both immunoprecipitated (IP) and input samples were processed to obtain Ratio.GFF files (log2 IP/input). All samples passed QC metrics provided by Roche NimbleGen (Roche). The Ratio.GFF files from triplicates for each experiment were merged to obtain average values for histone modification enrichment. These were then converted to wiggle files for visualization in an Integrative Genomics Viewer (IGV) genome browser. Initial peak calling was performed using NimbleScan software (Roche), and initial calls were further filtered to obtain significant peaks [log2 ratio>1 and false discovery rate (FDR) <5%]. The filtered peaks were subsequently mapped to overlapping features 5,000 bp upstream and 1,000 bp downstream of the nearest transcription start site (TSS), and a peak report was generated. Promoter information and coordinates were used for downstream analysis.
Average histone profiles versus distance to TSS
The average log2 ratio was computed for each histone modification for every 100 bp window from 3,000 bp upstream to 1,000 bp downstream for all the TSSs. The enrichment values were plotted on the Y axis in relation to the distance to the TSS on the X axis.
Generation of unique gene lists, clustering and functional annotation
To remove redundancy, promoter lists were generated for each histone modification containing unique lists of NM accession numbers and gene symbols in each cell line. Total promoter counts were established based on TSS, gene accessions, or gene symbols. Promoter comparisons between the two cell types were conducted using the Data Overlapping and Area-Proportional Venn Diagram Tool (BioInfoRX) to generate unique or overlapping lists for each histone modification. Box-plots were generated with DF histone enrichment values as a reference. DChIP software was used for clustering. For this, the peak height value for each histone modification was obtained for all promoters. Values corresponding to significant peaks (log2 ratio>1 and FDR<5%, value is 0 if there is no significant peak) were used for unsupervised hierarchical clustering to group promoters and samples. A total of 4,479 promoters were selected from 30,173 promoters [variation across samples: 0.50<standard deviation/mean<1,000.00; peak height (log2 ratio) ≥1.00 in ≥30% samples].
GO analysis for promoters enriched with each histone modification was performed using DAVID (http://david.abcc.ncifcrf.gov/). Functional annotation clustering for gene lists was performed with high classification stringency and an EASE score of 0.06.
Identification of promoters having enrichment for both H3K4me3 and H3K27me3. For each cell line, H3K4me3 and H3K27me3 peaks were examined for overlaps, in such a way that they have at least one nucleotide base in common. These overlap sites were identified as potential bivalent sites and were further mapped to individual promoters.
Chromatin immunoprecipitation analysis
ChIP assays were performed as described above with control, 7-day induced and 14-day induced DF and DP progenitor cells as experimental groups. All DNA samples were diluted to a concentration of 2 ng/μL. Real-time quantitative polymerase chain reaction (PCR) was performed on an ABI 7500 FAST machine with 4 ng of ChIP DNA, and the total input was used as an internal reference for normalization. Enrichment with the total histone H3 was used as a positive control, while beads alone served as a negative control. Data presented for each primer pair were obtained after subtracting the values obtained from the corresponding negative controls. The primer sequences are shown in Table 1.
Table 1.
Oligodeoxynucleotides
| Oligos for ChIP | ||
|---|---|---|
| MSX2 | Forward | TGCACACTTGCACTGTCTTG |
| Reverse | GAGTGCCTCTCTCCCAAATG | |
| DLX5 | Forward | CCTTGTCTCCTTCCCCACTT |
| Reverse | GCTTGCAAAAAGAGCCAAAC | |
| RUNX2 | Forward | AGCAGTTTGCAACCAGACCT |
| Reverse | TGGCTGGATTCCTTCTGTTT | |
| OSX | Forward | AAATTGGTGCCTACCTGCTG |
| Reverse | CAATTCCTTGCTCCTTTTGC | |
| COL I | Forward | TTGGGTGTGGCTGTAAGAGA |
| Reverse | GGGATCTTTCATGGTTTCCA | |
| IBSP | Forward | TGAGCTGACCTCACATGCAC |
| Reverse | TCATACCACTGAGAGGCAGATTT | |
| BGLAP | Forward | TTTTCAGGCTGGGATGTTCT |
| Reverse | GGAGCACGAAGATGGAGTGT | |
| DSPP | Forward | GCGGTTTTCCAGAATTCTTTT |
| Reverse | AACGTATTCTGAGCCCCAAA | |
| BMP2 | Forward | GCGTATGGATATGAAAATAAATGC |
| Reverse | CTGTTTCCCCGGAATAGGAG | |
| BMP4 | Forward | TCTCTGGCACACAGTTCTGC |
| Reverse | AGAAAGGAGCTGTTGGATCG | |
| BMP7 | Forward | CAGCAATTCCAGACAGCAAG |
| Reverse | CCAGAGACCTCCAAGACCTG | |
| Oligos for real-time PCR analysis | ||
|---|---|---|
| MSX2 | Forward | CGGAAAATTCAGAAGATGGAG |
| Reverse | GAGGAGCTGGGATGTGGTAA | |
| DLX5 | Forward | TTTGCCATTCACCATTCTCA |
| Reverse | CGCTAGCTCCTACCACCAGT | |
| RUNX2 | Forward | GTGCCTAGGCGCATTTCA |
| Reverse | GCTCTTCTTACTGAGAGTGGAAGG | |
| OSX | Forward | TACCCCATCTCCCTTGACTG |
| Reverse | GCAACAGGGGATTAACCTGA | |
| COL I | Forward | GGAGCTCCAAGGACAAGAAA |
| Reverse | ATGAAGGCAAGTTGGGTAGC | |
| IBSP | Forward | AACCTACAACCCCACCACAA |
| Reverse | CGTACTCCCCCTCGTATTCA | |
| BGLAP | Forward | GACTGTGACGAGTTGGCTGA |
| Reverse | AGCAGAGCGACACCCTAGAC | |
| DSPP | Forward | CCAGTTCCTCAAAGCAAACC |
| Reverse | GGCATTTAACTCATCCTGTACTGA | |
| BMP2 | Forward | TCAAGCCAAACACAAACAGC |
| Reverse | AGCCACAATCCAGTCATTCC | |
| BMP4 | Forward | CTTTACCGGCTTCAGTCTGG |
| Reverse | ATGTTCTTCGTGGTGGAAGC | |
| BMP7 | Forward | CAGAGCATCAACCCCAAGTT |
| Reverse | GTTCTGGCTGCGCTGTTT | |
| ALP | Forward | CAACCCTGGGGAGGAGAC |
| Reverse | GCATTGGTGTTGTACGTCTTG | |
| NANOG | Forward | GCAGAGAAGAGTGTCGCAAA |
| Reverse | ATCTGCTGGAGGCTGAGGTA | |
| OCT4 | Forward | GACAGGGGGAGGGGAGGAGCTAGG |
| Reverse | CTTCCCTCCAACCAGTTGCCCCAAAC | |
| SOX2 | Forward | GGGAAATGGGAGGGGTGCAAAAGAG |
| Reverse | TTGCGTGAGTGTGGATGGGATTGGTG | |
| GAPDH | Forward | ACAGTCAGCCGCATCTTCTT |
| Reverse | ACGACCAAATCCGTTGACTC | |
PCR, polymerase chain reaction; ALP, alkaline phosphatase.
RNA extraction and real time-PCR
Total RNAs were isolated from DF and DP cells using the TRIzol LS Reagent (Life Technologies) according to the manufacturer's instructions. Two micrograms of total extracted RNA was applied toward cDNA generation with the Sprint RT Complete kit® (Clontech). To quantify the mRNA expression levels of transcription factors and bone marker genes, real-time PCR primers were designed based on EMBL/GenBank searches (shown in Table 1). Real-time PCR was performed with sequence-specific primers, using SYBR green Master Mix and the ABI Prism 7000 Sequence detection system (Applied Biosystems). Reaction conditions were as follows: 2 min at 50°C (1 cycle), 10 min at 95°C (1 cycle), 15 s at 95°C, and 1 min at 60°C (40 cycles). Samples were normalized to levels of GAPDH. The analyses were performed in triplicate for three independent experiments to confirm reproducibility of the results. Relative expression levels were calculated using the 2−ΔΔCt method [39], and values were graphed as the mean expression level±standard deviation (SD). In some cases, PCR products were electrophoresed on 2% agarose gels, stained with ethidium bromide, and visualized under UV light.
Statistical analysis
Quantitative data are presented as means±SD from three independent experiments and compared with one-way ANOVA statistical analysis tests. The difference between groups was considered statistically significant at P<0.05.
Results
Compared to DF cells, DP cells expressed higher levels of pluripotency-associated genes and exhibited a faster rate of mineralization
DF and DP cells are cranial neural crest-derived multipotent MSCs [40] that differentiate into mineralized tissue secreting AB osteoblasts, cementoblasts, and odontoblasts during the final period of normal tooth development [1,41–43]. Here we have characterized DF and DP cells after one and 2 weeks of culture and in response to osteogenic induction. At the onset of culture, both DF and DP cells were typical mesenchymal cells with spindle-like and polygonal morphology (Fig. 1A). After 7 and 14 days of culture, DF cells became long and elongated, while DP cells displayed a tightly packed, cobble-stone morphology (Fig. 1A). In response to osteogenic induction, both cell types proliferated and exhibited a highly condensed morphology when compared to control cells.
FIG. 1.
Morphological and phenotypic comparison of neural crest-derived DF and DP odontogenic progenitors. DF and DP progenitors were compared with each other and subjected to differentiation induction in the mineralization medium for 7 and 14 days. (A) Morphological comparison of cytological changes accompanying osteogenic induction as revealed by phase-contrast microscopy. Note the fibroblast-like morphology and extensive proliferation of both cell types when subjected to the mineralization medium. After 14 days, DP progenitors displayed numerous dead cells floating in the culture dish. (B) Comparative analysis of the mineralization potential in DF and DP progenitors cultured in the mineralization medium for 0–14 days as revealed by ALP activity assay and calcium deposit detection via alizarin red staining. (C) Relative mRNA expression of the early stage osteoblast differentiation marker ALP in DF and DP progenitors by RT-qPCR. Note the increase in ALP transcripts levels at the 7-day time point followed by a reduction at day 14 in DF and DP cells. (D) Relative mRNA expression of key pluripotency-associated genes NANOG, OCT4, SOX2 in DF and DP progenitors by RT-qPCR. All three pluripotency markers were expressed at higher levels in DP cells of same-stage tooth organs. Statistical significance was determined by the Student's t test (*, P<0.05). DF, dental follicle; DP, dental pulp; ALP, alkaline phosphatase; ARS, alizarin red s; con, control cells; 7d and 14d, 7 and 14 days after induction with the mineralization medium; qPCR, quantitative polymerase chain reaction; RT, real time. Color images available online at www.liebertpub.com/scd
As a next step, osteoinductive conditions were used as a means to induce DF and DP progenitor differentiation [3,44] (Fig. 1B). Our in vitro studies indicated that both DP and DF displayed markers of mineralized tissue differentiation after one or 2 weeks of culture, including the differentiating osteoblast marker ALP [45] and also were positive for the presence of calcium deposits when stained with ARS [46,47]. ALP and ARS staining was higher in DP cells after 1 week of culture and in DF cells after 2 weeks of culture (Fig. 1B). Osteoinduction substantially increased ALP and ARS levels of both DF and DP cells that had been cultured for a week or more (Fig. 1B). Moreover, ALP transcript levels were elevated upon osteoinduction in DF and DP cells, with DP cells exhibiting higher levels of activation compared to DF cells (Fig 1C). Consistent with its role as an early mineralization marker, ALP transcripts decreased at day 14 in both DF and DP cells. Together, these studies indicate that DF and DP progenitors differentiate and form mineral deposits in vitro, and that osteoinductive culture conditions promote tissue-specific lineage differentiation.
The concept of DF and DP progenitors as multipotent stem cells for odontogenic tissues has been proposed in earlier studies [7,40,48]. To verify stem cell characteristics for subsequent epigenetic studies, the expression levels of key pluripotency factors OCT4, NANOG, and SOX2 [49] were determined in DF and DP progenitors using semiquantitative real time (RT)-PCR. These studies revealed that OCT4, NANOG, and SOX2 were expressed in both DF and DP progenitors, with higher transcript levels in DP cells when compared to DF cells (Fig. 1D).
Substantial repression of odontoblast lineage genes DSPP and DMP1 in DF cells, but not in DP cells
The chromatin state of the genome contributes to the cellular identities by regulating gene expression through epigenetic mechanisms, including DNA methylation and modifications of histone tails [50,51]. Here we have analyzed the global levels of key histone modifications in DF and DP cells using a promoter-based ChIP on the chip method. Immunoprecipitation was carried out on early DF and DP progenitors with antibodies against an active (H3K4me3) and two repressive (H3K9me3 and H3K27me3) histone marks. DNA enriched for these histone modifications was hybridized onto a promoter array tiling −3.2 kb to +0.8 kb relative to the TSS of 22,542 human promoters. The replicate data sets for each histone modification chip in DF and DP cells showed high levels of correlation and reproducibility.
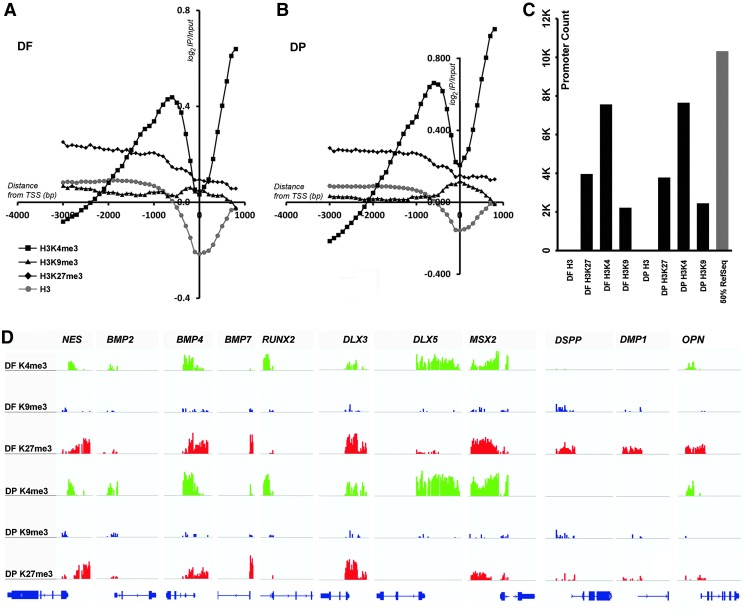
The average log2 ratio of ChIP/input obtained from replicates for each histone modification was plotted against the distance from the TSS for all promoters. Enrichment for H3K4me3 showed a pattern that peaked around 1,000 bp upstream of the TSS in both DF and DP cells (Fig. 2A, B). This histone modification is known to display a narrow and tall peak at gene promoters [52]. In contrast, the enrichment obtained with H3K9me3 and H3K27me3 gave rise to flatter and broader peaks (Fig. 2A, B) similar to those observed earlier [53]. The overall enrichment obtained with H3K9me3 was the least, while H3K4me3 gave the highest enrichment in both cell types. The histone H3 antibody used here as a control gave a negative peak at the TSS (Fig. 2A, B), which correlates with the nucleosome-free region observed at TSS of promoters [54]. Together, DF and DP TSS plots were similar to TSS plots of other progenitor populations [55], indicating that these odontogenic progenitors showed typical histone enrichment profiles on promoters.
FIG. 2.
Global ChIP-chip enrichment profiles for histone modifications: H3K4me3, H3K9me3, H3K27me3, and H3 from DF (A) and DP (B) progenitors at passage 3. The negative peak of the H3 antibody profile corresponded to the nucleosome-free region at the TSS (A, B). (C) Number of promoters enriched for histone modifications in DF and DP cells by ChIP-chip analysis. The percentage of RefSeq promoters is plotted alongside as a reference. (D) Representative view of histone enrichment profiles for select genes obtained using the IGV genome browser. Note the higher H3K4me3 enrichment levels at BMP2, BMP4, BMP7, RUNX2, DLX3, DLX5, MSX2, and OPN promoters in DP compared to DF cells. In contrast, DSPP and DMP1 promoters were enriched with H3K27me3 modifications only in DF cells. NES was used as a neural crest marker. TSS, transcription start site; IGV, Integrative Genomics Viewer; IP, immunoprecipitation; bp, base pairs; DMP1, dentin matrix protein 1; NES, Nestin. Color images available online at www.liebertpub.com/scd
As a next step, the total number of promoters from significant peaks of enrichment and corresponding to each histone modification (K4me3, K9me3, or K27me3) was compared between DF and DP cells. There were only marginal differences in the number of promoters enriched for each histone modification between DF and DP progenitors. Specifically, the H3K4me3 and H3K9me3 modification was more prominent in DP (7646 and 2446 in DP versus 7562 and 2215 in DF), while the H3K27me3 modification was slightly higher in DF (3966 versus 3781 in DP) (Fig. 2C).
Using the IGV genome browser [56], ChIP enrichment on specific gene promoter regions was visualized. This approach confirmed enrichment for key histone modifications H3K4me3, H3K9me3, and H3K27me3 in both DF and DP cells at the majority of promoters investigated (Fig. 2D). To focus on a select number of promoters of interest, a number of genes regulating either craniofacial development and patterning or mineralization were chosen, including BMP2, BMP4, BMP7, RUNX2, DLX3, DLX5, MSX2, DSPP, DMP1, and OPN. In addition, Nestin (NES) was selected as a neural crest marker. Interestingly, enrichment for the active H3K4me3 mark was higher in DP cells on BMP2,4,7, DLX3,5, and MSX2 promoters, while enrichment for silencing the H3K27me3 mark was higher in DF on BMP4, DLX5, and MSX2 promoters (Fig. 2D). DF cells were highlighted by substantial levels of enrichment with the repressive H3K27me3 mark on promoters for the three odontoblast-related genes DSPP, OPN, and DMP1, while this mark was essentially absent on the same promoters in DP cells (Fig. 2D). In contrast, the neural crest lineage marker NES displayed a similar enrichment profile in both DF and DP cells for all three histone modifications studied here (Fig. 2D).
Together, these studies indicated that DF and DP cells featured similar histone modification profiles indicative of their similar origin, with the exception of repressive histone modifications on key odontoblast lineage marker genes.
In both DF and DP progenitors, early mineralization genes (RUNX2, MSX2, DLX5) were predominantly marked with H3K4me3 active marks, while late mineralization markers (OSX, IBSP, BGLAP) were enriched for H3K9me3 or H3K27me3
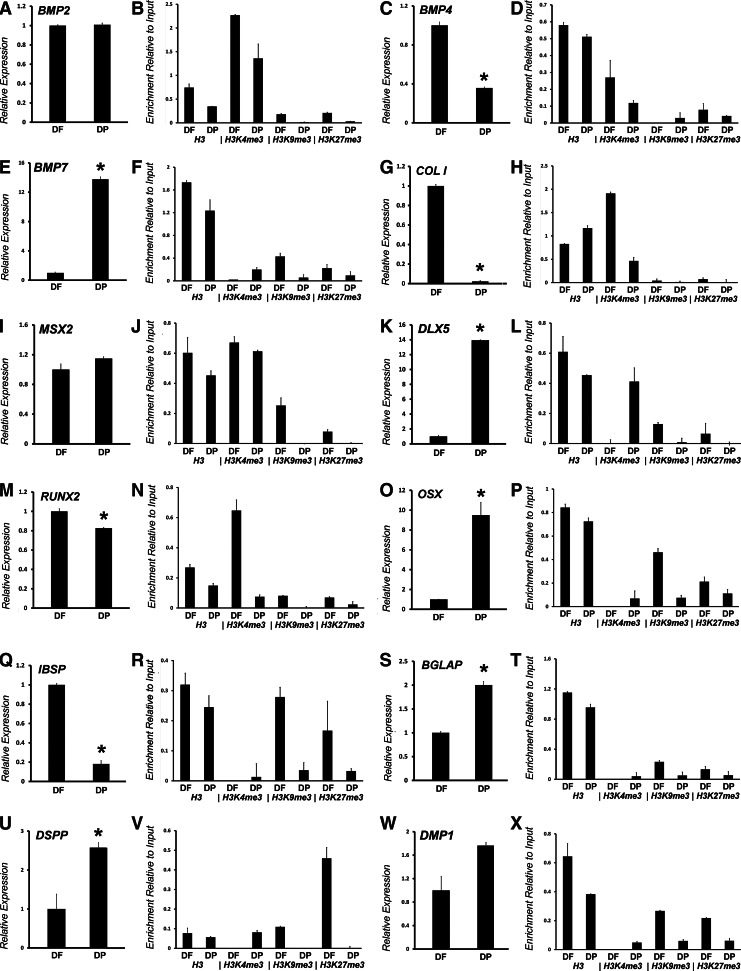
For an in-depth comparison of mineralization and craniofacial patterning, gene regulation between DF and DP progenitors, real-time RT-PCR, and ChIP analyses of 12 key genes were performed, including BMP2, BMP4, BMP7, COL I, MSX2, DLX5, RUNX2, OSX, IBSP, BGLAP, DSPP, and DMP1 (Fig. 3A–X). Our selection of genes once more focused on mineralization and patterning genes, and this time includes four additional genes related to the extracellular matrix and bone metabolism COL I, IBSP, BGLAP, and OSX, to conduct a comprehensive comparison of epigenetic changes related to mineralized tissue differentiation between DF and DP progenitors (Fig. 3A–X).
FIG. 3.
Comparison between DF and DP progenitors based on differential gene expression and histone modification patterns. Relative expression of the following mineralization-related genes was compared: BMP2 (A), BMP4 (C), BMP7 (E), COL I (G), MSX2 (I), DLX5 (K), RUNX2 (M), OSX (O), IBSP (Q), BGLAP (S), DSPP (U), and DMP1 (W). Relative gene expression comparisons were paired with ChIP-qPCR analyses of H3K4me3, H3K9me3, and H3K27me3 on gene promoters of early passage DF and DP cells. Histone H3 was used as a control. Representative graphs showing relative enrichment compared to input DNA after subtracting the no antibody background value for the following genes: BMP2 (B), BMP4 (D), BMP7 (F), COL I (H), MSX2 (J), DLX5 (L), RUNX2 (N), OSX (P), IBSP (R), BGLAP (T), DSPP (V), and DMP1 (X). Both ChIP and RT-qPCRs were performed at least three times with similar results, and one representative experiment is presented [±standard deviation (SD)]. (*P<0.05). DF and DP progenitors between passage 3 and 6 were used for ChIP and RT-qPCR experiments.
In general and indicative of the involvement of both DF and DP cells in mineralized tissue formation, the BMP family members, BMP2 and BMP4, the extracellular matrix gene, COL I, and the craniofacial homeobox-containing gene, MSX2, were predominantly marked with the active H3K4me3 histone modification, while displaying lower enrichment levels for the repressive H3K9me3 and H3K27me3 in both DF and DP cells (Fig. 3B, D, H, J, L, N). A second general trend observed among all promoters analyzed was a higher level of H3K9me3 and H3K27me3 enrichment in DF cells when compared to DP cells (Fig. 3B, F, H, J, L, N, P, R, T, V, X).
There were a number of unique enrichment patterns for individual genes in the two intermediate progenitor cells studied here. As an example, DF cells exhibited higher levels of H3K4me3 at the promoters of BMP2 and BMP4 when compared to DP cells (Fig. 3B, D). Moreover, DP cells featured high and lower levels of H3K4me3 at the DLX5 and RUNX2 promoters, respectively (Fig. 3L, N), while the MSX2 promoter exhibited similar enrichment for H3K4me3 in both cells types (Fig. 3J). The Osterix (SP7) gene, which functions downstream to RUNX2, and the bone marker gene IBSP displayed higher levels of H3K9me3 and H3K27me3 in DF cells when compared to DP cells (Fig. 3P, R). Collagen I (COL I), the major matrix protein of odontogenic tissues, displayed higher levels of H3K4me3 in DF cells compared to DP cells (Fig. 3H). Surprisingly, in the case of BGLAP, there was hardly any enrichment for all three histone modifications analyzed even though this region revealed histone H3 occupancy in ChIP, ruling out experimental errors and suggesting alternate mechanisms of gene regulation (Fig. 3T).
Our studies revealed a high level of correlation between histone modification levels at the promoter and downstream gene expression. For example, BMP2 and MSX2 expression were not significantly different between DF and DP cells (Fig. 3A, I), while BMP4, COL I, and RUNX2 expression were higher in DF cells (Fig. 3C, G, M), and BMP7, DLX5, and OSX were higher in DP cells (Fig. 3E, K, O), a trend that was matched by higher H3K4me3 enrichment at the respective gene promoters. Further validating our IGV plot analysis (Fig. 2D), the promoter of odontoblasts-specific genes, DSPP and DMP1, were enriched for repressive histone marks (H3K9me3 and H3K27me3) in DF cells, while they were enriched for the active H3K4me3 mark in DP cells (Fig. 3V, X). In accordance with the histone modification data, both DSPP and DMP1 exhibited higher expression levels in DP cells when compared to DF cells (Fig. 3U, W).
GOs reveal substantial overlap among genes enriched for individual histone modifications between DF and DP cells and an overall higher enrichment for the active H3K4me3 mark in DP cells
Histone methylation marks affect the accessibility of a gene promoter by either opening its structure for transcriptional activation or repressing access and thereby effectively blocking the transcriptional machinery [35,37]. Gene promoters may harbor either active or repressive marks or a combination of both, and the number of genes affected by active or repressive histone modifications is a useful identifier to evaluate the transcriptional state of a cell population [29,57,58]. We therefore conducted a systematic comparison of promoters enriched for each of the three histone modifications examined in the present study between DF and DP progenitors.
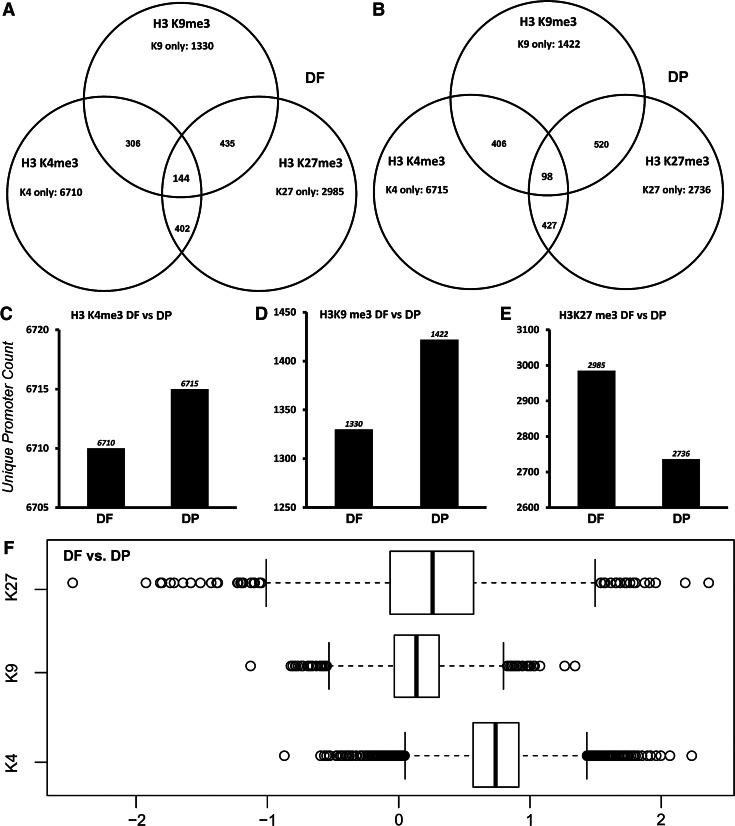
Our analysis identified a large number of genes that were unique to each histone modification studied within DF and DP cells (Fig. 4A, B). Based on our GO analysis, DF and DP progenitors shared a number of similar gene families in the K4, K9, and the K27 groups (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). For example, zinc finger proteins and other genes related to transcriptional activation, helicase activity, cell division, RNA splicing, and protein transport were H3K4me3 enriched in both DF and DP cells. The DP cells, however, displayed a higher enrichment for this active mark at many of the individual genes examined, including histone deacetylases, ankyrin repeat containing proteins, chromodomain proteins, and SET domain containing proteins (data not shown). The group of promoters enriched for the heterochromatic H3K9me3 mark featured a large number of genes responsible for the olfactory receptor activity in both cell types and also included cell adhesion genes (Cadherins), keratin genes, immunoglobulin domain containing genes, and few zinc finger containing genes (Supplementary Table S1). The other heterochromatic marker analyzed in our study, H3K27me3, was also associated with similar gene families in DF and DP cells, namely, genes encoding ionic channels, immunoglobulin domains, potassium transport, keratin genes, and genes regulating neurogenesis. The homeobox genes (HOX) were significantly enriched for the repressive H3K27me3 histone modification and to a lesser extent for the H3K9me3 modification in both cell types. Interestingly, genes of the WNT superfamily, including WNT1, WNT3A, WNT4, WNT6, and WNT10A, were also enriched for the silencing H3K27me3 modification in both cell types.
FIG. 4.
Bioinformatics comparison of gene promoter numbers associated with key histone modifications in DF and DP cells. (A, B) are Venn diagram analyses illustrating the distribution of H3K4me3, H3K9me3, and H3K27me3 modification marks within DF (A) and DP (B) progenitors. The number of genes that are unique for each histone modification group and the number of genes with overlapping histone modification marks are displayed. (C–E) Graphical comparison of promoter numbers unique for H3K4me3 (C), H3K9me3 (D), and H3K27me3 (E) between DF and DP progenitors. The number of promoters enriched for each histone modification was fairly similar when comparing DF and DP cell types. (F) Box plot comparison of global histone modification distribution in DP cells with DF cells as reference. Note the increased levels of H3K4me3 enrichment in DP cells when compared to DF cells.
As a next step, genes that were either unique for DF or DP cells when filtered for association with any of the K4, K9, or K27 modifications were identified and compared (Fig. 4C–E). There was a remarkable similarity among the K4-enriched genes between the DF and DP groups. Even though the K9 and K27 unique gene comparisons between DF and DP cells resulted in similar ontologies, there was a difference in significance, enrichment score, and actual genes present in each GO term. For example, the homeobox cluster list for unique K27-enriched genes featured BARX1, NANOG, DLX4, DLX6, and NKX3-1 promoters in DF cells and IRX3, ONECUT1, SIX2, SIX6, SHOX, and NKX2-4 in DP cells.
A comparison of global distribution of the three histone modifications between the two cell types revealed that DP cells had an overall higher level of enrichment for H3K4me3 when compared to DF cells (Fig. 4F). In contrast, global levels of H3K9me3 and H3K27me3 were fairly similar between DF and DP cells (Fig. 4F).
Unsupervised sample clustering analysis grouped promoters with the same histone modification in samples from DF and DP cells, and identified common histone methylation signatures
To identify clusters of genes based on levels of enrichment, promoters enriched for the three histone modifications H3K4me3, H3K9me3, and H3K27me3 in DF and DP cells were grouped using a hierarchical clustering approach [59]. This unsupervised sample clustering analysis resulted in neighbor assignments pairing DF and DP columns sorted for each of the three histone modifications (Fig. 5), that is, the promoters belonging to the H3K4me3 group in DF cells clustered with those from the H3K4me3 group in DP cells, DF H3K9me3 promoters clustered with DP H3K9me3 promoters, and DF H3K27me3 promoters clustered with DP H3K27me3 promoters. Interestingly, the two clusters of genes silenced either via H3K9me3 or H3K27me3 marks clustered together, indicating that the promoters of genes marked by these repressive modifications were closely related to each other in DF and DP cells and different from H3K4me3-activated genes (Fig. 5).
FIG. 5.
Unsupervised hierarchical clustering analysis of genes enriched for key histone modifications when comparing DF and DP progenitor cells. Peak height values were used as input to group both samples and promoters. The variation across samples was set to 0.50<standard deviation/mean<1,000.00 with the peak height (log2 ratio) ≥1.00 in ≥30% of the samples (FDR<5%). Peak height values ranged between −3 (dark red) to +3 (dark blue), while a value of 0 (white) indicates absence of a significant peak. Each column represents a sample type, and each line within a column represents a single promoter. Patterns within clusters were subjected to GO analysis, resulting in the identification of enriched GO terms for the promoters in the H3K27me3 group (A), H3K4me3 group (B), and H3K9me3 group (C). Significantly enriched GO terms were obtained with high classification stringency and an EASE score set at 0.06. FDR, false discovery rate; GO, gene ontology. Color images available online at www.liebertpub.com/scd
GOs for the individual clusters revealed unique insights into the molecular signatures common to DF and DP cells. Apart from homeobox-containing genes and genes regulating nervous system development, the K27 cluster also featured genes responsible for ear morphogenesis and sensory perception of sound (Fig. 5A). The K4 cluster featured a large set of genes classified as an intracellular organelle lumen cluster, which contains a diverse list of genes ranging from mediator complex subunits, zinc finger proteins, and other transcriptional regulators to histone proteins and histone modifying enzymes (Fig. 5B). Other K4 cluster genes identified in our DF/DP hierarchical cluster-sorting approach includes genes responsible for RNA splicing, protein synthesis, and cell cycle regulation (Fig. 5B). Finally, the K9 cluster common to both cell types featured cadherins, C2H2-type zinc fingers, transcription factors with the KRAB domain, and genes responsible for olfactory transduction (Fig. 5C). A number of individual genes were associated with higher enrichment values for the K4 active modification in DP cells, including the BMP receptors, BMPR1A and BMPR1B, genes involving calcium regulation such as CALCA, CALCB, and CALCR, the T-Box gene TBX1, and the WNT inhibitor DKK1, among others (data not shown).
Mineralized tissue formation-inductive conditions resulted in steep increases of mineralization and patterning gene expression levels in DP cells and highly dynamic histone modification changes in DF cells
Our initial studies (Fig. 1) demonstrated that osteoinductive cell culture conditions caused differentiation of DF and DP progenitors into mineralized tissue forming cells after 7 days of culture and increased mineral deposition thereafter. Here, this in vitro approach was used to map genetic and epigenetic changes during terminal differentiation of mesenchymal odontogenic progenitors and subsequent mineralized tissue secretion. We have once more focused on a combination of key patterning and mineralization genes, namely, BMP2, BMP4, MSX2, DLX5, RUNX2, OSX, IBSP, and BGLAP.
Our gene expression analysis indicated that BMP2, MSX2, DLX5, and OSX displayed a gradual increase in expression upon osteoinduction in both cell types, DF and DP (Fig. 6A, I, M, U). While RUNX2 expression in DP cells gradually increased at days 7 and 14, its expression in DF cells initially peaked at day 7 and subsequently decreased at day 14 (Fig. 6Q). BMP4 levels decreased in DF cultures after 14 days of induction, while in DP cells, they steeply increased from day 0 to 14 (Fig. 6E). In contrast, IBSP displayed a slow and gradual increase of expression in DF cells, while expression levels dramatically increased at day 7 in DP cells after osteoinduction, and then almost returned to baseline levels on day 14 in DP cells (Fig. 6Y). The other bone marker gene, BGLAP, exhibited lower levels of expression at 14 days in DF cells, while its expression increased significantly at day 7, and then dropped to near control levels by day 14 in DP cells (Fig. 6CC).
FIG. 6.
Correlation of histone dynamics and mRNA expression in DF and DP progenitors upon osteogenic induction. DF and DP cells cultured in the mineralization medium for 7 and 14 days were harvested for total RNA isolation and ChIP analysis. (A, E, I, M, Q, U, Y, CC) are RT-qPCR analyses of relative gene expression levels in DF and DP cells. The remaining figures are scatter plot analyses of histone modification changes upon osteoinduction in DF and DP cells; (B, F, J, N, R, V, Z, DD) display H3K4me3 changes, (C, G, K, O, S, W, AA, EE) show H3K9me3 changes, and (D, H, L, P, T, X, BB, FF) reveal H3K27me3 changes. ChIP and RT-qPCRs were performed at least three times with similar results, and one representative experiment is displayed [±standard deviation (SD)] (*P<0.05).
Our analysis of histone modification dynamics revealed a highly refined code of activation and repression of individual genes during odontogenic progenitor differentiation and mineralized matrix secretion. In general, histone dynamics were much more pronounced in DF progenitors when compared to DP counterparts, featuring a continuous rise in the active H3K4me3 mark, a steep decline in the H3K9me3 mark, and a U-shaped curve with a dip at day 7 in the H3K27me3 mark as overarching trends. In contrast, histone dynamics in DP progenitors remained much more at baseline levels, with the exception of a gradual reduction in K27 enrichment over the 14-day culture period and a gain in K4 enrichment on BMP2, BMP4, MSX2, DLX5, and RUNX2 promoters during osteogenic induction culture (Fig. 6B–D, F–H, J–L, N–P, R–T).
The H3K4me3 modification levels gradually increased in the early mineralization genes, BMP4, MSX2, and DLX5 in both DF and DP cells (Fig. 6F, J, N). H3K4me3 enrichment at the BMP2 promoter followed a hyperbolic curve in DF cells and a U-shaped curve in DP cells, indicating that BMP2 K4 activation played a more significant role in early osteogenic differentiation of DF cells, while it affected DP cells more during the mineral deposition period (Fig. 6B). The promoters of the bone-specific transcription factors RUNX2 and OSX and the late mineralization markers IBSP and BGLAP also exhibited higher levels of H3K4me3 upon induction in DF cells, while the K4 changes on these promoters were not significant in DP cells (Fig. 6R, V, Z, DD). The increased enrichment for H3K4me3 correlated with the expression increases of BMP2, BMP4, MSX2, DLX5, and RUNX2 in both cell types. In spite of the dramatic increase in expression levels of IBSP in DP cells, there was no matching increase in H3K4me3 levels on the promoter (Fig. 6Y, Z), suggesting an alternative mechanism of gene regulation. H3K9me3 enrichment values at the promoters of MSX2, DLX5, RUNX2, OSX, and IBSP decreased initially at day 7 and increased thereafter toward day 14 in DF cells (Fig. 6K, O, S, W, AA). In contrast, H3K9me3 enrichment levels in DP cells were much lower for all promoters studied (Fig. 6C, G, K, O, S, W, AA, EE).
GO analysis of bivalent marks unique for each cell type highlighted cell–cell attachment genes in DP cells and neurogenesis genes in DF cells
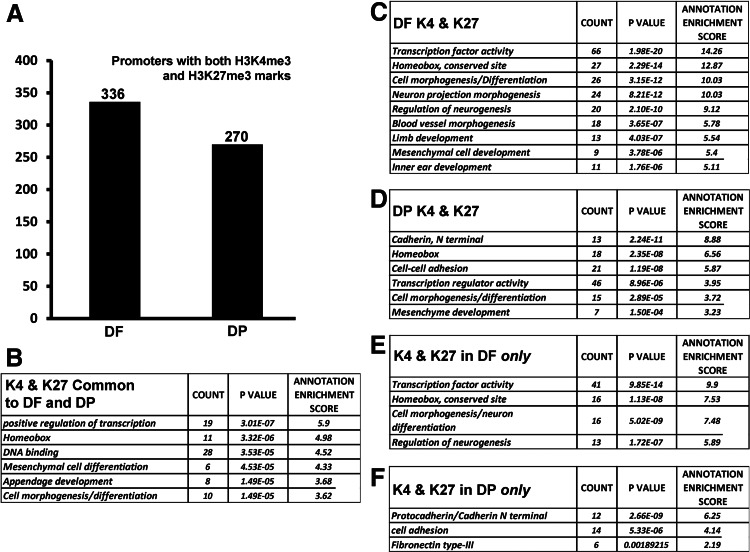
Gene promoters marked with both active and repressive histone modifications (H3K4me3 and H3K27me3) are known as bivalent genes. These promoters are considered to be in a transcriptionally poised state, implying their readiness for further activation or repression upon lineage commitment [60].
Our analysis for bivalent genes identified a total of 336 promoters in DF cells and 270 promoters in DP cells (Fig. 7A). GO analysis was used to identify unique and common gene clusters between DF and DP cells. Our GO analysis associated the homeobox-containing GO with bivalent genes in both DF and DP cells (Fig. 7B–D). Bivalent genes of the DF were associated with GOs related to the transcription factor activity, differentiation, neurogenesis, and inner ear development (Fig. 7C), while GOs in DP cells included cadherins, genes responsible for cell–cell adhesion, transcription regulation, and differentiation (Fig. 7D). Comparison of these bivalent genes between DF and DP cells revealed that genes responsible for the transcription factor activity and neurogenesis were unique to DF cells, while the protocadherin/cadherin genes and the cell adhesion and fibronectin type III genes were unique to DP cells (Figs. 7E, F).
FIG. 7.
GO analysis of bivalent promoters in DF and DP progenitors. (A) Comparison of total number of bivalent promoters identified in DF and DP cells. GO analysis illustrating enriched GO terms in both DF and DP cells (B), in DF cells (C), in DP cells (D), only in DF cells (E), and only in DP cells (F). Significantly enriched GO terms were obtained with high classification stringency and an EASE score set at 0.06.
Discussion
The present study is the first analysis of histone methylation profiles and dynamics in odontogenic progenitors. Our study focused on DP and DF progenitors as the two major ectomesenchymal progenitor populations that give rise to the developing tooth organ. This comparative analysis allowed us to dissect unique histone methylation events on gene promoters distinct for each of these two subsets of progenitors. Three key histone methylation marks were studied, the active H3K4me3 mark and two repressive marks, H3K9me3 and H3K27me3. All three marks are among the most frequently studied epigenetic indicators of gene activation and repression [33–35] and provide a first cross-sectional analysis of histone methylation in odontogenic progenitors. The most noteworthy outcome of this part of our study was the strong repression of two dentinogenesis gene promoters, DSPP and DMP1, by H3K27me3 marks in DF progenitors when compared to DP progenitors. Moreover, active histone marks were identified on many of the gene promoters traditionally associated with the induction of mineralization. As a next step, DP and DF cells were subjected to mineralization conditions as a differentiation-inducing environment to study and compare histone dynamics during the differentiation of odontogenic progenitors. While DP and DF cells are likely subject to different differentiation mechanisms, exposure to mineralizing conditions is the most well-studied mechanism to trigger differentiation in both populations [3,61]. These studies revealed remarkable histone modification dynamics in DF cells, which were not detected in DP counterparts. Finally, GO analysis of bivalent marks identified unique subsets of poised genes in each population: neurogenesis genes in DF progenitors and attachment/adhesion genes in DP progenitors. Together, this study provides a snapshot of the epigenetic signatures that define the two major mesenchymal odontogenic progenitor populations and of the regulatory mechanisms that take place during their differentiation.
Our data indicated high levels of H3K27me3 marks on DSPP and DMP1 promoters in DF cells in tandem with decreased levels of expression, while H3K27me3 marks on DSPP and DMP1 promoters in DP cells were almost absent and levels of gene expression were substantially higher. This progenitor-specific repression pathway appears to define two major subpopulations of odontogenic neural crest progenitors: those that give rise to pulp and dentin and do not repress DSPP and DMP1, and those that give rise to the DF, PDL, AB, and CEM and in which DSPP and DMP1 are repressed. It remains to be determined whether all craniofacial neural crest progenitors, or at least those that give rise to mineralized tissues, have the potential to express high levels of DSPP and DMP1, and whether this potential is selectively repressed outside of dentinogenic or pulp lineages. At least DMP1 has been detected in skeletogenic lineages outside of dentin [62], suggesting that DMP1 repression in those lineages may be less pronounced than in DF cells.
As a next step, we queried other mineralization-associated genes for their histone methylation profiles in DF and DP cells. ChIP on chip analysis identified an active histone mark on many of the gene promoters traditionally associated with the induction of bone formation, mineralized tissue patterning, and early preosteoblast differentiation, such as BMP2, BMP4, BMP7, RUNX2, COL I, DLX5, and MSX2. Our finding of active histone modifications on these early mineralization genes was supported by high gene expression levels in parallel studies. In contrast, typical SIBLING family mineralization gene IBSP, the bone marker BGLAP, and the osteoblast differentiation factor OSX remained in a silent state as indicated by repressive histone modifications (H3K9me3 and H3K27me3). This demarcation of early and late stage mineralization genes by antagonistic epigenetic modifications suggests that the developing tooth organs we sampled were representative of a developmental stage in which the early mineralization genes were in an ON state, ready to be induced, while the terminal markers remained silent, presumably until the onset of differentiation. In addition to those stage-specific differences between active and repressive marks on early and late stage mineralization genes, our study also highlighted progenitor-specific pathways to control mineralization when comparing DF and DP progenitors. Notably, BMP7, DLX5, and OSX transcript levels were higher in DP cells, while BMP4 and RUNX2 transcripts were present in higher amounts in DF cells. The overall higher expression levels of early mineralization genes in DP cells when compared to DF cells may explain the higher osteoinductive capacity of DP progenitors. However, two key mineralization factors, BMP2 and MSX2, were expressed in DF and DP cells at almost similar levels, indicating that both play important roles in the differentiation of odontoblasts, periodontal osteoblasts, and cementoblasts, as well as during subsequent mineralized tissue formation [63].
After identifying key patterns of odontogenic neural crest lineage specification through stage-specific and lineage-specific histone marks, we tested histone dynamics in a differentiation environment common to both DP and DF progenitors. These studies demonstrated that mineralized tissue formation-inductive conditions resulted in steep increases of mineralization and patterning gene expression levels in DP cells and highly dynamic histone modification changes in DF cells. The reasons for this fundamental difference in gene regulation between DP and DF progenitors may be found in their different biological functions and also in their different evolutionary origins. Comprehensive analyses consider dentin the cladistically primitive mineral relative to bone and likely related to the first emergence of vertebrate exoskeletal skeletogenic ability 500 MYA [64]. In contrast, the three-layered vertebrate tooth attachment apparatus consisting of AB, PDL, and root CEM is fairly recent, with a first wide-spread appearance only in modern crocodilians (200–65 MYA) [65,66] and a mineralized PDL in the mosasaurs (145–66 MYA) [67]. In principle, formation of mineralized denticles by odontogenic progenitors may have evolved as a simple, straight-forward mechanism involving the secretion of the mineralized matrix at the skeletogenic site. In contrast, complex tooth attachment involving a nonmineralized PDL has only emerged secondarily to ankylosis-based bony tooth attachment [65]. Moreover, complex periodontal tooth attachment as it occurs in mammals is subject to continuous remodeling under various functional pressures [68,69]. Here we propose that the basic mechanism of mineralized tissue formation in response to a mineral-inducing environment might have been highly conserved in DP progenitors, while DF progenitors have evolved more recently together with a highly flexible epigenetic regulatory machinery. A dynamic level of gene expression control via histone methylation might allow PDL progenitors to fine-tune the response to various mechanical loads and either form mineralized tissue for enhanced stability or inhibit mineralization to facilitate flexible ligamentous tooth anchorage in a site-specific fashion.
GO analysis of bivalent marks highlighted overall similar types of genes in DP and DF cells, with the exception of cell–cell attachment genes that were more prominent in DP cells and neurogenesis genes, which were preferentially identified in DF cells. The subsets of genes identified in this analysis were those poised for further activation or repression upon lineage commitment [61], implying that the group of genes most responsive to dynamic changes in the DP were attachment genes such as cadherins and protocadherins, and the corresponding set of genes in the DF were neurogenesis genes such as PAX6 and IRX3, as well as axogenesis and axon guidance genes such as Kalirin and ROBO2. Although expressed during human odontogenesis [70,71], cadherins are not expressed in permanent intact teeth, but their expression becomes activated in odontoblasts during the dentin repair process following trauma [72]. Thus, the bivalent nature of cadherins in DP cells identified in the present study would allow for rapid activation of cell adhesion mechanisms upon pulp cell injury and for the stimulation of dentin repair. Similarly, fibronectin, another bivalent gene in DP cells was shown to induce differentiation of DP cells into mineralized tissue forming cells [73]. The presence of bivalent marks on genes involved in adhesion and pulp repair illustrates the importance of DP reparative and protective mechanisms in the evolution of vertebrates with diphyodont and monophyodont dentitions. The preferential occurrence of bivalent marks on neurogenesis and axon guidance genes in the DF was a surprise finding that may be explained with the tight relationship between the PDL, mechanoreception, and jaw muscle function [74,75]. This finding was unexpected as there is good evidence suggesting that the DP functions as an ancestral neurosensory organ [76]. These data might indicate that the DF and its derivative, the PDL, require a high level of readiness for changes in occlusal stresses and loads imposed onto the periodontal attachment apparatus [77–79].
Supplementary Material
Acknowledgment
Funding by the NIDCR grant DE019463 is gratefully acknowledged.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Luan X. Dangaria S. Ito Y. Walker CG. Jin T. Schmidt MK. Galang MT. Druzinsky R. Neural crest lineage segregation: a blueprint for periodontal regeneration. J Dent Res. 2009;88:781–791. doi: 10.1177/0022034509340641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothova M. Peterkova R. Tucker AS. Fate map of the dental mesenchyme: dynamic development of the dental papilla and follicle. Dev Biol. 2012;366:244–254. doi: 10.1016/j.ydbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Yagyuu T. Ikeda E. Ohgushi H. Tadokoro M. Hirose M. Maeda M. Inagake K. Kirita T. Hard tissue-forming potential of stem/progenitor cells in human dental follicle and dental papilla. Arch Oral Biol. 2010;55:68–76. doi: 10.1016/j.archoralbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Ten Cate AR. The development of the periodontium—a largely ectomesenchymally derived unit. Periodontology 2000. 1997;13:9–19. doi: 10.1111/j.1600-0757.1997.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 5.Diekwisch TG. Berman BJ. Anderton X. Gurinsky B. Ortega AJ. Satchell PG. Williams M. Arumugham C. Luan X, et al. Membranes, minerals, and proteins of developing vertebrate enamel. Microsc Res Tech. 2002;59:373–395. doi: 10.1002/jemt.10218. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M. Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 7.Luan X. Ito Y. Dangaria S. Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15:595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tziafas D. Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Dangaria SJ. Ito Y. Luan X. Diekwisch TG. Differentiation of neural-crest-derived intermediate pluripotent progenitors into committed periodontal populations involves unique molecular signature changes, cohort shifts, and epigenetic modifications. Stem Cells Dev. 2011;20:39–52. doi: 10.1089/scd.2010.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesot H. Lisi S. Peterkova R. Peterka M. Mitolo V. Ruch JV. Epigenetic signals during odontoblast differentiation. Adv Dent Res. 2001;15:8–13. doi: 10.1177/08959374010150012001. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima M. Mizunuma K. Murakami T. Akamine A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11) Gene Ther. 2002;9:814–818. doi: 10.1038/sj.gt.3301692. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan K. Srinivas R. Ramachandran A. Hao J. Quinn B. George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci U S A. 2001;98:4516–4521. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S. Rani S. Wu Y. Unterbrink A. Gu TT. Gluhak-Heinrich J. Chuang HH. Macdougall M. Differential regulation of dentin sialophosphoprotein expression by Runx2 during odontoblast cytodifferentiation. J Biol Chem. 2005;280:29717–29727. doi: 10.1074/jbc.M502929200. [DOI] [PubMed] [Google Scholar]
- 14.Rios HF. Ye L. Dusevich V. Eick D. Bonewald LF. Feng JQ. DMP1 is essential for osteocyte formation and function. J Musculoskelet Neuronal Interact. 2005;5:325–327. [PubMed] [Google Scholar]
- 15.Goldberg M. Pulp healing and regeneration: more questions than answers. Adv Dent Res. 2011;23:270–274. doi: 10.1177/0022034511405385. [DOI] [PubMed] [Google Scholar]
- 16.Zhao M. Xiao G. Berry JE. Franceschi RT. Reddi A. Somerman MJ. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–1451. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]
- 17.Sena K. Morotome Y. Baba O. Terashima T. Takano Y. Ishikawa I. Gene expression of growth differentiation factors in the developing periodontium of rat molars. J Dent Res. 2003;82:166–171. doi: 10.1177/154405910308200304. [DOI] [PubMed] [Google Scholar]
- 18.Morsczeck C. Schmalz G. Reichert TE. Vollner F. Saugspier M. Viale-Bouroncle S. Driemel O. Gene expression profiles of dental follicle cells before and after osteogenic differentiation in vitro. Clin Oral Investig. 2009;13:383–391. doi: 10.1007/s00784-009-0260-x. [DOI] [PubMed] [Google Scholar]
- 19.Viale-Bouroncle S. Felthaus O. Schmalz G. Brockhoff G. Reichert TE. Morsczeck C. The transcription factor DLX3 regulates the osteogenic differentiation of human dental follicle precursor cells. Stem Cells Dev. 2012;21:1936–1947. doi: 10.1089/scd.2011.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li E. Bestor TH. Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 21.Ronemus MJ. Galbiati M. Ticknor C. Chen J. Dellaporta SL. Demethylation-induced developmental pleiotropy in Arabidopsis. Science. 1996;273:654–657. doi: 10.1126/science.273.5275.654. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez A. Vigorito E. Clare S. Warren MV. Couttet P. Soond DR. van Dongen S. Grocock RJ. Das PP, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaehres H. Scholer HR. Induction of pluripotency: from mouse to human. Cell. 2007;131:834–835. doi: 10.1016/j.cell.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 25.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 26.Morgan HD. Santos F. Green K. Dean W. Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(Spec No 1):R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 27.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 28.Turner BM. Defining an epigenetic code. Nat Cell Biol. 2007;9:2–6. doi: 10.1038/ncb0107-2. [DOI] [PubMed] [Google Scholar]
- 29.Lunyak VV. Rosenfeld MG. Epigenetic regulation of stem cell fate. Hum Mol Genet. 2008;17:R28–R36. doi: 10.1093/hmg/ddn149. [DOI] [PubMed] [Google Scholar]
- 30.Teven CM. Liu X. Hu N. Tang N. Kim SH. Huang E. Yang K. Li M. Gao JL, et al. Epigenetic regulation of mesenchymal stem cells: a focus on osteogenic and adipogenic differentiation. Stem Cells Int. 2011;2011:201371. doi: 10.4061/2011/201371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collas P. Programming differentiation potential in mesenchymal stem cells. Epigenetics. 2010;5:476–482. doi: 10.4161/epi.5.6.12517. [DOI] [PubMed] [Google Scholar]
- 32.Noer A. Lindeman LC. Collas P. Histone H3 modifications associated with differentiation and long-term culture of mesenchymal adipose stem cells. Stem Cells Dev. 2009;18:725–736. doi: 10.1089/scd.2008.0189. [DOI] [PubMed] [Google Scholar]
- 33.Bilodeau S. Kagey MH. Frampton GM. Rahl PB. Young RA. SetDB1 contributes to repression of genes encoding developmental regulators and maintenance of ES cell state. Genes Dev. 2009;23:2484–2489. doi: 10.1101/gad.1837309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peach SE. Rudomin EL. Udeshi ND. Carr SA. Jaffe JD. Quantitative assessment of chromatin immunoprecipitation grade antibodies directed against histone modifications reveals patterns of co-occurring marks on histone protein molecules. Mol Cell Proteomics. 2012;11:128–137. doi: 10.1074/mcp.M111.015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 36.Stewart MD. Li J. Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol Cell Biol. 2005;25:2525–2538. doi: 10.1128/MCB.25.7.2525-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz YB. Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 38.Lee TI. Johnstone SE. Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Thesleff I. Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 41.Thesleff I. Vaahtokari A. The role of growth factors in determination and differentiation of the odontoblastic cell lineage. Proc Finn Dent Soc. 1992;88(Suppl 1):357–368. [PubMed] [Google Scholar]
- 42.Ruch JV. Tooth crown morphogenesis and cytodifferentiations: candid questions and critical comments. Connect Tissue Res. 1995;32:1–8. doi: 10.3109/03008209509013699. [DOI] [PubMed] [Google Scholar]
- 43.Balic A. Aguila HL. Caimano MJ. Francone VP. Mina M. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone. 2010;46:1639–1651. doi: 10.1016/j.bone.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori G. Brunetti G. Oranger A. Carbone C. Ballini A. Lo Muzio L. Colucci S. Mori C. Grassi FR. Grano M. Dental pulp stem cells: osteogenic differentiation and gene expression. Ann N Y Acad Sci. 2011;1237:47–52. doi: 10.1111/j.1749-6632.2011.06234.x. [DOI] [PubMed] [Google Scholar]
- 45.Mikami Y. Asano M. Honda MJ. Takagi M. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells. J Cell Physiol. 2010;223:123–133. doi: 10.1002/jcp.22017. [DOI] [PubMed] [Google Scholar]
- 46.Ovchinnikov D. Alcian blue/alizarin red staining of cartilage and bone in mouse. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.prot5170. pdb prot5170. [DOI] [PubMed] [Google Scholar]
- 47.Wang X. Jin T. Chang S. Zhang Z. Czajka-Jakubowska A. Nor JE. Clarkson BH. Ni L. Liu J. In vitro differentiation and mineralization of dental pulp stem cells on enamel-like fluorapatite surfaces. Tissue Eng Part C Methods. 2012;18:821–830. doi: 10.1089/ten.tec.2011.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gronthos S. Mankani M. Brahim J. Robey PG. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L. Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–R27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 50.Fazzari MJ. Greally JM. Epigenomics: beyond CpG islands. Nat Rev Genet. 2004;5:446–455. doi: 10.1038/nrg1349. [DOI] [PubMed] [Google Scholar]
- 51.Feinberg AP. Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 52.Feng J. Liu T. Qin B. Zhang Y. Liu XS. Identifying ChIP-seq enrichment using MACS. Nat Protoc. 2012;7:1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brinkman AB. Gu H. Bartels SJ. Zhang Y. Matarese F. Simmer F. Marks H. Bock C. Gnirke A. Meissner A. Stunnenberg HG. Sequential ChIP-bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 2012;22:1128–1138. doi: 10.1101/gr.133728.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang C. Pugh BF. A compiled and systematic reference map of nucleosome positions across the Saccharomyces cerevisiae genome. Genome Biol. 2009;10:R109. doi: 10.1186/gb-2009-10-10-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorensen AL. Jacobsen BM. Reiner AH. Andersen IS. Collas P. Promoter DNA methylation patterns of differentiated cells are largely programmed at the progenitor stage. Mol Biol Cell. 2010;21:2066–2077. doi: 10.1091/mbc.E10-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorvaldsdottir H. Robinson JT. Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2012 doi: 10.1093/bib/bbs017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruyama R. Choudhury S. Kowalczyk A. Bessarabova M. Beresford-Smith B. Conway T. Kaspi A. Wu Z. Nikolskaya T, et al. Epigenetic regulation of cell type-specific expression patterns in the human mammary epithelium. PLoS Genet. 2011;7:e1001369. doi: 10.1371/journal.pgen.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Min IM. Waterfall JJ. Core LJ. Munroe RJ. Schimenti J. Lis JT. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 2011;25:742–754. doi: 10.1101/gad.2005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisen MB. Spellman PT. Brown PO. Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernstein BE. Mikkelsen TS. Xie X. Kamal M. Huebert DJ. Cuff J. Fry B. Meissner A. Wernig M, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 61.Heng BC. Cao T. Stanton LW. Robson P. Olsen B. Strategies for directing the differentiation of stem cells into the osteogenic lineage in vitro. J Bone Miner Res. 2004;19:1379–1394. doi: 10.1359/JBMR.040714. [DOI] [PubMed] [Google Scholar]
- 62.Albazzaz M. Narayanan K. Hao J. Impaired skeletal formation in mice overexpressing DMP1. Orthop Res Rev. 2009;1:1–10. [Google Scholar]
- 63.D'Souza RN. Aberg T. Gaikwad J. Cavender A. Owen M. Karsenty G. Thesleff I. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- 64.Smith MM. Hall BK. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev Camb Philos Soc. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 65.McIntosh JE. Anderton X. Flores-De-Jacoby L. Carlson DS. Shuler CF. Diekwisch TG. Caiman periodontium as an intermediate between basal vertebrate ankylosis-type attachment and mammalian “true” periodontium. Microsc Res Tech. 2002;59:449–459. doi: 10.1002/jemt.10222. [DOI] [PubMed] [Google Scholar]
- 66.Brochu CA. Osteology of Tyrannosaurus rex: insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. Journal of Vertebrate Paleontology. 2003;22(no.sup4):1–138. [Google Scholar]
- 67.Luan X. Walker C. Dangaria S. Ito Y. Druzinsky R. Jarosius K. Lesot H. Rieppel O. The mosasaur tooth attachment apparatus as paradigm for the evolution of the gnathostome periodontium. Evol Dev. 2009;11:247–259. doi: 10.1111/j.1525-142X.2009.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luan X. Ito Y. Holliday S. Walker C. Daniel J. Galang TM. Fukui T. Yamane A. Begole E. Evans C. Diekwisch TG. Extracellular matrix-mediated tissue remodeling following axial movement of teeth. J Histochem Cytochem. 2007;55:127–140. doi: 10.1369/jhc.6A7018.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henneman S. Von den Hoff JW. Maltha JC. Mechanobiology of tooth movement. Eur J Orthod. 2008;30:299–306. doi: 10.1093/ejo/cjn020. [DOI] [PubMed] [Google Scholar]
- 70.Luning C. Rass A. Rozell B. Wroblewski J. Obrink B. Expression of E-cadherin during craniofacial development. J Craniofac Genet Dev Biol. 1994;14:207–216. [PubMed] [Google Scholar]
- 71.Leonardi R. Spatio-temporal expression of E-cadherin during human odontogenesis. An immunohistochemical study. Minerva Stomatol. 1999;48:325–331. [PubMed] [Google Scholar]
- 72.Heymann R. About I. Lendahl U. Franquin JC. Obrink B. Mitsiadis TA. E- and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am J Pathol. 2002;160:2123–2133. doi: 10.1016/S0002-9440(10)61161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizuno M. Banzai Y. Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int Endod J. 2008;41:933–938. doi: 10.1111/j.1365-2591.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 74.Byers MR. Sensory innervation of periodontal ligament of rat molars consists of unencapsulated Ruffini-like mechanoreceptors and free nerve endings. J Comp Neurol. 1985;231:500–518. doi: 10.1002/cne.902310408. [DOI] [PubMed] [Google Scholar]
- 75.Brodin P. Turker KS. Miles TS. Mechanoreceptors around the tooth evoke inhibitory and excitatory reflexes in the human masseter muscle. J Physiol. 1993;464:711–723. doi: 10.1113/jphysiol.1993.sp019659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farahani RM. Simonian M. Hunter N. Blueprint of an ancestral neurosensory organ revealed in glial networks in human dental pulp. J Comp Neurol. 2011;519:3306–3326. doi: 10.1002/cne.22701. [DOI] [PubMed] [Google Scholar]
- 77.Lassila V. Koivumaa KK. Deteriorating effect of occlusal disorders on the periodontium of rats with experimental arteriosclerosis. Acta Odontol Scand. 1980;38:41–50. doi: 10.3109/00016358008997717. [DOI] [PubMed] [Google Scholar]
- 78.Reddy MK. Vandana KL. Three-dimensional finite element analysis of stress in the periodontium. J Int Acad Periodontol. 2005;7:102–107. [PubMed] [Google Scholar]
- 79.Walker CG. Ito Y. Dangaria S. Luan X. Diekwisch TG. RANKL, osteopontin, and osteoclast homeostasis in a hyperocclusion mouse model. Eur J Oral Sci. 2008;116:312–318. doi: 10.1111/j.1600-0722.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.