Abstract
Studies were performed to verify the physiological significance of attenuation in the life cycle of simian virus 40 and the role of agnoprotein in this process. For these purposes, nuclei were isolated at various times after infection and incubated in vitro in the presence of [alpha-32P]UTP under the standard conditions which lead to attenuation. Attenuation was evident by the production of a 94-nucleotide attenuator RNA, revealed by gel electrophoresis. In parallel, the synthesis of agnoprotein was studied at various times after infection by labeling the cells for 3 h with [14C]arginine, lysing them, and analyzing the labeled proteins by gel electrophoresis. Both attenuation and the synthesis of agnoprotein were predominant towards the end of the infectious cycle. At earlier times, there was almost no attenuation and no synthesis of agnoprotein. Moreover, there was almost no attenuation even at the latest times after infection in nuclei isolated from cells infected with simian virus 40 deletion mutants that do not synthesize agnoprotein. Finally, analysis by dot blot hybridization showed higher amounts of cytoplasmic viral RNA in cells infected with an agnoprotein gene insertion mutant, delta 79, that does not produce agnoprotein, compared with cells infected with wild-type virus. The present studies indicate that attenuation is temporally regulated and suggest that agnoprotein enhances attenuation in isolated nuclei and that may also enhance it in vivo.
Full text
PDF
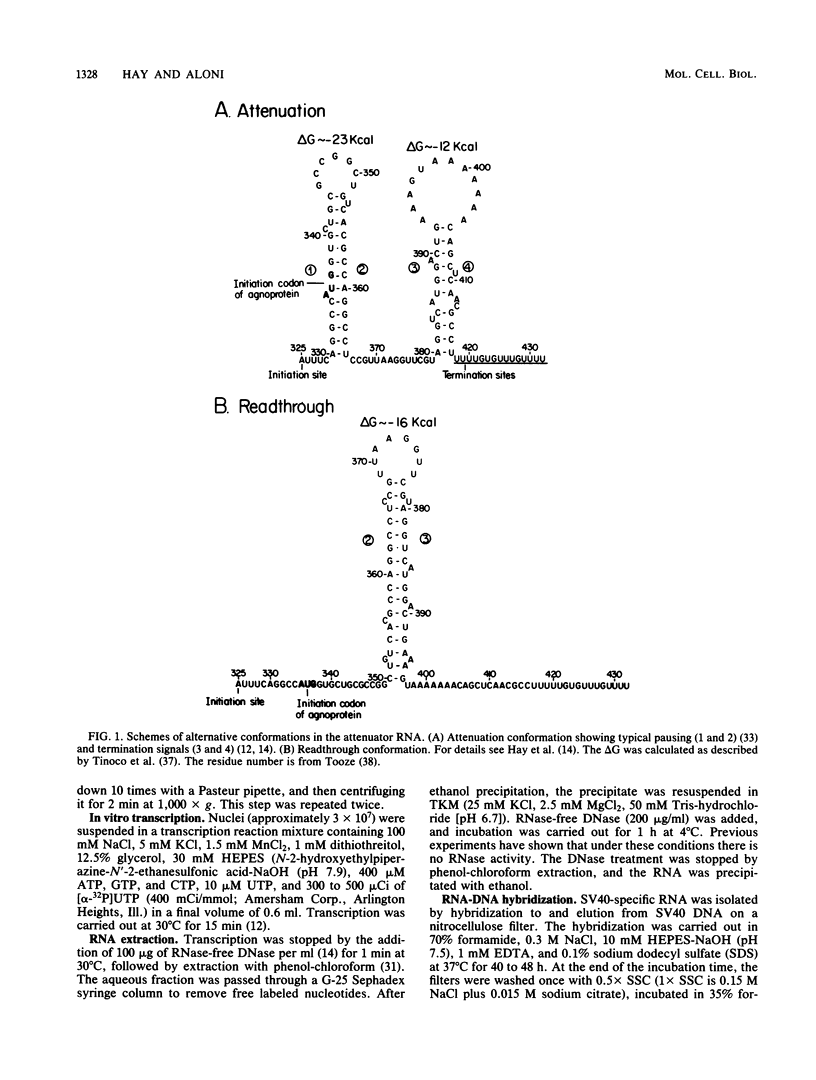


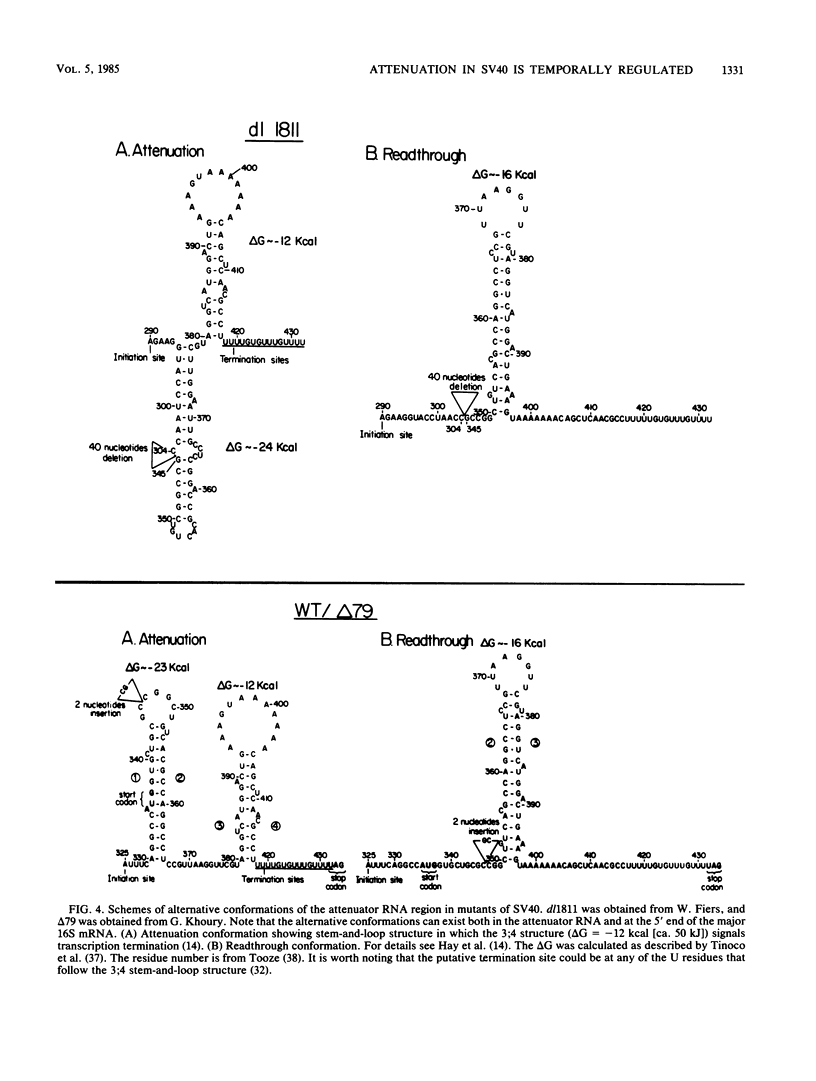



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abulafia R., Ben-Ze'ev A., Hay N., Aloni Y. Control of late simian virus 40 transcription by the attenuation mechanism and transcriptionally active ternary complexes are associated with the nuclear matrix. J Mol Biol. 1984 Feb 5;172(4):467–487. doi: 10.1016/s0022-2836(84)80018-2. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Hay N. Attenuation and modulation of mRNA secondary structure in a feedback control system regulating SV40 gene expression. Mol Biol Rep. 1983 May;9(1-2):91–100. doi: 10.1007/BF00777479. [DOI] [PubMed] [Google Scholar]
- Alwine J. C. Hybrid selection of small RNAs by using simian virus 40 DNA: evidence that the simian virus 40-associated small RNA is synthesized by specific cleavage from large viral transcripts. J Virol. 1982 Sep;43(3):987–996. doi: 10.1128/jvi.43.3.987-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Barkan A., Mertz J. E. DNA sequence analysis of simian virus 40 mutants with deletions mapping in the leader region of the late viral mRNA's: mutants with deletions similar in size and position exhibit varied phenotypes. J Virol. 1981 Feb;37(2):730–737. doi: 10.1128/jvi.37.2.730-737.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Asher E., Aloni Y. Transcription of minute virus of mice, an autonomous parvovirus, may be regulated by attenuation. J Virol. 1984 Oct;52(1):266–276. doi: 10.1128/jvi.52.1.266-276.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. W., Jr, Wydro R. M., Nadal-Ginard B., Dina D. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature. 1980 Dec 25;288(5792):665–669. doi: 10.1038/288665a0. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Iserentant D., Gheysen D., Fiers W. Characterization of the major altered leader sequence of late mRNA induced by SV40 deletion mutant d1-1811. Nucleic Acids Res. 1979 Dec 11;7(7):1799–1814. doi: 10.1093/nar/7.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Aloni Y. Attenuation in SV40 as a mechanism of transcription-termination by RNA polymerase B. Nucleic Acids Res. 1984 Feb 10;12(3):1401–1414. doi: 10.1093/nar/12.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. Use of whole-cell fixation to visualize replicating and maturing simian virus 40: identification of new viral gene product. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6081–6085. doi: 10.1073/pnas.78.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Evaluation of the "scanning model" for initiation of protein synthesis in eucaryotes. Cell. 1980 Nov;22(1 Pt 1):7–8. doi: 10.1016/0092-8674(80)90148-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eukaryotic ribosomes recognize the unique AUG initiator codon in messenger RNA? Biochem Soc Symp. 1982;47:113–128. [PubMed] [Google Scholar]
- Kozak M. Mechanism of mRNA recognition by eukaryotic ribosomes during initiation of protein synthesis. Curr Top Microbiol Immunol. 1981;93:81–123. doi: 10.1007/978-3-642-68123-3_5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Translation of insulin-related polypeptides from messenger RNAs with tandemly reiterated copies of the ribosome binding site. Cell. 1983 Oct;34(3):971–978. doi: 10.1016/0092-8674(83)90554-8. [DOI] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. VI. SV 40 DNA-RNA polymerase complex isolated from productively infected cells transcribed in vitro. Virology. 1976 Dec;75(2):346–354. doi: 10.1016/0042-6822(76)90033-7. [DOI] [PubMed] [Google Scholar]
- Margolskee R. F., Nathans D. Suppression of a VP1 mutant of simian virus 40 by missense mutations in serine codons of the viral agnogene. J Virol. 1983 Nov;48(2):405–409. doi: 10.1128/jvi.48.2.405-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Murphy A., Barkan A. Mutants deleted in the agnogene of simian virus 40 define a new complementation group. J Virol. 1983 Jan;45(1):36–46. doi: 10.1128/jvi.45.1.36-46.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Acheson N. H. Synthesis of prematurely terminated late transcripts of polyoma virus DNA is resistant to inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. J Gen Virol. 1982 Apr;59(Pt 2):367–376. doi: 10.1099/0022-1317-59-2-367. [DOI] [PubMed] [Google Scholar]
- Nomura S., Khoury G., Jay G. Subcellular localization of the simian virus 40 agnoprotein. J Virol. 1983 Jan;45(1):428–433. doi: 10.1128/jvi.45.1.428-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer P., Hay N., Pruzan R., Jakobovits E. B., Aloni Y. In vitro premature termination in SV40 late transcription. EMBO J. 1983;2(2):185–191. doi: 10.1002/j.1460-2075.1983.tb01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik-David H., Aloni Y. Pausing of RNA polymerase molecules during in vivo transcription of the SV40 leader region. EMBO J. 1983;2(2):179–184. doi: 10.1002/j.1460-2075.1983.tb01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik-David H., Hay N., Aloni Y. Site of premature termination of late transcription of simian virus 40 DNA: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1982 May;79(9):2743–2747. doi: 10.1073/pnas.79.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N. Segments of simian virus 40 DNA spanning most of the leader sequence of the major late viral messenger RNA are dispensable. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2556–2560. doi: 10.1073/pnas.76.6.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tweeten K. A., Molloy G. R. Induction of premature termination of transcription of the mouse beta-globin gene by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB). Nucleic Acids Res. 1981 Jul 24;9(14):3307–3319. doi: 10.1093/nar/9.14.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]






