Abstract
SUMMARY
Pore-forming toxins (PFTs) are the most common bacterial cytotoxic proteins and are required for virulence in a large number of important pathogens, including Streptococcus pneumoniae, group A and B streptococci, Staphylococcus aureus, Escherichia coli, and Mycobacterium tuberculosis. PFTs generally disrupt host cell membranes, but they can have additional effects independent of pore formation. Substantial effort has been devoted to understanding the molecular mechanisms underlying the functions of certain model PFTs. Likewise, specific host pathways mediating survival and immune responses in the face of toxin-mediated cellular damage have been delineated. However, less is known about the overall functions of PFTs during infection in vivo. This review focuses on common themes in the area of PFT biology, with an emphasis on studies addressing the roles of PFTs in in vivo and ex vivo models of colonization or infection. Common functions of PFTs include disruption of epithelial barrier function and evasion of host immune responses, which contribute to bacterial growth and spreading. The widespread nature of PFTs make this group of toxins an attractive target for the development of new virulence-targeted therapies that may have broad activity against human pathogens.
INTRODUCTION
Bacterial infections are a leading cause of morbidity and mortality worldwide, and bacteria can cause infections in nearly all host tissues. Furthermore, health care-associated urinary tract infections, pneumonia, skin and soft tissue infections, invasive bloodstream infections, and surgical-wound infections are increasingly common (1, 2). The usual method of treating bacterial infections is by local or systemic administration of broad-spectrum antibiotics. Excessive use of antibiotics is, however, common practice in many countries and is a leading cause of the rise of multidrug-resistant pathogenic bacterial strains (3).
Several well-known pathogenic bacteria have developed into highly antibiotic-resistant strains. Examples are Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, and Mycobacterium tuberculosis (1, 2). A common denominator of these drug-resistant strains, as well as of many other major pathogenic bacteria, is that they employ pore-forming toxins (PFTs) as virulence factors. PFTs are common among bacteria, and about 25 to 30% of cytotoxic bacterial proteins are PFTs, making them the single largest category of virulence factors (4, 5). Because of their nearly universal presence in bacterial pathogens, PFTs are a unique and important target for research into novel, broadly applicable antimicrobial prophylactics and therapeutics.
PFTs function to perforate membranes of host cells, predominantly the plasma membrane but also intracellular organelle membranes (6). They are classically hypothesized to do so in order to directly kill target cells, for intracellular delivery of other bacterial or external factors (7, 8), to release nutrients (9), or for phagosomal escape in the case of intracellularly acting PFTs (10). Loss of their PFTs generally causes pathogenic bacteria to be less virulent or completely avirulent (see below and Table 1). Conversely, transgenic expression of a PFT can turn an otherwise harmless bacterium into a parasite or a pathogen (224, 225).
TABLE 1.
PFTs with in vivo data supporting a role in bacterial virulence
| Species | PFT | Classa | Reference(s) for pore formationb | Reference(s) for in vivo virulencec |
|---|---|---|---|---|
| Actinobacillus pleuropneumoniae | ApxI | RTX | 11 | 12–14 |
| ApxII | RTX | 11 | 12–14 | |
| ApxIII | RTX | 11 | 11 | |
| ApxIV | RTX | 15 | 15 | |
| Aeromonas hydrophila | Aerolysind | β | 16, 17 | 18–20 |
| Hemolysin (HlyA) | 21 | 18, 20 | ||
| Aerolysin cytotoxic enterotoxin (ACT)d | 22 | 23 | ||
| Arcanobacterium pyogenes | Pyolysin (PLO) | CDC | 24, 25 | 26, 27 |
| Bacillus anthracis | Protective antigen (PA) | β | 28 | 29–31 |
| Anthrolysin O (ALO) | CDC | 32, 33 | 34 | |
| Bacillus cereus | Nonhemolytic enterotoxin (Nhe) | RTX | 35, 36 | 37 |
| Hemolysin BL (HBL)e | 38, 39 | 38, 40 | ||
| Cytotoxin K (CytK) | β | 41, 42 | 37 | |
| Hemolysin II (HlyII) | β | 43 | 37 | |
| Bacillus sphaericus | Sphaericolysin | CDC | 44 | 44 |
| Binary toxin (Bin) | β | 45–47 | 45 | |
| Bacillus thuringiensis | Crystal (Cry) toxins | α | 48–55 | 56–58 |
| Cytolytic (Cyt) toxins | 56, 59 | 56 | ||
| Bordetella pertussis | Adenylate cyclase toxin (ACT/CyaA)f | RTX | 60, 61 | 62, 63–66 |
| Clostridium bifermentans | Crystal (Cry) toxins | α | 67, 68 | 67 |
| Clostridium botulinum | Botulinolysin (BLY) | CDC | 69, 70 | 71, 72 |
| Clostridium perfringens | NetB | β | 73 | 73–75 |
| β-Toxin | β | 76 | 77, 78 | |
| ε-Toxin (ETX) | β | 79, 80 | 81, 82 | |
| Perfringolysin (PFO, θ-toxin) | CDC | 83–85 | 86, 87 | |
| Enterotoxin (CPE) | β | 88 | 89 | |
| Clostridium septicum | Alpha-toxin | β | 90 | 91–93 |
| Clostridium tetani | Tetanolysin | CDC | 94 | 95 |
| Enterococcus faecalis | Cytolysin (Cly)g | 96, 97 | 96, 97 | |
| Escherichia coli | Hemolysin A/α-hemolysin (HlyA) | RTX | 4 | 98–103 |
| Gardnerella vaginalis | Vaginolysin (VLY) | CDC | 104 | 105, 106 |
| Helicobacter pylori | TlyA | 107 | 107 | |
| VacAh | β | 108 | 109 | |
| Listeria monocytogenes | Listeriolysin O (LLO)i | CDC | 10, 110 | 111–116 |
| Moraxella bovis | MbxA | RTX | 117, 118 | 117 |
| Mycobacterium marinum | 6-kDa early secreted antigenic target (ESAT-6) | 119 | 119–121 | |
| Mycobacterium tuberculosis | 6-kDa early secreted antigenic target (ESAT-6) | 122, 123 | 121, 124 | |
| Pseudomonas aeruginosa | Exotoxin A (ETA)j | α | 16 | 125–130 |
| Salmonella entericak | Cytolysin A (ClyA) | α | 131 | 132, 133 |
| Serratia marcescens | Hemolysin (Shla) | 134–136 | 137–139 | |
| Staphylococcus aureus | Panton-Valentine leukocidin (PVL) | β | 140–142 | 143–149 |
| Alpha-toxin/α-hemolysin | β | 150, 151 | 152–160 | |
| LukGH/LukAB | β | 140, 161, 162 | 161 | |
| LukED | 163 | 164 | ||
| γ-Hemolysin | β | 142, 165, 166 | 152, 154, 167, 168 | |
| Streptococcus agalactiae (GBS) | CAMP factor (cocytolysin) | 169 | 170, 171 | |
| β-Hemolysin/cytolysin (β-h/c) | 172–174 | 175–179 | ||
| Streptococcus pneumoniae (Pneumococcus) | Pneumolysin (PLY) | CDC | 180 | 177, 181–185 |
| Streptococcus pyogenes (GAS) | Streptolysin O (SLO) | CDC | 5, 186 | 187–193 |
| Streptolysin S (SLS) | 194 | 188, 189, 191, 195, 196 | ||
| Streptococcus uberis | CAMP factor | 197 | 198 | |
| Vibrio cholerae | Cytolysin (VCC) | β | 199–202 | 203–210 |
| MARTXl | RTX | 211 | 207–210, 212 | |
| Vibrio parahaemolyticus | Thermostable direct hemolysin (TDH) | 213–216 | 217–219 |
α, α-PFT; β, non-CDC β-PFT; RTX, RTX family PFT; CDC, β-PFT of CDC subclass. If the field is blank, the PFT falls into a unique category, or no clear classification could be found in the literature. See the text for further details on the classifications.
References describe direct proof of pore formation, where available; otherwise, they contain considerable circumstantial evidence.
References describe a role in in vivo infection models or in virulence for the purified toxin or contain evidence that the PFT is expressed during infection.
Aerolysin and Act have been argued to be one and the same toxin (220).
HBL is a tripartite toxin consisting of three separately secreted proteins.
ACT is a fusion of a PFT and a calmodulin-activated adenylate cyclase enzyme.
Cly is a two-peptide toxin that is unique in that it is both a hemolytic toxin and a bacteriocin.
VacA is hypothesized to be an A-B toxin with a pore-forming instead of an enzymatic A subunit. The pore-forming domain is delivered to mitochondria of epithelial cells and induces apoptosis (221).
LLO is the subject of a recent, detailed review (222).
ETA is a fusion of a PFT and a toxin that inhibits protein translation; although there is a fair body of in vivo data, it is unclear which of ETA's effects are caused by its PFT function.
Exclusively serovars Typhi and Paratyphi A.
MARTX is an RTX family member but may not have pore-forming capability.
PFT Mechanism of Action
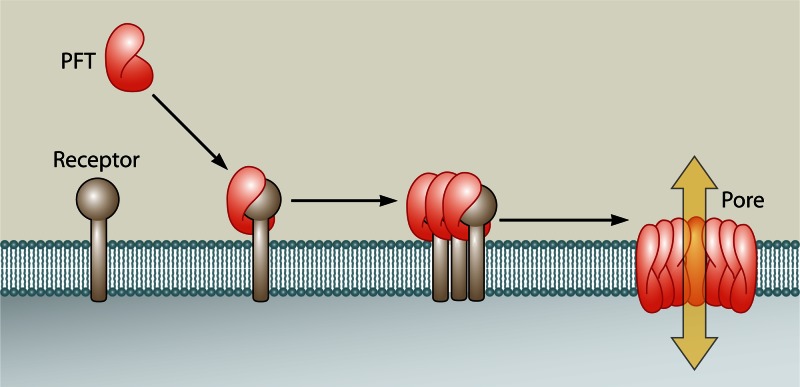
PFTs are generally secreted as water-soluble molecules. Recognition and binding to a specific receptor cause them to associate with the target membrane, form multimers, and undergo a conformational change, leading to the formation of an aqueous pore in the membrane (16, 226) (Fig. 1).
Fig 1.
Generalized mechanism of pore formation by PFTs. Soluble PFTs bind membrane receptors, which leads to oligomerization and insertion of an aqueous pore into the plasma membrane (5). Note that during the oligomerization step, some PFTs remain associated with their receptor, whereas others have already disassociated at this point.
PFTs can be classified based on the secondary structure of the regions that penetrate the host cell plasma membrane, which generally consist of either α-helices or β-barrels, and the specific toxins may be referred to as α-PFTs or β-PFTs. The majority of bacterial PFTs are β-PFTs, which also form the most-studied group (227). The large-pore-forming cholesterol-dependent cytolysins (CDCs), produced by Gram-positive and some Gram-negative bacteria, are a β-PFT subclass (4, 227, 228). The small-pore-forming repeat in toxin (RTX) toxins, produced by Gram-negative bacteria, form a large group of PFTs, but their classification and mechanism of pore formation remain unclear (4). Another useful form of classification, especially with regard to host defenses, is by the size of the pore that is formed (227). Bacterial PFTs generally form either small (0.5 to 5 nm) or large (20 to 100 nm) pores, and host cellular defenses against the different classes only partially overlap (229–232). Examples of the different classes of PFTs are shown in Fig. 2.
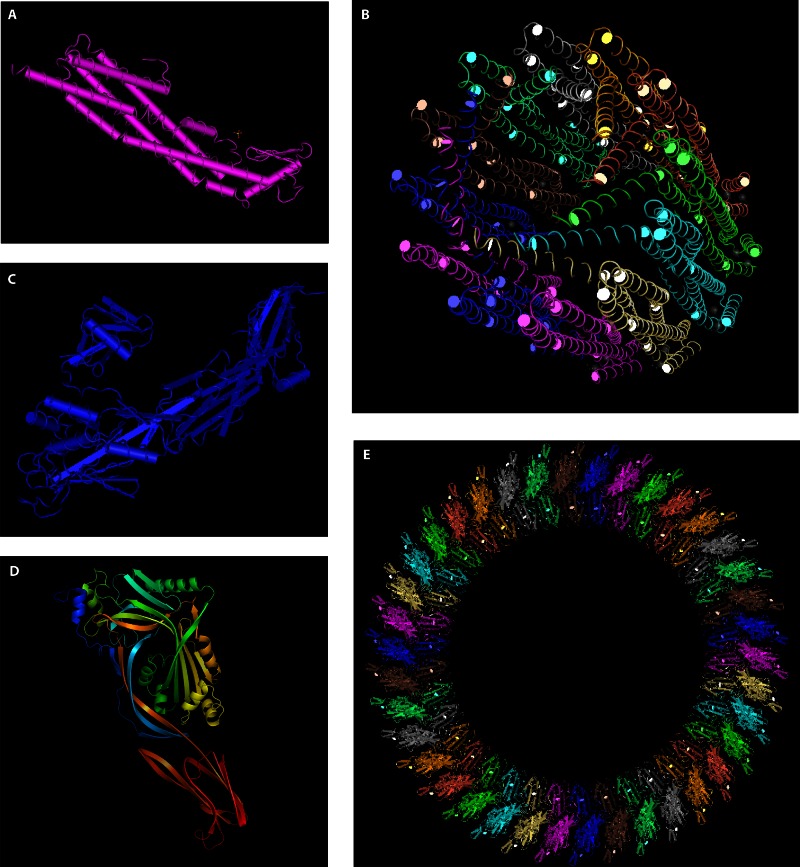
Fig 2.
Protein structures of various PFT classes. (A and B) Structures of a single molecule (A) and an assembled pore (B) of the E. coli α-PFT HlyE (295, 296) (Protein Data Bank [PDB] accession number 1QOY). (C) Structure of aerolysin, a β-PFT produced by A. hydrophila (485) (PDB accession number 1PRE). (D) PFO monomer (83) (PDB accession number 1PFO). (E) Hypothetical arrangement of CDC monomers into an assembled pore. This image was created by mapping PFO monomers onto a PLY cryo-electron microscopy (cryo-EM) image (180) (PDB accession number 2BK1). Structures were visualized using PyMOL (D) or MMDB (486) (A to C and E).
Various host cell receptors for PFTs have been identified, including glycosylphosphatidylinositol (GPI)-anchored proteins, other membrane proteins (e.g., ADAM10 and CCR5), lipids, and cholesterol (5, 233, 234). The target cell tropism of PFTs varies widely, one important cause of which is their various receptor specificities, and host or cell type specificity can be altered in vitro by genetic modification of PFTs (235–237).
Many bacteria produce toxins that are presumed to be PFTs based on their sequence or properties. A number of reviews have been written that extensively cover the biochemical properties and in vitro effects of bacterial and eukaryotic PFTs (4–6, 9, 16, 227, 229, 238, 239). For numerous human-pathogenic bacteria, as well as several economically important bacteria that are pathogenic to animals, there is direct proof or considerable circumstantial evidence that a PFT is expressed during infection and contributes significantly to virulence in vivo. We have listed such bacteria in Table 1. For a smaller group of bacteria, there are also in vivo data on the mechanisms through which their PFTs contribute to infection. After a summary of known PFT defense mechanisms, this review focuses on the contributions of PFTs to infections by 10 of the most-studied PFT-wielding bacterial pathogens, as determined by the study of in vivo and ex vivo infection models and clinical and epidemiological data. Although effects of PFTs are highly diverse, a number of common themes could be identified and provide the structure for this review. Relevant background information on the 10 bacteria is provided in Table 2.
TABLE 2.
Background information on bacteria and PFTs
| Species | Associated diseases | PFTs | Additional background | References |
|---|---|---|---|---|
| Streptococcus pneumoniae | S. pneumoniae is the leading cause of pneumonia, meningitis, otitis media, and bacteremia and a frequent cause of infection–related mortality in infants and the elderly. In 2000, it was responsible for an estimated 14.5 million serious infections worldwide and for 11% of all deaths in children under 5 years of age. | Pneumolysin (PLY), a CDC, is produced by nearly all clinical isolates. PLY is produced in vivo during infection and is required for virulence. Immunization against purified PLY protects against infection. | In certain host models, strains without PLY are 100–fold less virulent and grow at lower rates in vivo than strains with PLY, indicating that immunosuppression by PLY may stimulate growth directly or that the tissue damage provides conditions favorable for S. pneumoniae growth. | 180, 181, 184, 240–243 |
| Streptococcus pyogenes (GAS) | GAS causes skin and other soft tissue diseases (e.g., impetigo, pharyngitis, erysipelas, and cellulitis). Less frequently, it destroys fascia, adipose tissue, and muscle and can then be associated with necrotizing fasciitis and myositis. Untreated infections can cause acute rheumatic fever and subsequent rheumatic heart disease. Cellulitis, necrotizing fasciitis, or myositis can progress into toxic shock syndrome. In rare cases, GAS can cause bacteremia, endocarditis, or meningitis. | GAS produces streptolysin O (SLO), a PFT of the CDC family, and streptolysin S (SLS), a small-pore former. Both are important virulence factors during the early stages of infection. GAS can also produce CAMP factor, a PFT normally associated with group B Streptococcus, in which it may contribute to virulence. | 5, 169, 186, 189, 193–195, 244–249 | |
| Streptococcus agalactiae (GBS) | GBS is the leading cause of severe, invasive bacterial infection in neonates. In early-onset disease, it is vertically transferred from the mother and may cause pneumonia, bacteremia, and septic shock in the newborn. Late-onset disease may occur in infants up to several months of age and is characterized by a bacteremia that often progresses to meningitis and may give long-term neurological complications (seizures, cognitive impairment, or hearing loss). GBS is traditionally considered a neonatal pathogen, but increasingly, it is also an important cause of morbidity among pregnant women, nonpregnant adults with underlying medical conditions, and adults over 65 years of age. | GBS has two known PFTs, CAMP factor (named after Christie, Atkins, and Munch Petersen, who first described it) and β-hemolysin/cytolysin (β-h/c). The cylE gene is required for the production of β-h/c and the pigment granadaene. The pigment and toxin are often treated as two separate entities, but they remain to be separated convincingly. | GBS possesses a two-component signaling system, CsrRS. Inactivation of the csrR gene results in decreased expression of cfb, the gene encoding CAMP factor, and simultaneous increased expression of cylE, the gene responsible for β-h/c and granadaene, as well as increased hemolytic activity. When mice were challenged by intraperitoneal injection, strains with increased hemolytic activity showed reduced in vivo virulence. This may be due to decreased CAMP factor levels, but the pathogenicity of GBS lacking cfb (with cylE still present) was similar to that of isogenic, wild-type controls. | 169, 172–174, 178, 250–256 |
| Staphylococcus aureus | S. aureus is the leading cause of bacterial infections in the United States and a major cause of morbidity and mortality worldwide. It mostly causes skin and soft tissue infections but can cause severe invasive infections, including fatal sepsis and necrotizing fasciitis. It is likely best known for the potentially lethal necrotizing pneumonia caused by MRSA. S. aureus stands out for its ability to evade the immune system and its ability to infect fully immunocompetent hosts. The most-studied strains are Newman and MRSA strains USA400 and USA300. | S. aureus possesses multiple small-pore β-PFTs: alpha-toxin (or α-hemolysin), γ-hemolysins, LukHG (LukAB), LukED, and Panton-Valentine leukocidin (PVL). PVL and γ-hemolysins are expressed in vivo during infection, and all of these PFTs contribute to virulence. PVL is expressed by clinical isolates that cause necrotizing pneumonia, including USA300, and therefore has been studied extensively. However, the more abundant alpha-toxin has been argued to be the main virulence factor in MRSA pneumonia. | PVL, γ-hemolysins, LukED, and LukGH are bicomponent leukocidins. The three-gene hlg locus encodes one F and two S components that allow expression of two functional γ-hemolysins. The class S and F components of PVL and γ-hemolysins are able to recombine with each other, and all six possible cross-combinations cause inflammation and dermonecrosis, complicating interpretations of in vivo data. | 140, 142–145, 148, 155, 161, 162, 226, 257, 258–265 |
| Bacillus anthracis | B. anthracis is the causative agent of anthrax. Entry of spores into the body can cause cutaneous, gastrointestinal, or pulmonary infection. Early diagnosis of gastrointestinal and pulmonary forms is difficult, and these often develop into untreatable, fatal systemic infections, hallmarked by shock-like symptoms, sepsis, and respiratory failure. | The two main virulence factors of B. anthracis are the capsule and anthrax toxin. Anthrax toxin, responsible for the lethal toxic shock, consists of three components, namely, protective antigen (PA), lethal factor (LF), and edema factor (EF). PA is a small-pore β-PFT, whose main role is to mediate translocation of LF or EF into the target cell cytosol. EF then disrupts water homeostasis, and LF disrupts MAPK pathways. B anthracis also expresses anthrolysin O (ALO), a CDC. | The Bacillus cereus group (group 1 bacilli) consists of the closely related species B. cereus, B. anthracis, and B. thuringiensis (Table 1), which are often argued to be a single species. They differ mainly in the toxins they produce and have very different pathogenic properties. Normally harmless B. subtilis can convert to a pathogen when equipped with a B. thuringiensis PFT. This illustrates that pathogenicity, host specificity, and tissue preference can depend strongly on PFTs. | 7, 28, 32–34, 37, 225, 266–271 |
| Clostridium spp. | Many Clostridium species are pathogenic, and infections are often contracted via contaminated food or wound contamination. Although easily treatable and rare, these infections exhibit high mortality rates when improperly treated or untreated. C. tetani causes tetanus. C. perfringens causes myonecrosis (gas gangrene), dysentery, and enterotoxemia and has been implicated in enterocolitis. C. septicum causes enteric and wound infections (malignant edema), but the most lethal human diseases are nontraumatic myonecrosis and necrotic enteritis. C. botulinum causes botulism, which is hallmarked by muscular paralysis. | C. tetani tetanolysin is capable of locally damaging tissues, but the clinical syndrome of tetanus is caused by tetanus toxin. C. perfringens has the CDC perfringolysin O (PFO, or θ-toxin) and the β-PFTs β-toxin, ε-toxin (ETX), NetB, and enterotoxin (CPE), which are not all present in a single strain. C. septicum has alpha-toxin (O2-stable hemolysin) as well as the potential PFTs β-toxin (DNase and leukocidin) and δ-toxin (O2-labile hemolysin). C. botulinum has botulinolysin (BLY) but is better known for non-PFT neurotoxins, which are the cause of its clinical manifestations. | 69, 70, 73, 76, 79, 80, 83–85, 88, 90, 92, 94, 272, 273–278 | |
| Listeria monocytogenes | L. monocytogenes causes listeriosis, a rare but dangerous infection usually due to contaminated food. Immunocompetent individuals often develop febrile gastroenteritis. Immunocompromised adults can develop invasive listeriosis, characterized by septicemia or meningoencephalitis. Pregnant women are at increased risk for invasive listeriosis, which may be transmitted vertically to the fetus, resulting in fetal demise or invasive neonatal infection. The related species L. ivanovii is a pathogen mainly of ruminants. | L. monocytogenes has a CDC, listeriolysin O (LLO), and L. ivanovii has the closely related ivanolysin O (ILO). Pore formation has been shown for LLO, and the classification of ILO is based on sequence similarity. | The divergent virulence model of L. monocytogenes makes it questionable how much of the observed in vivo functions of LLO extend to other PFTs. One important exception may be Mycobacterium tuberculosis ESAT-6, which appears to function similarly to LLO. Additionally, the use of a mouse model to study listeriosis has shortcomings that restrict its applicability to human disease. | 110, 279–283 |
| Mycobacterium tuberculosis | M. tuberculosis causes tuberculosis, the seventh highest cause of mortality worldwide and the number one cause of death by a bacterial agent. Treatment is challenged by its chronic, often asymptomatic infection, exacerbation by HIV infection, and rising resistance to antibiotics. Mycobacterium bovis (a member of the M. tuberculosis complex) and M. marinum are closely related species that cause tuberculosis and tuberculosis-like disease in cattle and aqueous vertebrates, respectively, and that occasionally cause human disease as well. | Mycobacterium species all express the 6-kDa early secreted antigenic target (ESAT-6), which physically interacts with lipid bilayers, disrupts currents across and eventually destroys artificial membranes, allows a non-membrane-permeative dye to enter cells, and causes hemolysis that is blocked by osmoprotectants. This strongly argues that ESAT-6 is a PFT. | ESAT-6 expression is closely coupled to expression of the 10-kDa culture filtrate protein (CFP-10), which belongs to the same protein family as ESAT-6 and physically interacts with it. Both proteins bear no resemblance to any identified protein family. The M. bovis vaccine strain bacillus Calmette-Guérin (BCG) has attenuated virulence due to mutation of the RD1 region, which contains the ESAT6 and CFP-10 genes. This led to the identification of ESAT-6 as an important virulence factor of M. tuberculosis. | 119, 122, 123, 284–294 |
| Escherichia coli | E. coli is usually a harmless commensal in the gut, but it occasionally causes infections. Diarrheagenic E. coli such as enterohemorrhagic E. coli (EHEC) causes diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome. Treatment of EHEC with antibiotics is difficult because it causes increased release of the Shiga-like toxin associated with hemolytic-uremic syndrome, which can result in acute renal failure. Extraintestinal pathogenic E. coli (ExPEC) strains include uropathogenic E. coli (UPEC), which causes urinary tract infections, sepsis, and meningitis. ExPEC is also a common cause of nosocomial pneumonia. | E. coli has two PFTs, hemolysin A (HlyA; also called α-hemolysin), an RTX toxin, and hemolysin E (HlyE; also called cytolysin A [ClyA] or silent hemolysin A [SheA]), usually classified as an α-PFT. Multiple copies of HlyA may be present in UPEC, but within a single ExPEC strain, HlyA and HlyE usually do not occur together. O157, the most common EHEC serotype, possesses a third toxin, EHEC toxin (Ehx, or enterohemolysin [Ehly]). | 4, 100, 295–304 | |
| Vibrio cholerae | V. cholerae is the causative agent of the diarrheal disease cholera, which, if left untreated, leads to lethal dehydration and shock. Cholera is endemic in more than 50 countries worldwide, affecting 3 to 5 million people each year, with reported case fatality rates reaching 50% in some areas. The best-known serogroups, responsible for the currently ongoing pandemics, are O1 and O139. | Cholera toxin (CT) and the toxin-coregulated pilus (TcpA) are considered the main virulence factors of V. cholerae, but it has one confirmed PFT, V. cholerae cytolysin (VCC). V. cholerae δ-thermostable hemolysin (Vc-δTH) is a proposed PFT. Multifunctional, autoprocessing RTX toxin (MARTX) is an RTX family member but is unlikely to form pores. | VCC plays a role in infection in several in vivo models (Table 1). Compared to other V. cholerae virulence factors, VCC induces an especially strong T-cell proliferative response during human infection with O1. | 200–202, 211, 305–308 |
PFT EFFECTS AND CELLULAR DEFENSE MECHANISMS
The search for mechanisms of action shared by PFTs has involved predominantly in vitro studies on simplified target systems (lipid bilayers, cultured cells, and primary cells) and studies on the in vivo model involving the nematode Caenorhabditis elegans and the Bacillus thuringiensis crystal toxin PFT Cry5B. These studies have led to an understanding of the molecular requirements for attack, oligomerization, and pore formation. In addition, a number of important host defense and cell death pathways and membrane repair mechanisms have been identified. Nonetheless, several caveats deserve consideration in interpreting these studies, some of which extend to studies discussed in the section on in vivo PFT effects. First, because these studies use purified toxin, cells may be exposed to artificially high doses of toxin. Such doses may not be physiologically relevant and hence could result in responses that are not reflective of those seen during an infection. Second, purified PFTs may behave differently in isolation compared to their behavior in the presence of their pathogen or additional virulence factors. Third, PFTs can affect expression of other genes in their source bacteria (143) and hence may play roles that extend beyond their cytotoxic properties. Lastly, it can be unclear whether an observed response benefits the host, the pathogen, or both.
MAPK Pathways
Using C. elegans and Cry5B, the first functional molecular PFT defense pathways were identified, involving p38 mitogen-activated protein kinase (MAPK) and the c-Jun N-terminal (JNK)-like MAPK KGB-1 (309). p38 MAPK was shown to be important in mammalian cells in defense against the Aeromonas hydrophila PFT aerolysin and S. aureus alpha-toxin (230, 309). p38 activation is seen in vivo with B. thuringiensis Cry toxins and in vitro with numerous PFTs, including S. pneumoniae pneumolysin (PLY), S. aureus alpha-toxin, group A streptococcus (GAS) streptolysin O (SLO), Bacillus anthracis anthrolysin O (ALO) (230, 310), Gardnerella vaginalis vaginolysin (VLY) (104), A. hydrophila aerolysin (309), Listeria monocytogenes listeriolysin O (LLO) (311), and Lactobacillus iners inerolysin (ILY) (312). p38 was further shown to be activated by and required for defense against Cry toxin in vivo in the lepidopteran Manduca sexta and the dipteran Aedes aegypti (313). The activation of p38 in response to PFT is thus evolutionarily strongly conserved. Activity of JNK and extracellular signal-regulated kinase (ERK) MAPKs was also identified on several occasions in vitro (M. tuberculosis 6-kDa early secretory antigenic target [ESAT-6] [314], S. pneumoniae PLY [315], GAS SLO [316], A. hydrophila aerolysin, and L. monocytogenes LLO [311]) and in vivo, in C. elegans (with B. thuringiensis Cry5B) (231). Activator protein 1 (AP-1; Fos/Jun), functioning downstream of JNK, is involved in C. elegans in defenses against PFTs that form small pores (Cry5B) as well as against PFTs that form large pores (SLO). A role for AP-1 in defense against SLO was confirmed in vitro in mammalian cells (231). LLO and B. anthracis protective antigen (PA; a component of anthrax toxin and a PFT [Table 2]) can activate ERK, and both p38 and ERK can function to restore potassium homeostasis in cells damaged by PFTs (311). Thus, the p38, JNK, and perhaps ERK MAPK pathways are arguably the main mediators of physiological PFT defense pathways.
Potassium Efflux-Dependent Defenses, Including Inflammasome Activation
An important consequence of pore formation by PFTs is the efflux of cellular potassium. Aerolysin-induced potassium efflux was found to induce the activation of the Nod-like receptor pyrin domain-containing 3 (NLRP3) inflammasome and cysteine-aspartic protease 1 (caspase-1). Caspase-1 activates sterol regulatory element-binding proteins (SREBPs), which are central regulators of membrane lipid biogenesis, contributing to cellular survival (317). PFTs can also trigger apoptosis, which in the case of S. aureus alpha-toxin and aerolysin is dependent upon caspase-2. Preventing the PFT-associated efflux of potassium inactivated caspase-2, and inhibition of caspase-2 inhibited PFT-induced apoptosis (318). Potassium efflux was also found to be required for PFT-induced autophagy (311, 319) and to mediate p38 MAPK activation by S. aureus alpha-toxin, Vibrio cholerae cytolysin (VCC), SLO, and E. coli hemolysin A (HlyA) (320). As mentioned above, p38 and ERK activation promotes the recovery of disturbed potassium levels (311).
Other Cellular Defenses
The endoplasmic reticulum (ER) unfolded protein response (UPR) pathway is a key functional downstream factor of p38 MAPK in C. elegans defense against Cry5B, and the ER UPR was also activated in mammalian cells in response to aerolysin (321). The UPR may function to arrest protein synthesis, which has also been observed in vitro in response to L. monocytogenes LLO and B. anthracis PA, although in those cases the inhibition of protein synthesis occured independent of the UPR (311).
The hypoxia response pathway is also involved in C. elegans defense against B. thuringiensis Cry toxins (203). This pathway involves downregulation of hypoxia inducible factor 1α (HIF-1 in C. elegans) by prolyl hydroxylase, Von Hippel-Lindau tumor suppressor protein, and regulator of hypoxia-inducible factor (EGL-9, VHL-1, and RHI-1, respectively, in C. elegans). Mutations in egl-9, vhl-1, and rhi-1, which increase the activity of the hypoxia pathway, lead to resistance to Cry toxins, whereas a mutation in hif-1, which decreases pathway activity, leads to Cry toxin hypersensitivity. These results extended to the V. cholerae PFT cytolysin (VCC). Interestingly, however, whereas activity of the hypoxia pathway protected against a V. cholerae strain with VCC, it caused hypersensitivity to V. cholerae lacking VCC. Thus, whereas host factors may protect against one type of virulence factor, they may cause hypersensitivity to others (203), causing the host to face a difficult challenge. VCC was also shown to cause formation of vacuoles in C. elegans intestinal cells, consistent with earlier in vitro observations (204). The role of these vacuoles remains unclear.
Another pathway found to play a role in PFT defense is the insulin/insulin-like growth factor 1 (IGF-1) pathway. Loss of the insulin receptor, DAF-2, causes C. elegans to become resistant to Cry5B. This effect was found to depend not only on the canonical downstream forkhead transcription factor DAF-16 but also on a novel pathway arm involving WW domain protein 1 (WWP-1) (322). α-Defensins have been found to function in PFT defense in vitro (323).
Calcium-Dependent Membrane Repair Mechanisms
In addition to an efflux of potassium, PFT membrane pores often result in an influx of calcium. Ca2+ influx is a known trigger of apoptosis (324), a PFT response that has been observed in various cell types (238), and it can affect the vesicle trafficking machinery. GAS SLO-induced calcium influx triggers the exocytosis of lysosomes and extracellular release of the lysosomal enzyme acid sphingomyelinase. Acid sphingomyelinase was found to subsequently induce endocytosis, which contributed to membrane repair (325, 326). During endocytosis, PFT pores are taken up into the cells, ubiquitinated, and then, through activity of the ESCRT machinery, targeted to lysosomes for degradation (327). S. aureus alpha-toxin also enters cells via endocytosis, is transported via late endosomes, and then disappears from the cells. Alpha-toxin multimers, however, were not broken down in acidic compartments but were expelled from cells via exosome-like vesicles called toxosomes (328).
A recent study showed that vesicle trafficking pathways also protect cells against PFTs in vivo. Intoxication of C. elegans by Cry5B and V. cholerae VCC was found to trigger increased rates of endocytosis in intestinal cells. Loss of either of the two key Rab proteins (RAB-5 and RAB-11), master regulators of early endosome and recycling endosome functions, resulted in significant decreases in Cry5B-induced endocytosis in intestinal cells. Loss of RAB-5 and RAB-11 furthermore resulted in strong hypersensitivity of C. elegans to Cry5B, and both were required to restore the integrity of the plasma membranes of intestinal cells following Cry5B attack. This demonstrates a correlation between RAB-5, RAB-11, PFT-induced endocytosis, restoration of plasma membrane integrity, and survival of the whole organism. RAB-11 was additionally found to be required for PFT-induced expulsion of microvilli from the enterocyte cell surface, which is hypothesized to be part of the membrane repair mechanism and is also observed in vitro with other PFTs, including Vibrio parahaemolyticus thermostable direct hemolysin and GAS SLO (329–331).
ROLE OF BACTERIAL PFTs IN INFECTION AND HOST RESPONSES TO PFTs IN VIVO
Innate Immune Responses to PFTs
The innate immune system recognizes specific pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs) and Nod-like receptors (NLRs), which results in the triggering of innate immune responses, including cytokine signaling, phagosome maturation, inflammasome activation, and autophagy (332). The TLRs generally signal via the adaptor myeloid differentiation primary response gene 88 (MyD88) and several downstream cascades, including the p38 and JNK MAPK and NF-κB pathways, resulting in the expression of proinflammatory cytokines (333). The inflammasome is a multiprotein complex that can be activated by PRRs as well as other factors, such as potassium efflux (see PFT Effects and Cellular Defense Mechanisms). The inflammasome (the best-studied one is the NLRP3 inflammasome) is involved in activation of caspase-1, which promotes the maturation of interleukins (334).
The main cytokines studied in vivo in the context of PFTs are tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, which are produced mainly by mononuclear phagocytes (335). TNF-α is strongly induced by lipopolysaccharides (LPS) via TLR2 and TLR4, and it activates neutrophils and attracts granulocytes. IL-1β is activated by caspase-1; it has TNF-α-like effects and additionally induces proliferation of lymphocytes (335).
IL-6 is capable of stimulating both proinflammatory and anti-inflammatory signaling pathways, dependent upon a soluble receptor and signaling via the trans-signaling pathway and dependent on a membrane-bound receptor and signaling via the classic pathway, respectively (336, 337). IL-6 stimulates B-cell differentiation, activates T cells, and has several other proinflammatory effects (335). There is abundant evidence to suggest that PFTs induce IL-6 production, with subsequent stimulation of both the classic and trans-signaling pathways (175, 338–340). In in vivo experiments, PFTs trigger shedding of receptors for IL-6 independent of pore formation, which broadens the spectrum of IL-6-responsive host cells and skews the IL-6 response to proinflammatory signaling (341). It is hypothesized that the shedding of the membrane-bound IL-6 receptor may be due to activation of cell membrane metalloproteinases following PFT-induced membrane lipid rearrangements or PFT activation of neutral sphingomyelinase and generation of ceramide (342). Note that ceramide production thus contributes to defense against PFT within a single cell (induced by acid sphingomyelinase [326]; see PFT Effects and Cellular Defense Mechanisms) and, additionally, may sensitize other cells via IL-6 receptor shedding.
IL-1β and TNF-α stimulate each other's production as well as that of IL-6, while IL-6 may inhibit IL-1β and TNF-α production (335).
Inflammation.
Inflammation often results in epithelial damage. Cases where this was specifically assessed are discussed in “PFT-Induced Barrier Dysfunction.”
When injected intravenously into mice, S. pneumoniae induced increased levels of systemic IL-6, whereas injection of a PLY-deficient strain resulted in lower IL-6 levels. The strain lacking PLY established a chronic bacteremia, whereas the strain with PLY grew exponentially and quickly caused sepsis, likely induced by gamma interferon (IFN-γ). Interestingly, if a stable bacteremia was established with a PLY-deficient strain, later addition of PLY was unable to escalate this into sepsis, suggesting that the ability of PLY to affect the infection outcome in this model is limited to the first few hours of infection (343). Further work showed that the host resistance of mice developed during the first days after infection with the PLY-deficient strain depends on TNF-α and appears to be independent of IL-1β or IL-6. However, survival beyond the first days does depend on the ability to produce IL-1β (344).
The recently identified S. aureus PFT LukGH was found to cause skin inflammation in cynomolgus monkeys but to have little or no effect in mice and rabbits compared to that with Panton-Valentine leukocidin (PVL) (140). Skin abscesses in rabbits caused by LukGH-deficient and LukGH- and PVL-deficient strains were larger than those caused by USA300 controls, whereas an earlier study found that deletion of alpha-toxin led to formation of smaller abscesses (140, 161, 162, 345). In vitro, LukGH was cytotoxic to polymorphnuclear leukocytes (PMNs) from mice, rabbits, monkeys, and humans (140).
Purified PVL causes inflammation of the rabbit eye and mouse lungs and necrosis of rabbit skin (144, 257, 346, 347), and by itself can induce pneumonia in mouse and rabbit models by recruiting and lysing PMNs (145, 146). In a mouse model of soft tissue infections with S. aureus, it was found that PVL caused muscle but not skin injury. PVL-induced muscle injury was associated with increased levels of the proinflammatory keratinocyte-derived chemokine (KC), macrophage inflammatory protein 2 (MIP-2), and chemokine (C-C motif) ligand 5 (CC5; also called RANTES); TNF-α and IL-1β levels were unaffected (143). Animals that showed more severe lesions induced by PVL also showed increased MIP-2 and KC chemokine responses, showing that the damage was induced by the inflammatory response rather than directly by the toxin. Interestingly, animals that responded to PVL with a stronger inflammatory response were better able to clear the infections. Thus, there appears to be a trade-off between how quickly the infection is removed and the amount of damage that is caused to the host's own tissue in the process (143).
S. aureus alpha-toxin and γ-hemolysins (the toxins encoded by the three-gene hlg locus) (Table 2) functioned redundantly in a mouse model of septic arthritis, and when both PFTs were present, IL-6 expression levels were significantly higher than when both were absent (152). (IL-6 is an activator of osteoclasts, and its release is correlated with joint damage in arthritis [152].) Alpha-toxin appeared to be responsible in part for the typical arthritis symptoms, inflammation, pannus formation, and cartilage destruction (153).
GAS SLO and S. aureus alpha-toxin cause degranulation of mast cells. SLO is associated with a quick and transient skin inflammation in infected mice, whereas alpha-toxin induces a chronic inflammation. Mast cells are required for SLO-induced inflammation, but they limit the alpha-toxin-induced inflammatory response. This difference is hypothesized to be caused by the different pore sizes of SLO (a large-pore CDC) and alpha-toxin (small-pore β-barrel PFT) (232).
When the hlg locus (γ-hemolysins) was removed from the S. aureus Newman strain, although the strain was attenuated, it was still able to produce strong inflammation in a rabbit corneal infection model (167). A later study using the same model and S. aureus Newman strain but also including an alpha-toxin mutant found that alpha-toxin and γ-hemolysins were required for full virulence in a rabbit corneal infection model and were responsible for inflammation and ocular damage. However, corneal erosion was specifically associated with alpha-toxin, and an additional, uncharacterized virulence factor was likely also present (154). When purified F and S components of PVL and the γ-hemolysins (Table 2) were injected in various combinations intravitreally into the rabbit eye, all caused inflammation, and to some extent necrosis, of the posterior and anterior chambers and conjunctiva, eyelids, and annexes. Although the speed and intensity of the effects varied with the components, all cross-combinations between PVL and γ-hemolysin caused inflammation and necrosis. Pairs involving the γ-hemolysin S component HlgA were most potent (257). Loss of γ-hemolysins in the methicillin-resistant S. aureus (MRSA) strain USA300 did not lead to altered abscess formation in a mouse skin infection model, but virulence to neutrophils was reduced in vitro (168).
To study the effects of E. coli HlyA on immune responses, mice were infected intraperitoneally with HlyA-positive and HlyA-negative E. coli strains. It was found that lethal infection with the HlyA-positive strain caused a rise in (proinflammatory) IL-1α and IL-1β levels but did not affect TNF-α levels (98, 348). No changes in IL-1α and TNF-α were observed during infection with an HlyA-negative (nonlethal) strain. In contrast, E. coli LPS caused an increase of IL-1α as well as TNF-α (independent of lethality), and inhibition of TNF-α activity prevented LPS-induced lethality (348). However, inhibition of TNF-α activity failed to prevent HlyA-mediated lethality (98, 348). Ex vivo, in the lamina propria of human intestinal tissue, histamine secretion in response to E. coli was HlyA dependent, consistent with an increased degranulation of mast cells. This was also found in vitro, where mast cell activation was dependent on Ca2+ influx and p38 and ERK MAPKs (349). In renal epithelial cells in vitro, HlyA was found to induce a constant, low-frequency calcium oscillation response. This response was dependent on L-type calcium channels and intracellular stores gated by inositol triphosphate, and it induced IL-6 and IL-8 production (350). L. monocytogenes LLO similarly induces calcium oscillations (110).
Nonpathogenic E. coli J198 transformed to produce a high, moderate, or no level of HlyA was used for intraperitoneal challenge of rats. It was found that the presence of HlyA led to the formation of more and larger abscesses, a drop of the intraperitoneal pH (which remained unchanged in an infection with HlyA-negative E. coli), reduced viability of leukocytes, lysis of erythrocytes, and an impaired host defense as measured by the ability to remove other bacteria (Bacteroides fragilis) (99).
PRRs.
TLR2 is considered the main PRR for Gram-positive bacteria. One study showed a role for TLR2, but not TLR4, in clearance of S. pneumoniae infection (351). Another found that TLR2 is mostly dispensable for antibacterial defense, although it was found to play a role in the inflammatory response (352). In vitro, PLY directly binds TLR4 (353), and TLR4 appears to recognize other CDCs (ALO, PLY, LLO, SLO, and Clostridium perfringens perfringolysin O [PFO]) as well (354, 355). PLY stimulates TNF-α and IL-6 release from mouse macrophages, dependent on MyD88. Loss of TLR4 caused a diminished response to purified PLY, and TLR4 knockout mice were more susceptible than control mice to lethal infection after intranasal colonization (354). However, other studies found that TLR4 plays only a small role or is dispensable for the immune response to PLY (356, 357). In a mouse model of pneumonia, PLY-deficient S. pneumoniae can infect TLR2-deficient mice but not wild-type mice, consistent with a model where loss of TLR2 can be compensated for by PLY-induced TLR4 signaling (358). Nonetheless, the in vivo inflammatory response to purified PLY does appear to involve both TLR2 and TLR4 (359). Intranasally administered purified PLY resulted in inflammation in the lungs, i.e., an influx of neutrophils, release of proinflammatory cytokines and chemokines, and increased protein levels in bronchoalveolar lavage fluid (indicating barrier dysfunction). This inflammation was dependent on TLR4 but also, in part, on TLR2. At a low PLY dose, the level of inflammation was the same in wild-type, TLR2 knockout, and TLR4 knockout mice, and in all animals TNF-α and MIP-2 levels and total protein were upregulated, while IL-6 and IL-1β remained unchanged; KC was upregulated in control and TLR2 knockout mice but not in TLR4 knockout animals. At a high PLY dose, control animals showed macrophage and neutrophil influx, release of IL-6, IL-1β, TNF-α, and KC (MIP-2 was unaltered here), and increased total protein. In TLR4-deficient animals, fewer neutrophils, less IL-6, IL-1β, and KC, and lower total protein levels were observed. TLR2 knockout mice showed lower IL-6, KC, and total protein levels (359). TLR2 knockout mice exhibit a strongly reduced early inflammatory response during pneumonia caused by wild-type as well as PLY-deficient bacteria (352). In a mouse model of acute pneumonia, PLY promotes the production of the cytokines IFN-γ and IL-17A in the lungs, in a TLR4-independent manner (357). Purified PLY also induces production of IL-6, KC, and MIP-2. IL-6- and MIP-2-dependent influx of PMNs into the bronchoalveolar compartment requires PLY's cytotoxic properties (360).
A recent study found that sublytic concentrations of group B streptococcus (GBS) β-hemolysin/cytolysin (β-h/c) inhibited IL-12 and nitric oxide synthase 2 (NOS2) expression in mouse primary macrophages (361). β-h/c also activated JNK and p38 MAPKs, independently of TLR2, TLR4, the NLR protein NOD2, and the inflammasome. The suppression of proinflammatory IL-12 is dependent upon p38-induced expression of anti-inflammatory IL-10, while JNK, ERK, and IκB kinase (IKK; an activator of NF-κB) are dispensable for this effect. Although not affecting macrophage invasion or viability, the presence of β-h/c allows GBS to survive longer inside macrophages. Intraperitoneal infection with a wild-type or β-h/c mutant GBS strain in mice lacking p38 specifically in neutrophils and macrophages was performed. Surprisingly, loss of p38 in these cells increased the resistance of mice against invasive GBS infection (361). This contrasts with, for instance, the C. elegans-B. thuringiensis Cry5B model, where p38 is required for host (cell) defense (see PFT Effects and Cellular Defense Mechanisms).
The inflammation caused by S. aureus PVL or its LukS subunit was diminished in mice lacking cluster of differentiation 14 (CD14) and TLR2. In vitro experiments showed that PVL directly bound TLR2 and induced inflammation independent of pore formation (347).
Although TLR2 is the main receptor for Gram-positive bacteria, there are some Gram-positive infections for which TLR2 is dispensable. This phenomenon was studied in a mouse intraperitoneal infection model of L. monocytogenes. TLR2 knockout mice were found to respond normally to wild-type L. monocytogenes as well as to purified LLO but showed an impaired neutrophil response to LLO-deficient bacteria. Knockout mice for IL-1β, IL-18, and MyD88, on the other hand, were equally defective in their response to wild-type L. monocytogenes, LLO-deficient bacteria, and purified PFT. This suggests that although neutrophil recruitment in response to non-LLO bacterial factors is TLR2 dependent, LLO triggers recruitment independently of TLR2, through both MyD88-dependent and -independent pathways. Since the IL-1 receptor also uses MyD88 as an adaptor, it was suggested that an IL-1β–IL-18–MyD88 pathway, activated via the caspase-1-dependent inflammasome, mediates this response to LLO. No role for TLR4 was identified in the response to LLO (362).
V. cholerae VCC was found in vitro in mouse primary bone marrow-derived mast cells to be an agonist of TLR2, but not TLR3 or TLR4, that induced cytokine production (including that of IL-4, IL-6, and TNF-α) in a manner dependent on increased cytosolic Ca2+ (338).
Inflammasome.
Compared to control mice in a pneumonia model, NLRP3 knockout mice have a diminished ability to clear an infection with PLY-deficient S. pneumoniae and are completely incapable of clearing wild-type S. pneumoniae. In vitro, PLY induces IL-1β dependent on potassium influx, NLRP3, and phagosomal rupture. This suggests that during infection, NLRP3 protects against PLY-related aspects as well as against PLY-independent factors (357). Similar results were found in a contemporary study (363). IL-1β is also required for resistance to S. pneumoniae infection in mice (364).
Caspase-1 activation and IL-1β secretion were induced in macrophages by GAS, and they required expression of SLO. In vivo experiments in mice showed that the NLRP3 inflammasome is critical for IL-1β production but dispensable for survival in a GAS peritoneal infection model. Data further indicated that caspase-1 activation in response to GAS infection requires SLO and NF-κB but not TLR signaling (365). In vivo, murine macrophage expression of the cytokines TNF-α and IL-1β was suppressed by SLO (187).
In a mouse model of S. aureus pneumonia involving infection via the buccal cavity or intratracheally, alpha-toxin was found to induce IL-1β expression and acute pulmonary inflammation and injury, and these effects were abolished in NLRP3-deficient mice (366). The NLRP3 inflammasome did not control bacterial growth and did not affect the severity of the pneumonia, as bacterial burdens, body temperature, and attracted neutrophils were not influenced by inflammasome activity. IL-1 receptor-negative mice still showed lung injury, indicating that IL-1β is not likely to be responsible for this outcome (366).
Complement system.
Independent of its cytotoxic properties, S. pneumoniae PLY has the ability to activate the complement system, which is thought to lead to a depletion of serum opsonic activity (181). Intranasal challenge of mice with isogenic S. pneumoniae strains carrying point mutations in PLY to specifically target these two aspects showed that a defect in complement activation reduced virulence (including bacteremia) more strongly than a defect in cytotoxicity did (182). PLY's complement-activating property functions to reduce the accumulation of T cells, whereas its cytotoxicity increases neutrophil recruitment and contributes to T-cell suppression; neither has a major effect on the accumulation of B cells or macrophages (367). Simultaneously, PLY, aided by pneumococcal surface protein A (PspA), appears to be able to impair the complement system by inhibiting C3 deposition (368, 369).
Adaptive Immune Responses to PFTs
Several PFTs have been shown to be immunogenic, such as S. pneumoniae PLY, S. aureus alpha-toxin, M. tuberculosis ESAT-6, and L. monocytogenes LLO (370–373), but detailed studies on specific effects of PFTs on the adaptive immune system are scarce.
Acquired immunity to S. pneumoniae was long thought to be mediated through B-cell production of antibodies against its capsular polysaccharides but has more recently been found to be mediated mainly by major histocompatibility complex (MHC) class II-positive, CD4-positive T cells, possibly through the effects of PLY (351, 374, 375). T and B lymphocytes were found to be attracted to the site of infection, and the absence of PLY caused overall reduced bacterial growth as well as reduced recruitment of lymphocytes in vivo (376). A clinical study of CD4-positive T cells from previously exposed people showed that PLY, although not immunodominant, causes a distinct proinflammatory, Th1 profile of high IFN-γ, IL-12, and IL-17 levels and low IL-10 and IL-13 levels (377).
The Th17 response seen in mice in response to S. aureus lung infection is dependent, at least in part, upon alpha-toxin. Consistently, host IL-23 levels were found to be upregulated specifically in response to alpha-toxin in mice in vivo (also see “Other PFT Functions and Effects”) (378).
In addition to being a major virulence factor of L. monocytogenes, LLO is a major immunogen, as it is the target of CD4 and CD8 T-cell responses (379). However, when mice were inoculated subcutaneously with L. monocytogenes, strongly increased proliferation of CD4-positive T cells was observed with an LLO-negative strain, suggesting that LLO also plays a role in the inhibition of the adaptive immune response (380). In vitro work suggests that this inhibition likely occurs through the induction of apoptosis in T cells by LLO and that LLO's cytotoxic and immunogenic properties function independently (381, 382). It was found that during infection of mice with an LLO-negative L. monocytogenes strain, the production of antilisterial IgG and IgM antibodies takes place. This antibody response, as well as that against unrelated immunogenic factors, is repressed when mice are infected with an isogenic LLO-positive strain, indicating that LLO may downregulate the humoral immune response (383). LLO and the LLO-dependent escape of L. monocytogenes from the phagosome (see “Immune Evasion”) are required to induce IFN-γ expression, which induces a Th1-dependent immune response, in vivo (384, 385). Despite 80% amino acid sequence identity with LLO, Listeria ivanovii ivanolysin O (ILO) does not trigger IFN-γ production, which may be the reason that L. ivanovii cannot generate Th1-dependent protective immunity (385). ILO can nonetheless mostly fulfill LLO's role when transferred to L. monocytogenes (386).
PFT-Induced Barrier Dysfunction
An effect of PFTs that is often observed in vivo is a compromising of epithelial and endothelial layer integrity (barrier dysfunction), which can be caused by two mechanisms that are not mutually exclusive. The first mechanism consists of direct damage to epithelial or endothelial cells by the PFT, and the second consists of indirect damage caused by PFT-induced inflammatory effects. Often, however, the available data do not allow separation of these two mechanisms. Effects of PFTs on the vasculature other than inducing barrier dysfunction are discussed in “Other Effects of PFTs on the Vasculature.” Barrier dysfunction may result in the spreading of bacteria or bacterial virulence factors and can cause leakage of serum components into the affected tissues (e.g., the lungs and the intestinal lumen).
Barrier dysfunction in the lungs.
S. pneumoniae PLY-induced pulmonary edema in murine lungs ex vivo and in vitro induced gap formation between epithelial cells (387). Even at sublytic doses, PLY is capable of triggering the lethal effects of pneumonia—the destruction of lung tissue mediated by induction of apoptosis and recruitment of PMNs to the site of infection (183). PLY's cytotoxic property appears to be responsible for neutrophil recruitment (367). Purified PLY also caused increased alveolar epithelial permeability in mice after intratracheal administration. Coapplication of JI-34, a growth hormone-releasing hormone receptor agonist, reduced this effect. JI-34 did not affect proinflammatory cytokines or growth factors but did change the chemokine response of PLY-treated mice (388). This supports a hypothesis where JI-34's protective effect is mediated through the induction of cyclic AMP (cAMP), which in vitro was found to directly protect against PLY-induced changes in cellular Na+ uptake and membrane permeabilization (388). Purified PLY induced pulmonary microvascular barrier dysfunction and severe pulmonary hypertension in mice via direct toxic effects of PLY on the alveolus-capillary barrier, independent of resident or recruited immune cells (387, 389). Thus, it appears that PLY may cause barrier dysfunction via direct as well as indirect mechanisms. In a mouse model, the host factor deubiquitinating enzyme cylindromatosis (CYLD) mediates the PLY-induced barrier dysfunction (390) (discussed further in “Hijacking of Host Factors”).
In a neonatal rabbit model of GBS pneumonia, the PFT β-h/c was found to play a major role in breakdown of the pulmonary barrier. Animals challenged intratracheally with wild-type GBS showed increased bacterial loads, mortality, and bacteremia compared to animals infected with a β-h/c knockout GBS strain. Additionally, β-h/c was responsible for impaired lung compliance, but the mechanism was not determined (176).
Intratracheal instillation of S. aureus PVL in rabbits causes necrosis and edema of the lungs. PVL was shown to trigger increased local levels of IL-8 and monocyte chemotactic protein 1 (MCP-1), resulting in a more extensive PMN infiltration which is responsible for the observed necrosis, diffuse alveolar hemorrhage, and pulmonary edema. These results are consistent with a role for PVL in damaging the epithelium or endothelium (in this case indirectly, via PMNs), perhaps allowing systemic spread of S. aureus (145).
M. tuberculosis can invade pneumocytes, and ESAT-6 is proposed to help M. tuberculosis adhere to the basolateral plasma membrane, disrupt the cells, and allow dissemination through the alveolar wall (284, 391).
Intravenous injection of C. perfringens ε-toxin in calves resulted in a rapid (within 2 to 60 min) onset of neurological dysfunction (loss of consciousness, recumbency, convulsions, paddling, opistothonus, hyperesthesia, and dyspnea) and led to acute pulmonary edema. Histological examination further showed protein leakage in the brain, into the internal capsule, thalamus, and cerebral white matter (81). The acute nature of these effects appears consistent with direct effects of the toxin on the affected tissues rather than indirect effects mediated, for example, by the immune system.
In vitro, a low dose of E. coli HlyA induced neutrophil apoptosis via caspase-3 and -7, while a high dose caused necrosis. In a rat pneumonia model, HlyA mediated neutrophil necrosis and lung damage. Bronchoalveolar lavage yielded predominantly neutrophils, which appeared to be killed by necrosis in an HlyA-dependent fashion. HlyA further caused reduced oxygenation, leakage of albumin into the pulmonary compartment (barrier dysfunction), and histologically apparent damage to the lung tissue (100, 101). Additionally, HlyA was responsible for surfactant dysfunction, reducing the overall surface activity, which is a common characteristic of pneumonia (101). (Surface activity is the ability to lower surface tension, which increases pulmonary compliance and prevents lung collapse.)
Barrier dysfunction in the brain.
PLY is found in the cerebrospinal fluid of patients with S. pneumoniae meningitis (392). This PFT plays a critical role in mouse and rat meningitis models, causing worsened clinical outcomes, weight loss, and bacteremia (177, 393–395). In a rabbit meningitis model, PLY was detectable in the cerebrospinal fluid 24 h after intracisternal injection of S. pneumoniae, and in vitro data suggested that PLY-induced neurotoxicity involves Ca2+ influx and p38 MAPK activation in neuroblastoma cells (396). However, in an earlier study, it was found that although it stimulated the inflammatory response, PLY was not essential for virulence in this model (397). In a rat meningitis model involving infection through intracisternal injection, PLY did not affect the early kinetics of leukocyte influx and bacterial growth in the cerebrospinal fluid (177). Rather, PLY appears to be involved in breaching the endothelial layer, allowing S. pneumoniae to pass the blood-brain barrier (394, 398). The permanent neurological damage associated with pneumococcal meningitis is also caused at least in part by PLY. This is based on histological observations in the rat meningitis model and on the fact that it rapidly leads to extensive stabilization of microtubules, a known cause of axonal transport inhibition and neuropathy, in rabbits after intracisternal injection (177, 399). In a chinchilla model of acute pneumococcal otitis media, PLY (as well as PspA) is required for the associated sensorineural hearing loss (400).
GBS β-h/c was found to have similar effects to those of PLY in a rat neonatal meningitis model, i.e., it contributed to neuronal damage, resulted in a worsened clinical outcome and weight loss, and did not affect the early kinetics of leukocyte influx and bacterial growth in the cerebrospinal fluid (177). As with PLY-deficient S. pneumoniae, GBS lacking β-h/c showed reduced penetration of the blood-brain barrier compared with isogenic, wild-type controls in a mouse model of hematogenous streptococcal meningitis. The level of penetration for the wild-type strains was furthermore correlated with the amount of β-h/c produced by these strains. Strains lacking the cylE gene, which is essential for the production of β-h/c (172) (Table 2), still exhibited a significant level of penetration, suggesting that additional, partially redundant factors play a role (175).
C. perfringens ε-toxin is a highly toxic PFT that compromises several barriers as it spreads during infection, from the intestine via the bloodstream to the lungs, kidneys, and brain. Intoxication with ε-toxin causes neurological disorders associated with increased neurotransmitter release and neuronal cell death. ε-Toxin also binds to capillary endothelial cells and affects the blood-brain barrier. When mice were injected intravenously with labeled, functional ε-toxin, it was found to accumulate on endothelia in various organs, especially the kidneys and brain (401, 402). In the nervous system, ε-toxin associates with myelin structures (403). Although the neurotoxic effects were initially hypothesized to be caused by damage to brain blood vessels, later work showed that ε-toxin is also able to directly attack brain oligodendrocytes (82).
Barrier dysfunction in the intestine.
When S. aureus alpha-toxin was injected into the mesenteric artery in a rat ex vivo ileum, increased perfusion pressure and decreased mucosal hemoglobin oxygen saturation were observed. Coadministration of adrenomedullin (a peptide that induces vasodilation via cAMP and nitric oxide) abolished microvascular hyperpermeability and alpha-toxin-induced contraction of endothelial cells, as well as the subsequent barrier dysfunction (404).
In an ex vivo mouse model, it was found that B. anthracis compromised the intestinal barrier function dependent on ALO, likely via disrupting epithelial gap junctions, allowing passage of vegetative anthrax bacteria (405).
In piglet necrotic enteritis, C. perfringens β-toxin is an important virulence factor that causes necrosis of the intestinal epithelium and can lead to the disappearance of the brush border, which exposes underlying tissue to attack by C. perfringens and can lead to a β-toxin toxemia (77). β-Toxin was also found to be required for C. perfringens-induced necrotic enteritis in rabbit ileal loops, whereas PFO and (non-PFT) alpha-toxin were not (406). It is not known whether this was caused by direct effects of the PFT on the intestinal cells or indirectly, via, for example, the immune system.
In rabbit ex vivo ileal loops, V. cholerae VCC induces recruitment of PMNs, vascular alterations (edema and dilation of lymph vessels), necrosis and apoptosis of the epithelium, and congestion of the mucosa, all likely contributing to barrier dysfunction (205). Non-O1 and non-O139 serotype V. cholerae strains, which are usually cholera toxin (CT) and toxin-coregulated pilus A (TcpA) negative, can still cause watery diarrhea (205, 206). It was found that in such strains VCC induces a CT-like effect on excised human intestine, in that it causes leakage of Cl− ions, resulting in an outflow of Na+ and water (206).
Barrier dysfunction in other tissues.
After intraperitoneal infection of mice, GAS lacking both SLO and streptolysin S (SLS) resulted in reduced levels of resident macrophages, slower recruitment of neutrophils to the site of infection, less severe tissue damage, and decreased bacterial dissemination to the liver. These PFTs likely trigger oncosis (programmed cell death) of macrophages, which triggers an inflammatory response and attracts neutrophils (188). SLO and SLS were also both found to contribute to the formation of necrotic lesions in a mouse subcutaneous infection model, although it is not clear whether this is a direct (PFT action on epithelial cells) or indirect (mediated via, e.g., the immune system) effect (189). In an ex vivo model of porcine vaginal mucosa, purified GAS SLO and S. aureus alpha-toxin were both found to damage mucosal epithelia, mediating penetration of other virulence factors (190).
Isogenic S. aureus alpha-toxin deletion mutants cause smaller skin lesions than those seen with wild-type USA300 and Newman strains in mice. Alpha-toxin is further required for the infection to lead to dermonecrosis (370).
The intravenously injected labeled C. perfringens ε-toxin mentioned above aggregated most strongly in the kidneys, where it localized to vascular endothelia and renal distal tubules. The kidneys were the only organs where the labeled toxin also caused macroscopic changes: histological examination showed the medullae to be hemorrhagic, and degeneration of the distal tubules was observed (402). Ex vivo work showed that C. perfringens ε-toxin directly damages rat endothelial cells of the mesentery and thus increases vessel wall permeability (407).
In a mouse model of E. coli ascending urinary tract infection, HlyA was shown to cause shedding of the uroepithelial lining and hemorrhage of the bladder, leading to the hypothesis that HlyA is one of the major causes of the symptoms of cystitis in humans infected with uropathogenic E. coli (UPEC) (102).
Other Effects of PFTs on the Vasculature
In addition to compromising endothelial barrier integrity, PFTs can alter local or systemic blood pressure and perfusion and cause ischemic necrosis. Like the case with barrier dysfunction, these effects may be due to the PFTs affecting the endothelium directly or indirectly, via PFT effects on other host cells.
Vasoconstriction, vasodilation, and alteration of blood pressure.
When ventilated, blood-free perfused murine lungs were exposed intravascularly to S. pneumoniae PLY, a dose-dependent increase in vascular resistance was found. Immunohistochemistry showed that PLY was localized to the pulmonary arterial vessel, which displayed vasoconstriction (363).
In a model for sepsis and septic shock, S. aureus alpha-toxin and E. coli HlyA caused a strong coronary vasoconstrictive effect in isolated rat hearts (408), by inducing the release of thromboxane A2 (by alpha-toxin) or cysteinyl-leukotrienes (by HlyA) (409). This effect was thus caused by these PFTs' effects on eicosanoid production, not by direct endothelial damage (408), and likely contributed to the reduced cardiac output and systemic hypotension observed with sepsis (244).
C. perfringens ε-toxin caused contraction of an isolated rat aorta, and pharmacological experiments showed that this effect was likely mediated by the nervous system (410). In live rats, intravenous injection of ε-toxin transiently increases systemic blood pressure due to a vasoconstrictive effect on cutaneous vessels, but it does not affect heart rate or electrocardiogram (ECG) results (411). C. perfringens β-toxin also causes a transient increase in systemic blood pressure in rats, but here the effects are accompanied by an altered heart rate and a subsequent change of the ECG. The increased blood pressure can be counteracted by coadministering alpha-adrenergic and ganglionic blocking agents, indicating that β-toxin's effect is likely also neuronal and involves catecholamines (412). Consistently, sensory nerve-mediated mechanisms appear to be involved in β-toxin-induced plasma extravasation (413). C. perfringens PFO was found to reduce blood pressure and affect cardiac output, although not acting directly on the heart, thus causing lethal shock in rabbits (414). However, this could not be replicated in a different study (415). Intravenous injection of purified Clostridium botulinum botulinolysin (BLY) in rats caused a rapid drop in systemic blood pressure, which at low toxin doses was transient (71). BLY induces this effect by inhibiting acetylcholine-dependent relaxation of the aortic ring, thus causing a local, coronary vasoconstriction (72). Clostridium tetani tetanolysin also causes cardiac failure in mice and alters the ECGs of rhesus monkeys (95).
Effects of E. coli HlyA on microvasculature have been researched in an ex vivo rabbit ileum model. When low doses of HlyA were administered via the mesenteric artery, a quick and transient rise of blood pressure was observed (likely caused by vasoconstriction), with a concomitant drop in mucosal oxygen saturation. The homogeneous distribution of oxygen over the mucosa remained disrupted, causing an increase in the gap between mucosal and arterial CO2 partial pressures. In addition, an increase in the levels of hemoglobin in the mucosa was observed, as well as edema, which is suggestive of postcapillary vasoconstriction and capillary leakage (barrier dysfunction) (416). The altered oxygenation and CO2 pressure may serve to provide competitive growth conditions for E. coli in the mucosa.
Comparable results with regard to blood pressure were found in a prior ex vivo study where the effects of intravascular administration of HlyA were investigated in blood-free perfused rabbit lungs. In this case, a dose- and time-dependent release of thromboxane A2 and prostaglandin I2 into the circulating medium and the bronchoalveolar space was observed. The vasoconstrictive potency of thromboxane surpassed the vasodilatory effect of prostaglandin, as a net pulmonary hypertension was observed. The circulating medium further showed increased levels of potassium but not lactate dehydrogenase (LDH), indicating damage of cell membranes but likely not cell death. Furthermore, severe pulmonary edema was observed, which was independent of thromboxane's vasoconstrictive effect and was caused by increased permeability of the vasculature. These findings mimic events during acute respiratory failure in states of septicemia (417). When in similar experiments the lungs were primed by preexposure to LPS, the effects of HlyA on thromboxane release and blood pressure were 15-fold more severe than those without priming, indicating that the response to LPS, the release of TNF-α into the medium, synergizes with the effects of HlyA (418). In vitro studies showed that HlyA may be associated with E. coli outer membrane vesicles (OMVs), which may also contain additional bacterial factors, suggesting that such vesicles may alter cellular responses (419).
Vascular and ischemic necrosis.
Extensive ischemic necrosis is a primary feature of patients with GAS necrotizing fasciitis or myonecrosis. In a rat model of GAS-induced myonecrosis, laser Doppler flowmetry was used to assess the microcirculatory function following intramuscular injection of SLO, which revealed a dose-dependent decrease in local tissue perfusion at the injection site. Flow cytometry studies demonstrated a SLO-induced coaggregation of platelets and neutrophils, leading to the observed microvascular obstruction (420).
In a human case of lethal necrotic enteritis, C. perfringens β-toxin was found to be associated with the vascular endothelium, indicating that it may have been responsible for the observed vascular necrosis, similar to observations in infected piglets (421). Intradermally injected β-toxin causes dermonecrosis and plasma extravasation in mice. A histamine H1 receptor antagonist markedly inhibited β-toxin-induced plasma extravasation. Further data, however, suggested that β-toxin does not act on mast cells directly, so rather than histamine release from skin mast cells, it seems that sensory nerve-mediated mechanisms are involved in plasma extravasation (413).
Clostridial myonecrosis, which can be caused by C. perfringens and Clostridium septicum, is characterized by rapidly spreading tissue necrosis accompanied by thrombosis and leukostasis. Although PFO is not an essential virulence factor of C. perfringens, it does play a role in this process. C. perfringens lacking both (non-PFT) alpha-toxin and (PFT) PFO was essentially avirulent in a mouse intravenous challenge model, whereas reconstitution of either toxin led to the restoration of some (PFO) or most (alpha-toxin) virulence characteristics. Restoration of only alpha-toxin to the double mutant reconstituted most of the typical myonecrosis features. Interestingly, restoration of only PFO subsequently led to different virulence features, characterized by coagulative necrosis that was apparently enhanced by vascular disruption (86). For C. septicum, it has been shown that its ability to produce fulminant myonecrosis in mice is dependent on (PFT) alpha-toxin (91) (note that whereas C. perfringens alpha-toxin is not a PFT, C. septicum alpha-toxin is). The typical leukostasis is also dependent on C. septicum alpha-toxin. Whereas the paucity of leukocytes during C. perfringens gas gangrene is due to vascular leukostasis caused by the synergistic actions of alpha-toxin and PFO (see above), this appears not to be the cause of the absence of leukocytes during C. septicum myonecrosis. Instead, this absence is likely caused by direct impairment of PMN function or by PMN cytotoxicity (91). Results from a more recent study do suggest a role for C. septicum alpha-toxin, in conjunction with other virulence factors, in reducing vascular perfusion (92).
Immune Evasion
PFTs can help bacteria to evade the host immune system through several mechanisms. First, the immune responses discussed above may reflect effects that are beneficial to the bacterium or efficient mobilizing of defenses by the host. Described here are alternative mechanisms, which include direct cytotoxicity of PFTs toward immune cells, a contribution of PFTs to infiltration and intracellular survival in host cells, and the hijacking of host factors. Cytotoxicity may be due to direct lysing of cells through membrane damage or via activating controlled cell death signaling pathways (e.g., apoptosis); the mechanism cannot always be deduced from the available data.
Cytotoxicity toward immune cells.
During intraperitoneal infection in mice, GAS SLO and SLS are responsible, among other effects, for removing resident macrophages. In vitro, GAS strains lacking either SLO or SLS were as lethal to macrophages as wild-type bacteria; however, a double mutant lacking both PFTs showed attenuated killing. In addition, purified SLO and SLS were both cytotoxic to macrophages in vitro, indicating that they may function redundantly (188). Consistent with redundant functions for SLO and SLS, deletion of either did not influence host survival in a mouse subcutaneous GAS infection model, and deletion of SLO had only a small effect in an intraperitoneal infection model (191). Furthermore, when the capsule, which protects GAS from phagocytosis, was absent, loss of SLO completely attenuated killing, and loss of SLS significantly increased host survival (191), indicating that both SLO and SLS contribute to evasion of phagocytosis and apparently are not redundant in this case.
In a zebrafish model of lethal necrotic myositis, an SLS-deficient GAS strain was associated with decreased lethality and a robust recruitment of neutrophils. In mice after subcutaneous infection with GAS, SLS deficiency was associated with accelerated extravasation of neutrophils, indicating that SLS inhibits neutrophil migration (195). However, intraperitoneal challenge of mice with GAS showed that SLS was cytotoxic to newly recruited neutrophils, rather than inhibiting migration. In vitro, in primary cells, the cytotoxicity appeared to be due to induction of apoptosis (196).
GBS lacking the cylE gene (causing β-h/c deficiency) (Table 2) was cleared more easily than wild-type bacteria from the mouse bloodstream, human blood, and isolated macrophages and neutrophils. Persistence of GBS in the blood was linked to β-h/c's cytotoxic effects on phagocytes, as well as to a protective effect of the pigment granadaene to respiratory burst (i.e., the rapid release of reactive oxygen species [ROS]) killing mechanisms (178). In a zebrafish model, E. coli HlyA was found to be one of the major determinants of extraintestinal pathogenic E. coli (ExPEC) infection, functioning to prevent eradication by phagocytes (103). The ability to produce HlyA is coupled to a resistance of E. coli to the bactericidal activity of human blood serum (422).
Alpha-toxin was found to be dispensable for the survival of S. aureus USA300 in human blood; however, the toxin did specifically induce programmed cell death of monocytes, B cells, and T cells (423).
Ex vivo and in vitro data show that B. anthracis ALO can activate TLR4 to induce macrophage apoptosis (354, 355).
In mice infected with S. aureus via the retro-orbital venous plexus, LukED was found to contribute to bacterial replication by directly killing phagocytes recruited to colonized sites. LukED-deficient bacteria showed decreased growth in kidneys after 96 h but not after 16 h. This indicates that these bacteria are not defective in colonizing the kidneys and that LukED is required for long-term survival of S. aureus. Loss of LukED was further associated with lower serum levels of IL-6 and granulocyte colony-stimulating factor (G-CSF), which are markers of inflammation. When neutrophils were depleted, wild-type and LukED-deficient S. aureus strains showed similar levels of virulence, indicating that these cells are a primary target of this PFT (164).
Intracellular survival of pathogens.
Many bacterial pathogens can invade host cells, which may aid in evasion of the host immune system. For some of these pathogens, proof has been found that PFTs are required for invasion or intracellular survival.
L. monocytogenes can propagate in and spread between many host cells, thus allowing it to evade host immune defenses, and it enters these cells via phagocytosis or induced uptake. The bacterium then escapes from the phagosome and replicates in the host cell cytosol, although replication in macrophage vacuoles has also been observed (424, 425). LLO (as well as L. ivanovii ILO) appears uniquely tailored to attack the membranes of phagosomes while avoiding damage to the plasma membrane, thus facilitating the virulence of L. monocytogenes. The compartmentalization of LLO's function is dependent on the pH of the milieu surrounding the bacterium (10). LLO function is also regulated via reduction by the host phagosomal enzyme gamma interferon-inducible lysosomal thiol reductase (GILT). GILT is responsible for the activation of LLO in vivo, and mice lacking GILT (and which are deficient in generating MHC-II-restricted CD4-positive T-cell responses to relevant protein antigens) are resistant to L. monocytogenes infection (426). Interestingly, PFTs from several other bacteria also allow L. monocytogenes to escape from phagosomes; however, because these PFTs are not correctly regulated, they go on to attack the plasma membranes of host cells, thus impairing L. monocytogenes virulence in vivo (427, 428).
Note that L. monocytogenes is not an obligatorily intracellular pathogen (429), and the finding that anti-LLO antibodies can affect virulence may be consistent with a role for LLO in the extracellular milieu (430), which is supported by in vitro findings (110, 431), as well as potentially by the experiments with purified LLO described above. C. perfringens PFO has also been hypothesized to help the bacterium escape from the phagosome, but this PFT clearly has other functions as well (92), and hence a model where a single PFT would fulfill these different roles is not novel.
M. tuberculosis is classically assumed to survive inside phagosomes of host phagocytes. More recently, however, it has been proposed to be able to escape into the cytosol (122), as has also been observed for Mycobacterium marinum (432), in which this depends on ESAT-6 (433). In this model, ESAT-6 could function to help M. tuberculosis escape from phagosomes, like LLO for L. monocytogenes. This suggestion is based on, among other things, the in vitro findings that ESAT-6 and 10-kDa culture filtrate protein (CFP-10) can individually interact with artificial membranes but together form a complex that prevents their membrane interaction. The ESAT-6–CFP-10 complex is pH dependent and dissociates at a low pH (such as in a phagosome), releasing ESAT-6 to attack the phagosomal membrane, analogous to L. monocytogenes LLO (122). ESAT-6-binding cofactors such as CFP-10 may thus tailor its function for extracellular, intraphagosomal, or cytosolic use, an idea supported by in vitro data (391), but this hypothesis remains to be tested in vivo. Such a mechanism for M. tuberculosis is further supported by the fact that M. tuberculosis needs ESAT-6 to spread between macrophages (although, in contrast to LLO, it is not required for survival within the macrophage) (434). In an in vivo M. marinum-zebrafish infection model, the RD1 locus, encoding ESAT-6 and CFP-10, is additionally required to cause macrophage aggregation (granulomas), typically seen with tuberculosis, and death of the infected tissue (120).
β-h/c promotes GBS survival inside macrophages (361) (also see “Cytotoxicity toward immune cells”). Subcutaneous injection of S. aureus results in the presence of live bacteria in mast cells. Further in vitro experiments showed that internalization requires alpha-toxin and may allow S. aureus to evade the immune system (435).
Hijacking of Host Factors
In vivo in mice, the deubiquitinating enzyme CYLD is a negative regulator of host survival during S. pneumoniae infection, by allowing PLY-induced acute lung injury and bacterial translocation. It was found that CYLD's negative effect is based on a downregulation of plasminogen activator inhibitor 1 (PAI-1) induction. PAI-1 functions to reduce hemorrhage and is required for recovery from local tissue injury. PAI-1 activation was found to function through MAPK kinase 3 (MKK3) and p38 MAPK, as well as TLR4 and MyD88, but not through TLR2. Both S. pneumoniae and purified PLY were able to cause phosphorylation of p38 in vitro, and MKK3 was required in vivo for normal host survival with S. pneumoniae expressing PLY and purified PLY, consistent with an in vivo role for p38 in PLY defense (390). CYLD production is also highly induced by PLY, so it appears that this PFT hijacks a host factor (CYLD) to inhibit a host PFT defense pathway (p38). In addition, CYLD appears to be responsible for PLY-induced microvascular leakage (barrier dysfunction) (390).
The receptor for S. aureus alpha-toxin is A-disintegrin and metalloprotease 10 (ADAM10) (234). Disruption of this receptor in the lung tissue makes mice resistant to lethal pneumonia. In vitro work showed that ADAM10 is activated by alpha-toxin, as well as by PLY and potentially other PFTs, and that its metalloprotease activity leads to cleavage of E-cadherin and to epithelial barrier disruption. Consistent with this, bronchoalveolar lavage after in vivo infection with wild-type S. aureus showed a release of N-terminal fragments of E-cadherin, whereas an alpha-toxin-deficient mutant did not have this effect (436). When mice were treated with a metalloprotease inhibitor, they survived an otherwise lethal S. aureus lung infection and showed increased resistance to an intravenous challenge (436, 437). Thus, alpha-toxin causes barrier dysfunction through hijacking of a host molecular pathway.
Infection of mice with L. monocytogenes caused an LLO-dependent reduction of levels of Ubc9, an essential enzyme of the SUMOylation machinery (a posttranslational protein modification mechanism of eukaryotic cells, required for viability, that involves small ubiquitin-like modifier [SUMO] proteins). This result was found in vitro to extend to S. pneumoniae PLY and C. perfringens PFO. Further in vitro data showed that L. monocytogenes inhibition of SUMOylation resulted in an attenuated transforming growth factor beta (TGF-β) response and, consistent with this, that overexpression of SUMOylation components led to an increased resistance to bacterial invasion (111).
Other PFT Functions and Effects
Programmed cell death of nonimmune cells.
As mentioned above (see PFT Effects and Cellular Defense Mechanisms), programmed cell death forms an important aspect of the PFT response in vitro. In vivo, PFTs have been found to induce programmed cell death, mainly apoptosis, on several occasions in both immune cells (see “Immune Evasion”) and nonimmune cells.
Apoptosis appears to protect against S. pneumoniae PLY-induced lethality. PLY induces apoptosis in vivo in mouse airway epithelial cells. Rather than being caused by pore formation, apoptosis appears to depend on a direct, physical interaction between PLY and TLR4 but not TLR2, and TLR4-deficient mice show strongly reduced levels of apoptosis. Additionally, a pan-caspase inhibitor reduces this PLY-induced apoptosis, and inhibition of apoptosis causes increased invasive disease in the mouse model, resulting in increased lethality (353).
As mentioned in “Innate Immune Responses to PFTs,” caspase-1 is activated by GAS SLO. Another study also found SLO to induce macrophage apoptosis, partially dependent on caspase-1, in vivo in a mouse model. The apoptosis protects GAS from killing by macrophages and is required for full virulence (187).
In addition to apoptotic effects on immune cells (see “Immune Evasion”), GBS β-h/c causes apoptosis in rat neurons in vitro, apparently independently of caspase activity (177), as well as in vivo in rabbit hepatocytes, via caspase-3, in a sepsis model (179).
E. coli HlyA caused decreased expression of antiapoptotic bcl-x as well as the proapoptotic factor Fas in vivo in a rat pneumonia model. In this case, predominantly necrosis of neutrophils was seen. In vitro, a low dose of HlyA caused caspase-3 and -7-dependent apoptosis, while a high dose caused necrosis (100).
In human intestinal epithelial cells in vitro, V. cholerae VCC induces apoptosis due to its anion channel activity and via caspase-3, and in rabbit ileal loops ex vivo, it induces epithelial cell apoptosis and necrosis (205).
Intracellular delivery of additional virulence factors.
S. pneumoniae PLY may also alter host mucosal responses to other microbes during colonization. Colonization with PLY-expressing but not PLY-deficient S. pneumoniae leads to synergistic proinflammatory signaling and neutrophil recruitment in the setting of cocolonization with Haemophilus influenzae, and this effect promotes interspecies competition (438, 439). Based on a combination of in vitro and in vivo studies, the enhancement of signaling is thought to result from PLY allowing extracellular peptidoglycan fragments to access the host cell cytoplasm, where they can be detected by Nod-like receptors, in a pore-dependent manner (8, 440). Similarly, pneumococcal DNA enters airway epithelial and dendritic cells in a PLY-dependent fashion and then stimulates type I IFN signaling. IFN-α/β receptor null mice showed increased nasal colonization with S. pneumoniae, indicating a role for IFN signaling in the mucosal response to this pathogen (441).
B. anthracis PA is a PFT that allows the other components of anthrax toxin, lethal factor (LF) and edema factor (EF), access to the host cell cytosol (Table 2). Sometimes, however, models oversimplify PFTs as delivery vehicles for other virulence factors. For example, GAS SLO's pore-forming capability was hypothesized to function to translocate NAD-glycohydrolase (NADase), an important GAS virulence factor, across the host cell membrane (442, 443). This notion proved wrong when it was found that NADase translocation is independent of pore formation, although still requiring SLO, showing that SLO's cytotoxic and translocation activities can be separated (444).
Mucus production.
Mucus production is an important part of the response to S. pneumoniae infection, mediated by expression of mucin genes. It has a defensive effect, but when produced in quantities that are too large, mucus can cause obstruction of the airways (445). In vitro, PLY is capable of upregulating expression of the MUC5AC mucin gene via TNF-α and ERK- and p38 MAPK-dependent pathways. Furthermore, JNK MAPK acts as a negative regulator of MUC5AC expression, and MAPK phosphatase 1 (MKP1) is a positive regulator of PLY-induced MUC5AC expression via dephosphorylation of JNK. Consistent with this, it was found in vivo in mice that S. pneumoniae upregulates expression of MUC5AC and MKP1 expression and that chemical inhibition of JNK results in increased MUC5AC levels. These findings indicate a tightly controlled regulation of mucus production, allowing for a balance between its defensive effect and obstruction of the airways (446).
Antimicrobial compounds.
One aspect of the innate immune response against bacterial pathogens entails the release of antimicrobial compounds. As mentioned above (see “Immune Evasion”), the GBS pigment granadaene (Table 2) protects against reactive oxygen species released by immune cells (178).
In mice infected intravenously, L. monocytogenes accumulates in the liver, whereas an isogenic LLO mutant is mostly cleared from the liver after 48 h. However, in mice lacking the NOX2 NADP (NADPH) oxidase (gp91phox or phagocyte oxidase), the LLO deletion mutant is not cleared from the liver. NOX2 is responsible for the production of antimicrobial ROS. In primary macrophages, LLO inhibits ROS production by blocking NOX2 localization to the phagosomes, thus promoting intracellular survival of Listeria (447).
ATP depletion.
Phospholipid scramblase 1 (PLSCR1) was identified in vitro as a candidate gene to mediate a protective effect of IFN-α on cultured cells permeabilized by S. aureus alpha-toxin. IFN-α was found to protect these cultured cells by reducing the amount of ATP released extracellularly and allowing them to maintain sufficient levels of intracellular ATP. In vivo, loss of PLSCR1 increases sensitivity to alpha-toxin: PLSCR1 knockout mice display difficulty in restoring disturbed body temperature after inhaling alpha-toxin, and a significantly larger portion of PLSCR1 knockout mice than heterozygous littermates succumb to S. aureus infection (448).
Genomic responses to PFTs.
Using a mouse model of lung infection, host microarray analysis was performed on lung tissue after 4 and 24 h of infection with wild-type S. aureus or an alpha-toxin knockout (378). Interestingly, at the 4-h time point, no differences were found between genes up- or downregulated in response to the wild-type and alpha-toxin knockout strains. After 24 h, however, 1,281 genes were differentially regulated (540 up- and 741 downregulated) in response to the two strains. Further analysis identified that pathways involved in the extracellular matrix (consistent with the role for ADAM10 [see “Hijacking of host factors”] and including collagens, integrins, and syndecans [see “Other PFT Functions and Effects”]) and in cardiomyopathy were specifically differentially regulated in response to alpha-toxin. IL-23 and IL-6 were upregulated in an alpha-toxin-dependent manner.
A genomewide RNA interference (RNAi) screen in C. elegans identified 106 genes, or 0.5% of the genome, that are strongly involved in defense against the B. thuringiensis PFT Cry5B (231). Two MAPK pathways, p38 and JNK, were found to be at the center of the genetic PFT response network formed by these genes. Microarray analyses further showed that JNK, but not p38, is a central regulator of transcriptional PFT responses. Microarray analysis of C. elegans exposed to V. cholerae expressing or lacking VCC (both lacking the cholera toxin) showed differential expression of 743 genes specifically in response to VCC. The induced genes included C-type lectin genes, collagen genes, genes that function downstream of the insulin/IGF-1 pathway transcription factor gene daf-16 (see PFT Effects and Cellular Defense Mechanisms), prion-like (Q/N-rich) domain protein genes, and genes that are activated when the unfolded protein response (UPR) (see PFT Effects and Cellular Defense Mechanisms) is blocked (449). One hundred forty-four of these genes overlapped with those identified in the Cry5B microarray (231, 449).
Ectodomain shedding.
Mice infected with B. anthracis showed increased levels of syndecans in the systemic circulation. Syndecans are proteoglycans that function in cell spreading, adhesion, motility, and maintenance of intercellular contacts. Experiments in vitro showed that B. anthracis ALO induced syndecan shedding (as did the non-PFT factors AnlB and CnlA), which was linked to barrier dysfunction via disorganization of E-cadherin. The combination of PA and LF, called lethal toxin, also caused syndecan shedding, but to a lesser extent, and only when the components were applied in combination. Shedding in response to ALO and lethal toxin was dependent upon p38 MAPK activation (p38 is normally upregulated by ALO and downregulated by LF), but shedding induced by the non-PFT factors was independent of p38. ALO-induced shedding was further dependent upon the MEK1/2 ERK pathway but not the JNK pathway (34). The Pseudomonas aeruginosa elastase LasA has been shown to cause shedding of syndecans from the host cell surface in vivo (450, 451), and S. aureus alpha-toxin and β-toxin (sphingomyelinase) have been show to cause syndecan shedding in vitro (452). Notably, ceramide produced by sphingomyelinase has been shown to mediate the removal of PFT pores from the host cell plasma membrane (326) and may contribute to shedding of IL-6 receptors (342) (see PFT Effects and Cellular Defense Mechanisms and “Innate Immune Responses to PFTs,” respectively). It has been hypothesized that syndecan shedding benefits pathogenesis (451); however, there are currently no data directly demonstrating this. It is also unclear whether it is the increased amount of syndecans on ectodomains, the decreased amount of syndecans on the cell surface, or the associated barrier dysfunction (or more than one of the above) that is relevant to infection or host defense.
Various B. thuringiensis Cry toxins were found to cause shedding of GPI-anchored aminopeptidase and alkaline phosphatase from plasma membranes of midgut epithelial cells of gypsy moths, similar to the glycoprotein shedding induced by ALO and anthrax toxin. This shedding apparently did not result from proteolysis but was dependent upon cAMP and the ERK1/2 pathway, via cleavage of the GPI anchor by an endogenous GPI-specific phospholipase C (453), similar to what was found for B. anthracis (34). Since aminopeptidase and alkaline phosphatase are receptors for Cry toxins (454), the observed shedding may be a protective function that is under the control of the ERK1/2 (and p38) MAPK pathway.
Effects on host colonization and bacterial growth.
A number of the studies reviewed in the sections above also provide data on PFT contributions to host colonization and bacterial growth. Several for which the data are clearly interpretable are listed in this section.
After infection via intracisternal injection in a rat meningitis model, PLY did not affect bacterial growth in the cerebrospinal fluid (177), but it appears to be involved in breaching the blood-brain barrier (394, 398). After intracerebral infection in mice, however, PLY was dispensable for subsequent spreading to the spleen (395).
In mouse models, PVL was found to contribute to muscle but not skin injury after subcutaneous challenge with S. aureus. The bacterial loads in the different lesions were similar, however, showing that PVL does not cause increased bacterial growth (143). After intranasal challenge of mice, a requirement for PVL for bacterial growth in the lungs and the blood was found, which correlates with its complement-activating ability but not with its cytotoxic properties (182).
After intravenous inoculation into mice, LukED-deficient bacteria showed decreased growth in kidneys 96 h, but not 16 h, after infection. LukED is thus required for long-term survival of S. aureus but not for breaching of endothelial barriers or colonization (164).
In a neonatal rabbit model of GBS pneumonia involving intratracheal installation, wild-type GBS caused increased bacterial loads in the lungs and the blood compared to those seen with a β-h/c knockout GBS strain (176). In a rat neonatal meningitis model involving intracisternal injection, β-h/c did not affect the early kinetics of bacterial growth in the cerebrospinal fluid (177) and was required for efficient penetration of the blood-brain barrier (175).
During intratracheal infection of rabbits with S. aureus, PVL was found to be dispensable for initial colonization and bacterial growth rates (145). In a rabbit bacteremia model involving intravenous injection, PVL was found to contribute to early bacterial spreading to the kidney but not to bacterial growth at later time points (147).
M. tuberculosis lacking ESAT-6 and CFP-10 or failing to secrete these proteins initially showed decreased colonization of the lungs of intravenously challenged mice after intratracheal infection. Later in the infection, the growth rates caught up with those of mutants that secreted the proteins normally, but host killing was attenuated if ESAT-6 and CFP-10 were not secreted (124).
E. coli HlyA did not affect bacterial growth during the early phase of infection in a rat ExPEC pneumonia model involving intratracheal installation (101). Similarly, in a mouse model of ascending urinary tract infection, HlyA was found to have no influence on bacterial colonization of the bladder or kidneys after intraurethral inoculation via a catheter (102).
In a mouse model, V. cholerae VCC and MARTX were found to contribute to establishing a prolonged colonization of the small intestine and were the main virulence factors causing lethality, whereas CT and TcpA appeared not to be required for colonization or lethality. It was suggested that after establishment of this prolonged colonization, CT and TcpA cause diarrhea. In the absence of VCC or MARTX, the fractions of colonized mice were smaller, but when colonization did take place in the absence of either toxin, counts of recovered bacteria were similar to those for control bacteria. Thus, VCC and MARTX contribute to colonization of the intestinal lining but do not affect subsequent bacterial growth. VCC was also found not to be responsible for bacterial spreading to the spleen and liver in this model (207–209).
Thus, PFTs on some occasions are found to contribute specifically to colonization or early stages of bacterial growth, while in other studies no such effects are found, sometimes even for the same PFT. Many experimental factors could influence these findings, such as the host model, host immune status, and route of infection (further discussed below).
CONCLUSIONS
Two Main Effects of PFTs during Infection
A condensed overview of regularly observed PFT effects is provided in Table 3. Generalizing the in vivo PFT data from the available literature described above, two broad results of PFT-induced effects during in vivo infections become apparent. First, many PFT effects result in compromised integrity of epithelial and endothelial layers. Second, PFT action results in a disrupted immune response. PFTs can contribute to the disruption of the host immune response by (i) preventing the attraction of immune cells, (ii) destroying immune cells (by direct lysis or by inducing programmed cell death), (iii) aiding bacterial invasion of host cells and intracellular survival, and (iv) hijacking host molecular defense pathways. PFTs can induce barrier dysfunction through (i) direct attack of epithelial and endothelial cells, (ii) damage caused by PFT-driven inflammation, (iii) local vascular effects (mediated either by affecting endothelial cells directly or via modulation of the nervous system), and (iv) the hijacking of host cell molecular pathways that regulate the extracellular matrix. A schematic overview of the global pathogenic effects of PFTs in vivo is provided in Fig. 3.
TABLE 3.
PFT-induced effects observed in vivo
| Pathogena | PFTa | Presence of PFT effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Innate immune responses |
Barrier dysfunction | Vasoconstriction and -dilation and altered blood pressure | Vascular and ischemic necrosis | Immune evasion |
Hijacking of host factors | Programmed cell death of nonimmune cells | |||||
| Inflammation | PRRs involved | Inflammasome activation | Cytotoxicity toward immune cells (direct and via programmed cell death) | Intracellular survival of pathogen | |||||||
| S. pneumoniae | PLY | × | × | × | × | × | × | × | |||
| GAS | SLO | × | × | × | × | × | × | ||||
| SLS | × | × | |||||||||
| GBS | β-h/c | × | × | × | × | ||||||
| S. aureus | PVL | × | × | × | |||||||
| Alpha-toxin | × | × | × | × | × | × | |||||
| γ-hemolysin | × | ||||||||||
| LukED | × | ||||||||||
| LukGH | × | ||||||||||
| B. anthracis | ALO | × | × | × | |||||||
| C. botulinum | BLY | × | |||||||||
| C. perfringens | β-toxin | × | × | × | |||||||
| ε-toxin | × | × | |||||||||
| PFO | × | × | |||||||||
| C. septicum | Alpha-toxin | × | |||||||||
| C. tetani | Tetanolysin | × | |||||||||
| L. monocytogenes | LLO | × | × | ||||||||
| M. tuberculosis | ESAT-6 | × | × | ||||||||
| E. coli | HlyA | × | × | × | × | ||||||
| V. cholerae | VCC | × | × | ||||||||
See Table 2 for details on these pathogens and PFTs.
Fig 3.
Overview of global in vivo effects of PFTs. Note that not all pathways are relevant to all toxins and hosts.
In vitro, differential effects of and host responses to PFTs that form small pores and PFTs that form large pores have been observed (229). From the in vivo data reviewed here, no such differences are obvious for the PFTs of the 10 pathogens that are the focus of this article (Table 3).
The method and timing of PFT release into the extracellular (from the bacterial viewpoint) milieu likely affect the role of PFTs in pathogenesis. The vast majority of PFTs are actively secreted as monomeric proteins into the extracellular milieu via one of the many well-described bacterial secretion systems. However, there are some unique mechanisms for toxin release and processing that are worth noting. The E. coli hemolysin E (HlyE) toxin oligomerizes and forms active pore assemblies in bacterial OMVs prior to their release from the bacterial cells (455). PLY distinguishes itself from all other members of the CDC family by lacking a signal peptide for export outside the cell (456, 457), and it was hypothesized that PLY was confined to the bacterial cytoplasm unless bacterial autolysis occurred (181). More recent data, however, indicate that PLY can additionally be secreted from the cell, in which case it appears to remain localized to the bacterial cell wall (458, 459). The precise export mechanism remains uncharacterized but is known to depend upon domain 2 of PLY (460). LLO and ILO, produced by Listeria spp., are released within host cells after bacterial invasion. This intracellular release is essential for bacterial escape from the host cell phagosome, enabling further replication and invasion of neighboring cells by these intracellular pathogens (10, 461). Other PFTs are synthesized as inactive precursors requiring additional processing or cofactors for activation. The conversion to the active state may occur within the bacterial cytoplasm prior to extracellular transport (HlyA) (462) or extracellularly at the target cell surface (aerolysin) (463). The crystal toxins produced by B. thuringiensis remain inactive until they are ingested by the host, solubilized in the midgut, and further processed by host proteases (464). The toxic effects of the staphylococcal bicomponent leukocidins are dependent upon the recombination of two distinct proteins (one each from the class S and class F families of proteins) that remain inactive until both bind the target cell surface (223).
Several of the 10 bacteria that are the focus of this review (Table 2) are not always pathogenic and can be harmless commensals. In this situation, their PFTs may still have effects that give these bacteria a competitive edge over other species. Some examples are the case of S. pneumoniae and H. influenzae, where synergistic inflammatory effects promote competition between the two species (438, 439) (see “Other PFT Functions and Effects”), and the finding that E. coli HlyA impairs the host defenses against B. fragilis (99) (see “Innate Immune Responses to PFTs”). This is speculative, however, and whether PFTs are also expressed in vivo under nonpathogenic conditions, to our knowledge, remains to be determined.
Induction of Barrier Dysfunction
Damage to epithelial and endothelial barriers is especially obvious in pneumonia, where PFTs by themselves can cause the clinical manifestations of the disease, as observed with S. pneumoniae PLY (387, 389) and S. aureus PVL (145, 146). Examples of PFTs that directly damage epithelial cells are GAS SLO, S. aureus alpha-toxin (190), B. thuringiensis Cry5B (330), C. perfringens β-toxin (77) and ε-toxin (81), and V. cholerae VCC (205, 206). Findings in vitro for GAS, B. anthracis, and C. perfringens further support the notion that PFTs induce barrier dysfunction (465–467).
PFTs can also kill epithelial cells by inducing programmed cell death, as observed in vivo for S. pneumoniae PLY (183) and V. cholerae VCC (205). Another factor that induces barrier dysfunction is the disruption of cell-cell junctions, as seen with S. aureus alpha-toxin, and possibly PLY (436), and B. anthracis ALO (34, 405).
Also, in many cases, the PFT-induced damage to epithelia is secondary, caused by the actions of neutrophils that are recruited to the site of infection (described for S. pneumoniae PLY [183], GAS SLO and SLS [188], S. aureus PVL [145, 146], and V. cholerae VCC [205]) or by ischemic effects due to PFT-induced vascular damage (Table 3). Several PFTs were found to affect the vasculature (Table 3). These effects were either direct damage or damage caused by recruited PMNs, and in some cases they involved vasoconstrictive effects and affected blood pressure. Local vasoconstriction of coronary vessels may lead to myocardial infarction and subsequent shock. The destruction of epithelia and endothelia and the alteration of blood flow are also often responsible for the necrotic lesions that can be associated with the bacterial infections described here.
One main function of compromising epithelial barrier integrity is to allow bacteria or their toxins access to the circulation and other sections of the host body. A contribution of the PFT to bacterial or toxin spreading was confirmed for S. pneumoniae PLY (394, 398), GAS SLO and S. aureus alpha-toxin (190), S. aureus PVL (147, 182), V. cholerae VCC (207), and GBS β-h/c (175).
The edema, reduced ventilation, and protein leakage resulting from pneumonia-associated barrier dysfunction (see “PFT-Induced Barrier Dysfunction” and “Other Effects of PFTs on the Vasculature”) or the altered local tissue oxygenation (as with E. coli HlyA in the intestine [416] and lungs [100, 101]) may alter local growth or competition conditions for the infecting bacteria. In other cases, the purpose of cytotoxicity may be to extract specific nutrients. For example, E. coli HlyA destroys red blood cells in vivo (99), and HlyA expression is known to be (inversely) controlled by the availability of iron (468). Although these are attractive hypotheses, they often seem to be contradicted by in vivo data (see “Other PFT Functions and Effects”). Exceptions and ambiguous findings exist (e.g., S. pneumoniae PLY and S. aureus PVL), and the inconsistent use and definition of terms such as “colonization” and “initial growth” further complicate interpretation. An additional caveat is that when PFTs are concluded to be required for initial bacterial growth, an alternative explanation may in fact be that the PFT is required to breach an anatomical barrier to allow access to the site of colonization rather than to provide nutrients. In one study, the effect on M. tuberculosis growth of ESAT-6 secretion was measured in the lungs, but the bacterium was administered intravenously (124), so in fact the authors may have measured a requirement for ESAT-6 to breach epithelial barriers. Such situations may in fact account for discrepancies found for some of these pathogens regarding a requirement for their PFTs in colonization or early growth. In looking at the experimental methods for several (but not all) bacteria, the differences may well have to do with the route of infection, i.e., whether or not the bacteria need to pass an epithelial or endothelial layer to reach the target organ. Among the examples listed in “Other PFT Functions and Effects,” this is illustrated by S. aureus PVL (143, 182) and GBS β-h/c (175–177). It thus appears that if PFTs damage host cells with the express purpose of obtaining nutrients or altering growth conditions, this is limited to establishing an infection or affecting spreading.
Disruption of the Host Immune Response
PFTs have the ability to disrupt host immune responses. In some cases, PFTs cause an exacerbated inflammatory response that leads to extensive host tissue damage (e.g., with S. aureus PVL [143]). In many cases, PFTs impair immune defenses, and this is accomplished through several different mechanisms. These include allowing bacteria to physically hide from the immune system and survive phagocytosis (e.g., L. monocytogenes LLO [424, 425], M. tuberculosis ESAT-6 [122], GBS β-h/c [361], S. aureus alpha-toxin [435], and C. perfringens PFO [92]), preventing the activation of immune pathways (e.g., complement activation by S. pneumoniae PLY [181] or inhibition of the IgG and IgM response by LLO during L. monocytogenes infection [383]), and preventing the actions of antimicrobial compounds (178, 447).
Another mechanism is decreasing or preventing the recruitment of phagocytic cells to the site of infection (reported for GAS SLO and SLS [188, 195]). However, in other cases, PFTs are instead found to increase leukocyte infiltration (S. aureus PVL [145, 146], V. cholerae VCC [205], and GAS SLO and SLS [188]). PFTs can induce programmed cell death in immune cells, seen in vivo or ex vivo for GAS SLO (187), S. aureus alpha-toxin (423), B. anthracis ALO (355), and Bordetella pertussis ACT (62) (Table 1) and in vitro (among others) for GBS β-h/c (178), L. monocytogenes LLO (10), M. tuberculosis ESAT-6 (123), and E. coli HlyA (100, 101). Often, when a lack of leukocytes at the site of infection is observed, the available data do not allow for distinction between reduced recruitment and killing of recruited cells upon arrival. In addition, as discussed for GAS SLS, which in mice inhibited neutrophil migration after subcutaneous infection (195) but was cytotoxic to newly recruited neutrophils after intraperitoneal challenge (196) (see “Immune Evasion”), the specific site of infection (or other experimental factors) may influence which mechanism is used to disable immune cells.
Host Pathways Involved in Defense against PFTs
Our current understanding of generalized host responses to bacterial PFTs comes largely from in vitro work and studies involving the nematode Caenorhabditis elegans, and hence comprise mostly cellular and innate immune defense mechanisms, as discussed in PFT Effects and Cellular Defense Mechanisms. Important aspects of cellular host defenses against PFTs in vitro are MAPK activation, activation of the inflammasome and programmed cell death mechanisms, and activation of membrane repair mechanisms involving the vesicle trafficking machinery. With the exception of membrane repair mechanisms, which have not been determined clearly in vivo except for in C. elegans (330), all of these mechanisms were also identified in vivo in 1 or more of the 10 pathogens discussed here (Table 2). A role in PFT defense, or at least PFT-dependent activation, was seen for MAPKs with S. pneumoniae PLY (p38) (390), GBS β-h/c (p38 and JNK) (361), B. anthracis ALO (p38 and ERK) (34), and B. thuringiensis Cry toxins (p38 and JNK) (231, 313). A role for the inflammasome was observed for S. pneumoniae PLY (357, 363), S. aureus alpha-toxin (366), and possibly GAS SLO (365) and L. monocytogenes LLO (362). Programmed cell death was induced in vivo or ex vivo by several PFTs (Table 3). Although in many cases the controlled cell death appears to benefit the pathogen, motivations for the host to trigger apoptosis after PFT attack could include the preservation of tissue structure by removing compromised cells. The fact that several of the in vitro findings extend to in vivo models provides confidence regarding their relevance to human infection.
With regard to PRRs and cytokines, no uniform response to PFTs appears to exist, and published findings on occasion appear to contradict each other (especially for TLR2- and TLR4-mediated responses to S. pneumoniae and PLY). Both TLR2 and TLR4 have been found to directly bind at least one PFT in vitro (S. aureus PVL and S. pneumoniae PLY, respectively) (347, 353), and both have been reported to be involved in the defense against PFT-mediated aspects of infections (see “Innate Immune Responses to PFTs”). The most-discussed proinflammatory cytokines are all upregulated by several PFTs in vivo (seen for TNF-α by S. pneumoniae PLY [359] and V. cholerae VCC [338], for IL-1β by PLY [359], E. coli HlyA [98, 348], and S. aureus alpha-toxin [366], and for IL-6 by PLY [343, 354, 359, 360], S. aureus alpha-toxin and γ-hemolysins [152], and VCC [338]). TNF-α and IL-1β were both found to be downregulated by GAS SLO (187). IL-1β is required for defense against LLO-mediated effects during L. monocytogenes infection (362), and possibly against PLY-mediated effects (344, 364).
Two recent articles reviewed here add additional insight to the general picture of host PFT defense mechanisms. The first is a study showing that the GHRH agonist JI-34 increases host cell cAMP levels, stabilizing the membrane during an attack by S. pneumoniae PLY (388). The second found that TNF-α-dependent PLSCR1 helps to maintain intracellular ATP levels while cells are under attack by S. aureus alpha-toxin (448). Both mechanisms involve adenosine phosphates, which may hint at an important role for cellular energy levels (which would be consistent with the fact that many metabolic genes were found to be expressed differentially in C. elegans in response to B. thuringiensis Cry5B exposure [231]) or (not mutually exclusive) a role for ATP- or cAMP-dependent signaling pathways.
All in all, the exact mechanisms of action of these pathways, i.e., precisely how they exert their protective effects, remain mostly unclear. In addition, much PFT research, especially in vitro studies, is performed using isolated or purified toxins. Experimental doses used may be significantly higher than those observed during an infection. Also, defense mechanisms may function differently in the context of an intact pathogen (as with the hypoxia response pathway, which helps C. elegans to defend against VCC but increases its sensitivity to VCC-deficient V. cholerae [203]). An overview of known PFT defense pathways and mechanisms is provided in Fig. 4.
Fig 4.
Overview of host pathways that are activated by PFTs. Note that not all pathways are relevant to all toxins and hosts.
Cautionary Notes
In vivo research on PFTs in several cases appears to be plagued by inconsistencies. Many of these paradoxical findings, generally debating the contribution of a PFT to virulence, are likely explained by insufficient host specificity of the PFT, the use of different host species, and the presence of redundantly functioning PFTs in the bacterium. A prime example of this is S. aureus PVL, whose role in virulence is surrounded by controversy. Introduction of PVL into a laboratory S. aureus strain changed the expression of other virulence factors (143), but deletion of PVL from the USA300 and USA400 clinical isolates had no influence on global gene expression (147). Experiments on mice and rabbits have usually confirmed a role for PVL in virulence (143, 145–149, 469), whereas studies using rats and primates, and occasionally mice and rabbits, have not identified a role for PVL in infection (155, 345, 470, 471). Given the relative insensitivity of mouse and rat PMNs to PVL, rabbits appear to be a better model for studying the effects of PVL, as their PMNs are more sensitive to PVL, more closely resembling the human situation (145). This is especially important because PMNs seem to be a prime target of PVL. Many of the conflicting findings regarding PVL's role in virulence appear to be attributable to details of the experimental setup, such as differences in susceptibility between the various animal models, genetic differences between hosts of the same species, and differences in the concentrations of inocula used and in the route of infection (e.g., intravenous, subcutaneous, or intranasal). Importantly, most of these studies did not account for the presence of other PFTs, such as alpha-toxin and the γ-hemolysins. These aspects of S. aureus biology are the subject of a recent review article (472).
It follows from the above observations that results from animal studies should be assessed with care with regard to their direct relevance to human disease, since affinities of PFTs for different hosts may affect the outcomes of studies (as with S. aureus PVL) or completely change the way that bacteria behave (as with Bacillus cereus, B. anthracis, and B. thuringiensis) (Table 2). Furthermore, note that the majority (if not all) of the bacteria discussed here use additional, non-PFT virulence factors, which may function synergistically with PFTs. Such effects have been observed for PLY and hyaluronidase during S. pneumoniae infection (473) and are analogous to the different subunits of multicomponent toxins such as B. anthracis anthrax toxin and B. pertussis ACT (Tables 1 and 2). Observed differences in an infection model between a PFT-positive and a PFT-negative bacterial strain may therefore be more than just the effects of holes in host cell plasma membranes, e.g., the PFT may affect expression of other virulence factors, or the PFT may give other factors access to the host cell cytosol. Additionally, bacteria likely possess different mechanisms through which they can infect hosts and that may or may not function in parallel. Which of the virulence mechanisms at its disposal that a bacterial pathogen will employ likely depends upon factors such as the host species and immune status.
PFTs as Targets for Antimicrobial Prophylactics and Therapeutics
The large majority of newly introduced antibiotics over the last 50 years are variations on a few common core mechanisms. Thus, the development of new scaffolds, narrower-spectrum or virulence-targeted antimicrobial prophylactics and therapeutics, and combination therapies is a requirement for the continued treatment of increasingly resistant bacteria (1). The PFT class of virulence factors may thus be a candidate target, as a PFT-targeting compound would fit several of these criteria. From this review, it is clear that there are numerous commonalities between PFT-induced effects during infections by different bacteria.
PFTs may be viable targets for vaccination against bacterial infection, as observed, for instance, for S. pneumoniae, S. aureus, M. tuberculosis, and L. monocytogenes (370–373). Although active immunization against ALO protected mice from purified ALO, it did not protect them from B. anthracis infection (474). Thus, successful immunization against a PFT is not always an effective vaccination against disease. Even if effective, a vaccine will function to prevent infection involving only one specific PFT rather than being broadly applicable against many bacterial infections. Additionally, vaccines function exclusively to prevent disease, not to cure. Other approaches to targeting PFTs are thus worth pursuing.
The disabling of PFTs may contribute to eradicating S. aureus infection (475) and has been shown to inhibit cellular invasion by GBS in vitro (476). Inhibiting the effects of PFTs on host epithelia also increases survival of the host during S. pneumoniae infection (436). Further examples are cyclodextrin derivatives, which can disable B. anthracis PA pores and S. aureus alpha-toxin pores in vitro and protect mice from killing by anthrax in vivo (477, 478), and pore-dead PFTs or other compounds used as competitive inhibitors of live PFTs (479, 480). Other, hypothetical mechanisms include the administration of decoy membranes (330) and methods to inhibit expression or release of PFTs. In the case of C. elegans and B. thuringiensis Cry PFTs, the host even appears to possess a native mechanism that functions as a competitive interactor with the PFT receptor (481). Another class of drugs could function specifically to boost host defenses. For example, known defensive molecular pathways, such as MAPK pathways, could be activated.
The various steps in the pore formation process, as outlined in Fig. 1 and also including the multistep processing pathways sometimes required to activate PFTs (see “Two Main Effects of PFTs on Infection”), may be potential intervention points for drugs. Additionally, PFT function could be targeted by counteracting the effects of PFTs (i.e., preventing loss of epithelial barrier function or fortifying the immune system) or by specifically boosting or preactivating host defenses that neutralize PFTs. A recent study found that barrier dysfunction in vitro induced by thrombin or histamine was mediated via the tyrosine kinase Abl-related gene (Arg) and could be prevented by administering imatinib, a drug used to treat leukemia and gastrointestinal stromal cancers, which inhibits Arg. In vivo in mice, imatinib was able to protect against vascular endothelial growth factor (VEGF)-induced vascular leakage and pulmonary edema (482). Besides providing a lead for studying mechanisms through which PFTs may induce barrier dysfunction, imatinib may be a compound to pursue in the search for drugs that block specific PFT effects.
PFT-targeting drugs could help to limit the extent of infection, aid in preventing systemic spreading when a localized infection is present, and prevent PFT-mediated tissue destruction (e.g., in S. pneumoniae or S. aureus pneumonia or clostridial myonecrosis). Such drugs could also be used to prevent problematic nosocomial infections (e.g., preventive administration during surgery or the use of catheters). Additionally, the introduction of a novel class of antimicrobial prophylactic or therapeutic agents would open up possibilities for adjunctive therapy (e.g., coadministration with existing antibiotics), which may result in synergistic effects. The use of existing bactericidal antibiotics can lead to increased release of PFTs from bacteria (as observed, for example, during treatment of S. pneumoniae infection [392]), and adjunctive therapy could also limit the damage caused by the released PFTs (e.g., in line with the S. pneumoniae example, anti-PLY antibodies can limit PLY effects [483], and administration of antibodies that inhibit B. anthracis ALO's cytotoxic properties have a protective effect in B. anthracis-infected mice [484]).
Suggestions for Future Research
Based on the work reviewed here and our conclusions, we put forward several suggestions with regard to future PFT studies. First of all, with regard to the technical aspects of PFT research, we feel that the notion of PFTs as unique, individually operating virulence factors needs to be revised. Rather, PFTs should be studied as a class of toxins, with a focus on the common ground between different PFTs. As we hope has become clear from this review, PFTs are widespread among bacterial pathogens and share several core functions and effects. Also, and following from the above, different PFTs within a single pathogen are likely to function (at least partially) redundantly. Therefore, using the classical approach of excising a single virulence factor to study its function may cause aspects of PFT functions to remain obscured, as these may be masked by other, functionally redundant PFTs expressed by the same pathogen. Additionally, for several PFTs, it has become clear that the host specificity of the toxins and the choice of model organisms strongly influence study outcomes. Thus, in vivo PFT studies will benefit from a careful assessment of the applicability of specific host animals to model PFT function during human infection.
It is our opinion that the goals for future PFT research should be 2-fold. First, there are currently many holes in our knowledge that warrant further fundamental studies, most notably the detailed workings of host defense mechanisms and the generalization, or cross-species translation, of PFT functions in pathogenesis. Such research could focus on testing the general applicability of hypotheses such as those outlined in this review to other important, PFT-wielding human pathogens, using multiple host systems. For example, future PFT studies could incorporate such outcomes as whether levels of syndecans and shed membrane proteins are elevated in host blood or lavage fluid, whether PFTs aid in epithelial barrier dysfunction and bacterial spreading, which of the common host responses occur, and so on.
As outlined in the previous section, novel drugs specifically targeting PFT function are worth pursuing in the development of new classes of antimicrobial therapeutics and will likely be completely independent in function and structure from any current class of antibiotics. Second, therefore, applied research should focus on finding novel treatments that impair PFTs from a broad range of bacterial pathogens so that we may continue treating bacterial infectious diseases in the future.
ACKNOWLEDGMENTS
We are very grateful to Maarten Los for assistance with literature research and to Frans Los and Cheng-Yuan Kao for critical readings of the manuscript.
Biographies

Ferdinand Los is a postdoctoral scientist at Columbia University, in the lab of Adam Ratner. He received an M.Sc. degree in general biology from Utrecht University (Netherlands) in 2004 and was a volunteer scientist in the lab of Hendrik Korswagen at the Hubrecht Institute (Utrecht, Netherlands) in 2005 and 2006. Dr. Los received his doctoral training in the lab of Raffi Aroian at the University of California San Diego from 2006 to 2010 and defended his Ph.D. thesis at Utrecht University in 2011. At the University of California San Diego, Dr. Los researched mechanisms involved in repair of the host cell plasma membrane after attack by bacterial PFTs and defensive neuronal host responses to PFTs, using the nematode Caenorhabditis elegans. Currently, he studies the role of orphan nuclear receptors in host defense against PFTs in human cells.

Tara M. Randis is an Assistant Professor of Pediatrics at Columbia University Medical Center and a member of the Division of Neonatology and Perinatology. She is a graduate of The University of Scranton and received her M.D. from MCP Hahnemann University School of Medicine, Philadelphia, PA. She completed her pediatric residency training at St. Christopher's Hospital for Children, Philadelphia, PA, where she served an additional year as Chief Resident before pursuing her fellowship training in neonatal-perinatal medicine at Columbia University. In 2011, she earned an M.S. degree in biostatistics from the Mailman School of Public Health at Columbia University. In 2005, Dr. Randis was awarded The Young Investigator Grant from the American Academy of Pediatrics Neonatal Resuscitation Program, and in 2011 she received a Mentored Patient-Oriented Research Development Award from the National Institute of Child Health and Human Development for her work investigating the link between vitamin D deficiency and bacterial vaginosis in pregnant women. Her current research focuses on bacterial colonization of the female reproductive tract as it relates to adverse pregnancy outcomes, including chorioamnionitis, preterm labor, and early-onset neonatal sepsis.

Raffi V. Aroian received his Ph.D. from the California Institute of Technology. His postdoctoral studies were carried out at the University of California San Francisco, where he was a Helen Hayes Whitney Fellow and a Senior Postdoctoral Fellow of the American Cancer Society. He is currently a Professor of Biological Sciences at the University of California San Diego, where his group is (i) studying the genetics and cell biology of cellular defenses against pore-forming toxins and (ii) developing new treatments for soil-transmitted helminths (intestinal roundworms), which are leading causes of childhood disease, affecting 400,000,000 children worldwide. He was a recipient of a New Investigators Award in Toxicological Sciences from the Burroughs-Wellcome Foundation and a Beckman Foundation Young Investigator.

Adam J. Ratner is an Associate Professor of Pediatrics and a member of the Division of Pediatric Infectious Diseases at Columbia University. He received M.D. and M.P.H. degrees from Columbia University. He then completed training in pediatrics at Columbia University and in pediatric infectious diseases at the Children's Hospital of Philadelphia. His postdoctoral work, carried out under the mentorship of Jeffrey Weiser, examined mechanisms of interspecies competition during mucosal colonization, determined pathways by which mucosal epithelial cells sense sublethal concentrations of bacterial pore-forming toxins, and delineated a new link between combinations of extracellular pathogens and host cytoplasmic pattern recognition receptors. His current research focuses on mechanisms of bacterial colonization of human mucosal surfaces, with a particular interest in pathogens that cause perinatal and neonatal infections and interbacterial interactions in the vaginal microbiota.
REFERENCES
- 1. Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woodford N, Livermore DM. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 59(Suppl 1):S4–S16 [DOI] [PubMed] [Google Scholar]
- 3. Huttner B, Goossens H, Verheij T, Harbarth S. 2010. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect. Dis. 10:17–31 [DOI] [PubMed] [Google Scholar]
- 4. Alouf JE. 2003. Molecular features of the cytolytic pore-forming bacterial protein toxins. Folia Microbiol. (Prague) 48:5–16 [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B. 2008. Bacterial pore-forming toxins: the (w)hole story? Cell. Mol. Life Sci. 65:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iacovache I, Bischofberger M, van der Goot FG. 2010. Structure and assembly of pore-forming proteins. Curr. Opin. Struct. Biol. 20:241–246 [DOI] [PubMed] [Google Scholar]
- 7. Collier RJ. 2009. Membrane translocation by anthrax toxin. Mol. Aspects Med. 30:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ratner AJ, Aguilar JL, Shchepetov M, Lysenko ES, Weiser JN. 2007. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cell. Microbiol. 9:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geny B, Popoff MR. 2006. Bacterial protein toxins and lipids: pore formation or toxin entry into cells. Biol. Cell 98:667–678 [DOI] [PubMed] [Google Scholar]
- 10. Schnupf P, Portnoy DA. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9:1176–1187 [DOI] [PubMed] [Google Scholar]
- 11. Frey J. 1995. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 3:257–261 [DOI] [PubMed] [Google Scholar]
- 12. Reimer D, Frey J, Jansen R, Veit HP, Inzana TJ. 1995. Molecular investigation of the role of ApxI and ApxII in the virulence of Actinobacillus pleuropneumoniae serotype 5. Microb. Pathog. 18:197–209 [DOI] [PubMed] [Google Scholar]
- 13. Tascon RI, Vazquez-Boland JA, Gutierrez-Martin CB, Rodriguez-Barbosa I, Rodriguez-Ferri EF. 1994. The RTX haemolysins ApxI and ApxII are major virulence factors of the swine pathogen Actinobacillus pleuropneumoniae: evidence from mutational analysis. Mol. Microbiol. 14:207–216 [DOI] [PubMed] [Google Scholar]
- 14. Inzana TJ, Todd J, Ma JN, Veit H. 1991. Characterization of a non-hemolytic mutant of Actinobacillus pleuropneumoniae serotype 5: role of the 110 kilodalton hemolysin in virulence and immunoprotection. Microb. Pathog. 10:281–296 [DOI] [PubMed] [Google Scholar]
- 15. Schaller A, Kuhn R, Kuhnert P, Nicolet J, Anderson TJ, MacInnes JI, Segers RP, Frey J. 1999. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology 145:2105–2116 [DOI] [PubMed] [Google Scholar]
- 16. Parker MW, Feil SC. 2005. Pore-forming protein toxins: from structure to function. Prog. Biophys. Mol. Biol. 88:91–142 [DOI] [PubMed] [Google Scholar]
- 17. Wilmsen HU, Pattus F, Buckley JT. 1990. Aerolysin, a hemolysin from Aeromonas hydrophila, forms voltage-gated channels in planar lipid bilayers. J. Membr. Biol. 115:71–81 [DOI] [PubMed] [Google Scholar]
- 18. Wong CY, Heuzenroeder MW, Flower RL. 1998. Inactivation of two haemolytic toxin genes in Aeromonas hydrophila attenuates virulence in a suckling mouse model. Microbiology 144:291–298 [DOI] [PubMed] [Google Scholar]
- 19. Chakraborty T, Huhle B, Hof H, Bergbauer H, Goebel W. 1987. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect. Immun. 55:2274–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heuzenroeder MW, Wong CY, Flower RL. 1999. Distribution of two hemolytic toxin genes in clinical and environmental isolates of Aeromonas spp.: correlation with virulence in a suckling mouse model. FEMS Microbiol. Lett. 174:131–136 [DOI] [PubMed] [Google Scholar]
- 21. Erova TE, Sha J, Horneman AJ, Borchardt MA, Khajanchi BK, Fadl AA, Chopra AK. 2007. Identification of a new hemolysin from diarrheal isolate SSU of Aeromonas hydrophila. FEMS Microbiol. Lett. 275:301–311 [DOI] [PubMed] [Google Scholar]
- 22. Ferguson MR, Xu XJ, Houston CW, Peterson JW, Coppenhaver DH, Popov VL, Chopra AK. 1997. Hyperproduction, purification, and mechanism of action of the cytotoxic enterotoxin produced by Aeromonas hydrophila. Infect. Immun. 65:4299–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu XJ, Ferguson MR, Popov VL, Houston CW, Peterson JW, Chopra AK. 1998. Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: development of transposon and isogenic mutants. Infect. Immun. 66:3501–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imaizumi K, Matsunaga K, Higuchi H, Kaidoh T, Takeuchi S. 2003. Effect of amino acid substitutions in the epitope regions of pyolysin from Arcanobacterium pyogenes. Vet. Microbiol. 91:205–213 [DOI] [PubMed] [Google Scholar]
- 25. Donkor AD, Su Z, Mandal HS, Jin X, Tang X. 2009. Carbon nanotubes inhibit the hemolytic activity of the pore-forming toxin pyolysin. Nano Res. 2:517–525 [Google Scholar]
- 26. Jost BH, Songer JG, Billington SJ. 1999. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infect. Immun. 67:1723–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jost BH, Trinh HT, Songer JG, Billington SJ. 2003. Immunization with genetic toxoids of the Arcanobacterium pyogenes cholesterol-dependent cytolysin, pyolysin, protects mice against infection. Infect. Immun. 71:2966–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young JA, Collier RJ. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243–265 [DOI] [PubMed] [Google Scholar]
- 29. Brossier F, Weber-Levy M, Mock M, Sirard JC. 2000. Role of toxin functional domains in anthrax pathogenesis. Infect. Immun. 68:1781–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loving CL, Khurana T, Osorio M, Lee GM, Kelly VK, Stibitz S, Merkel TJ. 2009. Role of anthrax toxins in dissemination, disease progression, and induction of protective adaptive immunity in the mouse aerosol challenge model. Infect. Immun. 77:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pezard C, Berche P, Mock M. 1991. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 59:3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klichko VI, Miller J, Wu A, Popov SG, Alibek K. 2003. Anaerobic induction of Bacillus anthracis hemolytic activity. Biochem. Biophys. Res. Commun. 303:855–862 [DOI] [PubMed] [Google Scholar]
- 33. Mosser EM, Rest RF. 2006. The Bacillus anthracis cholesterol-dependent cytolysin, anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol. 6:56 doi: 10.1186/1471-2180-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Popova TG, Millis B, Bradburne C, Nazarenko S, Bailey C, Chandhoke V, Popov SG. 2006. Acceleration of epithelial cell syndecan-1 shedding by anthrax hemolytic virulence factors. BMC Microbiol. 6:8 doi: 10.1186/1471-2180-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fagerlund A, Lindback T, Storset AK, Granum PE, Hardy SP. 2008. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 154:693–704 [DOI] [PubMed] [Google Scholar]
- 36. Haug TM, Sand SL, Sand O, Phung D, Granum PE, Hardy SP. 2010. Formation of very large conductance channels by Bacillus cereus Nhe in Vero and GH(4) cells identifies NheA+B as the inherent pore-forming structure. J. Membr. Biol. 237:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 38. Beecher DJ, Schoeni JL, Wong AC. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beecher DJ, Wong AC. 1994. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect. Immun. 62:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beecher DJ, Olsen TW, Somers EB, Wong AC. 2000. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect. Immun. 68:5269–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hardy SP, Lund T, Granum PE. 2001. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 197:47–51 [DOI] [PubMed] [Google Scholar]
- 42. Lund T, De Buyser ML, Granum PE. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254–261 [DOI] [PubMed] [Google Scholar]
- 43. Andreeva ZI, Nesterenko VF, Fomkina MG, Ternovsky VI, Suzina NE, Bakulina AY, Solonin AS, Sineva EV. 2007. The properties of Bacillus cereus hemolysin II pores depend on environmental conditions. Biochim. Biophys. Acta 1768:253–263 [DOI] [PubMed] [Google Scholar]
- 44. Nishiwaki H, Nakashima K, Ishida C, Kawamura T, Matsuda K. 2007. Cloning, functional characterization, and mode of action of a novel insecticidal pore-forming toxin, sphaericolysin, produced by Bacillus sphaericus. Appl. Environ. Microbiol. 73:3404–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pauchet Y, Luton F, Castella C, Charles JF, Romey G, Pauron D. 2005. Effects of a mosquitocidal toxin on a mammalian epithelial cell line expressing its target receptor. Cell. Microbiol. 7:1335–1344 [DOI] [PubMed] [Google Scholar]
- 46. Schwartz JL, Potvin L, Coux F, Charles JF, Berry C, Humphreys MJ, Jones AF, Bernhart I, Dalla Serra M, Menestrina G. 2001. Permeabilization of model lipid membranes by Bacillus sphaericus mosquitocidal binary toxin and its individual components. J. Membr. Biol. 184:171–183 [DOI] [PubMed] [Google Scholar]
- 47. Srisucharitpanit K, Inchana P, Rungrod A, Promdonkoy B, Boonserm P. 2012. Expression and purification of the active soluble form of Bacillus sphaericus binary toxin for structural analysis. Protein Expr. Purif. 82:368–372 [DOI] [PubMed] [Google Scholar]
- 48. Brunet JF, Vachon V, Juteau M, Van Rie J, Larouche G, Vincent C, Schwartz JL, Laprade R. 2010. Pore-forming properties of the Bacillus thuringiensis toxin Cry9Ca in Manduca sexta brush border membrane vesicles. Biochim. Biophys. Acta 1798:1111–1118 [DOI] [PubMed] [Google Scholar]
- 49. Gazit E, La Rocca P, Sansom MS, Shai Y. 1998. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis delta-endotoxin are consistent with an “umbrella-like” structure of the pore. Proc. Natl. Acad. Sci. U. S. A. 95:12289–12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL, Brousseau R, Cygler M. 1995. Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation. J. Mol. Biol. 254:447–464 [DOI] [PubMed] [Google Scholar]
- 51. Li JD, Carroll J, Ellar DJ. 1991. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature 353:815–821 [DOI] [PubMed] [Google Scholar]
- 52. Masson L, Schwab G, Mazza A, Brousseau R, Potvin L, Schwartz JL. 2004. A novel Bacillus thuringiensis (PS149B1) containing a Cry34Ab1/Cry35Ab1 binary toxin specific for the western corn rootworm Diabrotica virgifera virgifera LeConte forms ion channels in lipid membranes. Biochemistry 43:12349–12357 [DOI] [PubMed] [Google Scholar]
- 53. Schwartz JL, Garneau L, Masson L, Brousseau R. 1991. Early response of cultured lepidopteran cells to exposure to delta-endotoxin from Bacillus thuringiensis: involvement of calcium and anionic channels. Biochim. Biophys. Acta 1065:250–260 [DOI] [PubMed] [Google Scholar]
- 54. Slatin SL, Abrams CK, English L. 1990. Delta-endotoxins form cation-selective channels in planar lipid bilayers. Biochem. Biophys. Res. Commun. 169:765–772 [DOI] [PubMed] [Google Scholar]
- 55. Tran LB, Vachon V, Schwartz JL, Laprade R. 2001. Differential effects of pH on the pore-forming properties of Bacillus thuringiensis insecticidal crystal toxins. Appl. Environ. Microbiol. 67:4488–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bravo A, Gill SS, Soberon M. 2007. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ghelardi E, Celandroni F, Salvetti S, Fiscarelli E, Senesi S. 2007. Bacillus thuringiensis pulmonary infection: critical role for bacterial membrane-damaging toxins and host neutrophils. Microbes Infect. 9:591–598 [DOI] [PubMed] [Google Scholar]
- 58. Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV. 2003. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. U. S. A. 100:2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li J, Koni PA, Ellar DJ. 1995. Crystallization of a membrane pore-forming protein with mosquitocidal activity from Bacillus thuringiensis subspecies kyushuensis. Proteins 23:290–293 [DOI] [PubMed] [Google Scholar]
- 60. Vojtova J, Kamanova J, Sebo P. 2006. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr. Opin. Microbiol. 9:69–75 [DOI] [PubMed] [Google Scholar]
- 61. Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. 1988. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 7:3997–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gueirard P, Druilhe A, Pretolani M, Guiso N. 1998. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect. Immun. 66:1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, Iwakura Y, Tschopp J, Sebo P, Mills KH. 2010. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J. Immunol. 185:1711–1719 [DOI] [PubMed] [Google Scholar]
- 64. Goodwin MS, Weiss AA. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khelef N, Sakamoto H, Guiso N. 1992. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb. Pathog. 12:227–235 [DOI] [PubMed] [Google Scholar]
- 66. Oldenburg DJ, Gross MK, Smith AL, Storm DR. 1993. Virulence of a Bordetella pertussis strain expressing a mutant adenylyl cyclase with decreased calmodulin affinity. Microb. Pathog. 14:489–493 [DOI] [PubMed] [Google Scholar]
- 67. Barloy F, Delecluse A, Nicolas L, Lecadet MM. 1996. Cloning and expression of the first anaerobic toxin gene from Clostridium bifermentans subsp. malaysia, encoding a new mosquitocidal protein with homologies to Bacillus thuringiensis delta-endotoxins. J. Bacteriol. 178:3099–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barloy F, Lecadet MM, Delecluse A. 1998. Cloning and sequencing of three new putative toxin genes from Clostridium bifermentans CH18. Gene 211:293–299 [DOI] [PubMed] [Google Scholar]
- 69. Haque A, Sugimoto N, Horiguchi Y, Okabe T, Miyata T, Iwanaga S, Matsuda M. 1992. Production, purification, and characterization of botulinolysin, a thiol-activated hemolysin of Clostridium botulinum. Infect. Immun. 60:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sekiya K, Danbara H, Futaesaku Y, Haque A, Sugimoto N, Matsuda M. 1998. Formation of ring-shaped structures on erythrocyte membranes after treatment with botulinolysin, a thiol-activated hemolysin from Clostridium botulinum. Infect. Immun. 66:2987–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sugimoto N, Haque A, Horiguchi Y, Matsuda M. 1995. Coronary vasoconstriction is the most probable cause of death of rats intoxicated with botulinolysin, a hemolysin produced by Clostridium botulinum. Toxicon 33:1215–1230 [DOI] [PubMed] [Google Scholar]
- 72. Sugimoto N, Haque A, Horiguchi Y, Matsuda M. 1997. Botulinolysin, a thiol-activated hemolysin produced by Clostridium botulinum, inhibits endothelium-dependent relaxation of rat aortic ring. Toxicon 35:1011–1023 [DOI] [PubMed] [Google Scholar]
- 73. Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4:e26 doi: 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cooper KK, Songer JG. 2010. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet. Microbiol. 142:323–328 [DOI] [PubMed] [Google Scholar]
- 75. Smyth JA, Martin TG. 2010. Disease producing capability of netB positive isolates of C. perfringens recovered from normal chickens and a cow, and netB positive and negative isolates from chickens with necrotic enteritis. Vet. Microbiol. 146:76–84 [DOI] [PubMed] [Google Scholar]
- 76. Steinthorsdottir V, Halldorsson H, Andresson OS. 2000. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 28:45–50 [DOI] [PubMed] [Google Scholar]
- 77. Springer S, Selbitz HJ. 1999. The control of necrotic enteritis in sucking piglets by means of a Clostridium perfringens toxoid vaccine. FEMS Immunol. Med. Microbiol. 24:333–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15–30 [DOI] [PubMed] [Google Scholar]
- 79. Cole AR, Gibert M, Popoff M, Moss DS, Titball RW, Basak AK. 2004. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat. Struct. Mol. Biol. 11:797–798 [DOI] [PubMed] [Google Scholar]
- 80. Petit L, Maier E, Gibert M, Popoff MR, Benz R. 2001. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J. Biol. Chem. 276:15736–15740 [DOI] [PubMed] [Google Scholar]
- 81. Uzal FA, Kelly WR, Morris WE, Assis RA. 2002. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. J. Comp. Pathol. 126:71–75 [DOI] [PubMed] [Google Scholar]
- 82. Lonchamp E, Dupont JL, Wioland L, Courjaret R, Mbebi-Liegeois C, Jover E, Doussau F, Popoff MR, Bossu JL, de Barry J, Poulain B. 2010. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS One 5:e13046 doi: 10.1371/journal.pone.0013046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685–692 [DOI] [PubMed] [Google Scholar]
- 84. Shepard LA, Heuck AP, Hamman BD, Rossjohn J, Parker MW, Ryan KR, Johnson AE, Tweten RK. 1998. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry 37:14563–14574 [DOI] [PubMed] [Google Scholar]
- 85. Shepard LA, Shatursky O, Johnson AE, Tweten RK. 2000. The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry 39:10284–10293 [DOI] [PubMed] [Google Scholar]
- 86. Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood JI. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904–7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Awad MM, Bryant AE, Stevens DL, Rood JI. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191–202 [DOI] [PubMed] [Google Scholar]
- 88. Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H, Horiguchi Y. 2011. Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J. Biol. Chem. 286:19549–19555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Caserta JA, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA. 2011. Development and application of a mouse intestinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect. Immun. 79:3020–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Melton JA, Parker MW, Rossjohn J, Buckley JT, Tweten RK. 2004. The identification and structure of the membrane-spanning domain of the Clostridium septicum alpha toxin. J. Biol. Chem. 279:14315–14322 [DOI] [PubMed] [Google Scholar]
- 91. Kennedy CL, Krejany EO, Young LF, O'Connor JR, Awad MM, Boyd RL, Emmins JJ, Lyras D, Rood JI. 2005. The alpha-toxin of Clostridium septicum is essential for virulence. Mol. Microbiol. 57:1357–1366 [DOI] [PubMed] [Google Scholar]
- 92. Hickey MJ, Kwan RY, Awad MM, Kennedy CL, Young LF, Hall P, Cordner LM, Lyras D, Emmins JJ, Rood JI. 2008. Molecular and cellular basis of microvascular perfusion deficits induced by Clostridium perfringens and Clostridium septicum. PLoS Pathog. 4:e1000045 doi: 10.1371/journal.ppat.1000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kennedy CL, Lyras D, Cheung JK, Hiscox TJ, Emmins JJ, Rood JI. 2009. Cross-complementation of Clostridium perfringens PLC and Clostridium septicum alpha-toxin mutants reveals PLC is sufficient to mediate gas gangrene. Microbes Infect. 11:413–418 [DOI] [PubMed] [Google Scholar]
- 94. Rottem S, Groover K, Habig WH, Barile MF, Hardegree MC. 1990. Transmembrane diffusion channels in Mycoplasma gallisepticum induced by tetanolysin. Infect. Immun. 58:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hardegree MC, Palmer AE, Duffin N. 1971. Tetanolysin: in-vivo effects in animals. J. Infect. Dis. 123:51–60 [DOI] [PubMed] [Google Scholar]
- 96. Coburn PS, Gilmore MS. 2003. The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 5:661–669 [DOI] [PubMed] [Google Scholar]
- 97. Cox CR, Coburn PS, Gilmore MS. 2005. Enterococcal cytolysin: a novel two component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr. Protein Pept. Sci. 6:77–84 [DOI] [PubMed] [Google Scholar]
- 98. Gleason TG, Houlgrave CW, May AK, Crabtree TD, Sawyer RG, Denham W, Norman JG, Pruett TL. 1998. Hemolytically active (acylated) alpha-hemolysin elicits interleukin-1beta (IL-1beta) but augments the lethality of Escherichia coli by an IL-1- and tumor necrosis factor-independent mechanism. Infect. Immun. 66:4215–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. May AK, Gleason TG, Sawyer RG, Pruett TL. 2000. Contribution of Escherichia coli alpha-hemolysin to bacterial virulence and to intraperitoneal alterations in peritonitis. Infect. Immun. 68:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Russo TA, Davidson BA, Genagon SA, Warholic NM, Macdonald U, Pawlicki PD, Beanan JM, Olson R, Holm BA, Knight PR., 3rd 2005. E. coli virulence factor hemolysin induces neutrophil apoptosis and necrosis/lysis in vitro and necrosis/lysis and lung injury in a rat pneumonia model. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L207–L216 [DOI] [PubMed] [Google Scholar]
- 101. Russo TA, Wang Z, Davidson BA, Genagon SA, Beanan JM, Olson R, Holm BA, Knight PR, 3rd, Chess PR, Notter RH. 2007. Surfactant dysfunction and lung injury due to the E. coli virulence factor hemolysin in a rat pneumonia model. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L632–L643 [DOI] [PubMed] [Google Scholar]
- 102. Smith YC, Rasmussen SB, Grande KK, Conran RM, O'Brien AD. 2008. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 76:2978–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wiles TJ, Bower JM, Redd MJ, Mulvey MA. 2009. Use of zebrafish to probe the divergent virulence potentials and toxin requirements of extraintestinal pathogenic Escherichia coli. PLoS Pathog. 5:e1000697 doi: 10.1371/journal.ppat.1000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. 2008. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 190:3896–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cauci S, Scrimin F, Driussi S, Ceccone S, Monte R, Fant L, Quadrifoglio F. 1996. Specific immune response against Gardnerella vaginalis hemolysin in patients with bacterial vaginosis. Am. J. Obstet. Gynecol. 175:1601–1605 [DOI] [PubMed] [Google Scholar]
- 106. Cauci S, Driussi S, Monte R, Lanzafame P, Pitzus E, Quadrifoglio F. 1998. Immunoglobulin A response against Gardnerella vaginalis hemolysin and sialidase activity in bacterial vaginosis. Am. J. Obstet. Gynecol. 178:511–515 [DOI] [PubMed] [Google Scholar]
- 107. Martino MC, Stabler RA, Zhang ZW, Farthing MJ, Wren BW, Dorrell N. 2001. Helicobacter pylori pore-forming cytolysin orthologue TlyA possesses in vitro hemolytic activity and has a role in colonization of the gastric mucosa. Infect. Immun. 69:1697–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Czajkowsky DM, Iwamoto H, Cover TL, Shao Z. 1999. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc. Natl. Acad. Sci. U. S. A. 96:2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Necchi V, Sommi P, Ricci V, Solcia E. 2010. In vivo accumulation of Helicobacter pylori products, NOD1, ubiquitinated proteins and proteasome in a novel cytoplasmic structure. PLoS One 5:e9716 doi: 10.1371/journal.pone.0009716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Repp H, Pamukci Z, Koschinski A, Domann E, Darji A, Birringer J, Brockmeier D, Chakraborty T, Dreyer F. 2002. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell. Microbiol. 4:483–491 [DOI] [PubMed] [Google Scholar]
- 111. Ribet D, Hamon M, Gouin E, Nahori MA, Impens F, Neyret-Kahn H, Gevaert K, Vandekerckhove J, Dejean A, Cossart P. 2010. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 464:1192–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gaillard JL, Berche P, Sansonetti P. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun. 52:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Glomski IJ, Decatur AL, Portnoy DA. 2003. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect. Immun. 71:6754–6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Joyce SA, Gahan CG. 2010. Molecular pathogenesis of Listeria monocytogenes in the alternative model host Galleria mellonella. Microbiology 156:3456–3468 [DOI] [PubMed] [Google Scholar]
- 115. Kathariou S, Metz P, Hof H, Goebel W. 1987. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J. Bacteriol. 169:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schnupf P, Hofmann J, Norseen J, Glomski IJ, Schwartzstein H, Decatur AL. 2006. Regulated translation of listeriolysin O controls virulence of Listeria monocytogenes. Mol. Microbiol. 61:999–1012 [DOI] [PubMed] [Google Scholar]
- 117. Postma GC, Carfagnini JC, Minatel L. 2008. Moraxella bovis pathogenicity: an update. Comp. Immunol. Microbiol. Infect. Dis. 31:449–458 [DOI] [PubMed] [Google Scholar]
- 118. Clinkenbeard KD, Thiessen AE. 1991. Mechanism of action of Moraxella bovis hemolysin. Infect. Immun. 59:1148–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, Liu J, McDonald KL, Szyk A, LaRonde-LeBlanc N, Gao LY. 2008. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect. Immun. 76:5478–5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Volkman HE, Clay H, Beery D, Chang JC, Sherman DR, Ramakrishnan L. 2004. Tuberculous granuloma formation is enhanced by a Mycobacterium virulence determinant. PLoS Biol. 2:e367 doi: 10.1371/journal.pbio.0020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ulrichs T, Munk ME, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro ML, Kaufmann SH. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur. J. Immunol. 28:3949–3958 [DOI] [PubMed] [Google Scholar]
- 122. De Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honore N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J. Bacteriol. 189:6028–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Derrick SC, Morris SL. 2007. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell. Microbiol. 9:1547–1555 [DOI] [PubMed] [Google Scholar]
- 124. Stanley SA, Raghavan S, Hwang WW, Cox JS. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. U. S. A. 100:13001–13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hirakata Y, Furuya N, Tateda K, Kaku M, Yamaguchi K. 1993. In vivo production of exotoxin A and its role in endogenous Pseudomonas aeruginosa septicemia in mice. Infect. Immun. 61:2468–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Miyazaki S, Matsumoto T, Tateda K, Ohno A, Yamaguchi K. 1995. Role of exotoxin A in inducing severe Pseudomonas aeruginosa infections in mice. J. Med. Microbiol. 43:169–175 [DOI] [PubMed] [Google Scholar]
- 127. Schumann J, Angermuller S, Bang R, Lohoff M, Tiegs G. 1998. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and TNF. J. Immunol. 161:5745–5754 [PubMed] [Google Scholar]
- 128. Schultz MJ, Speelman P, Zaat SA, Hack CE, van Deventer SJ, van der Poll T. 2000. The effect of Pseudomonas exotoxin A on cytokine production in whole blood exposed to Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 29:227–232 [DOI] [PubMed] [Google Scholar]
- 129. Pillar CM, Hobden JA. 2002. Pseudomonas aeruginosa exotoxin A and keratitis in mice. Invest. Ophthalmol. Vis. Sci. 43:1437–1444 [PubMed] [Google Scholar]
- 130. Muhlen KA, Schumann J, Wittke F, Stenger S, Van Rooijen N, Van Kaer L, Tiegs G. 2004. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J. Immunol. 172:3034–3041 [DOI] [PubMed] [Google Scholar]
- 131. Oscarsson J, Westermark M, Lofdahl S, Olsen B, Palmgren H, Mizunoe Y, Wai SN, Uhlin BE. 2002. Characterization of a pore-forming cytotoxin expressed by Salmonella enterica serovars Typhi and Paratyphi A. Infect. Immun. 70:5759–5769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. von Rhein C, Hunfeld KP, Ludwig A. 2006. Serologic evidence for effective production of cytolysin A in Salmonella enterica serovars Typhi and Paratyphi A during human infection. Infect. Immun. 74:6505–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. von Rhein C, Bauer S, Lopez Sanjurjo EJ, Benz R, Goebel W, Ludwig A. 2009. ClyA cytolysin from Salmonella: distribution within the genus, regulation of expression by SlyA, and pore-forming characteristics. Int. J. Med. Microbiol. 299:21–35 [DOI] [PubMed] [Google Scholar]
- 134. Hertle R. 2002. Serratia marcescens hemolysin (ShlA) binds artificial membranes and forms pores in a receptor-independent manner. J. Membr. Biol. 189:1–14 [DOI] [PubMed] [Google Scholar]
- 135. Konninger UW, Hobbie S, Benz R, Braun V. 1999. The haemolysin-secreting ShlB protein of the outer membrane of Serratia marcescens: determination of surface-exposed residues and formation of ion-permeable pores by ShlB mutants in artificial lipid bilayer membranes. Mol. Microbiol. 32:1212–1225 [DOI] [PubMed] [Google Scholar]
- 136. Schonherr R, Hilger M, Broer S, Benz R, Braun V. 1994. Interaction of Serratia marcescens hemolysin (ShlA) with artificial and erythrocyte membranes. Demonstration of the formation of aqueous multistate channels. Eur. J. Biochem. 223:655–663 [DOI] [PubMed] [Google Scholar]
- 137. Kurz CL, Chauvet S, Andres E, Aurouze M, Vallet I, Michel GP, Uh M, Celli J, Filloux A, De Bentzmann S, Steinmetz I, Hoffmann JA, Finlay BB, Gorvel JP, Ferrandon D, Ewbank JJ. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22:1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Marre R, Hacker J, Braun V. 1989. The cell-bound hemolysin of Serratia marcescens contributes to uropathogenicity. Microb. Pathog. 7:153–156 [DOI] [PubMed] [Google Scholar]
- 139. Konig W, Faltin Y, Scheffer J, Schoffler H, Braun V. 1987. Role of cell-bound hemolysin as a pathogenicity factor for Serratia infections. Infect. Immun. 55:2554–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J. Infect. Dis. 206:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Finck-Barbancon V, Duportail G, Meunier O, Colin DA. 1993. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta 1182:275–282 [DOI] [PubMed] [Google Scholar]
- 142. Menestrina G, Dalla Serra M, Comai M, Coraiola M, Viero G, Werner S, Colin DA, Monteil H, Prevost G. 2003. Ion channels and bacterial infection: the case of beta-barrel pore-forming protein toxins of Staphylococcus aureus. FEBS Lett. 552:54–60 [DOI] [PubMed] [Google Scholar]
- 143. Tseng CW, Kyme P, Low J, Rocha MA, Alsabeh R, Miller LG, Otto M, Arditi M, Diep BA, Nizet V, Doherty TM, Beenhouwer DO, Liu GY. 2009. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS One 4:e6387 doi: 10.1371/journal.pone.0006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Prevost G, Cribier B, Couppie P, Petiau P, Supersac G, Finck-Barbancon V, Monteil H, Piemont Y. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63:4121–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U. S. A. 107:5587–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130–1133 [DOI] [PubMed] [Google Scholar]
- 147. Diep BA, Palazzolo-Ballance AM, Tattevin P, Basuino L, Braughton KR, Whitney AR, Chen L, Kreiswirth BN, Otto M, DeLeo FR, Chambers HF. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 3:e3198 doi: 10.1371/journal.pone.0003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cremieux AC, Dumitrescu O, Lina G, Vallee C, Cote JF, Muffat-Joly M, Lilin T, Etienne J, Vandenesch F, Saleh-Mghir A. 2009. Panton-Valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One 4:e7204 doi: 10.1371/journal.pone.0007204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vandenesch F, Couzon F, Boisset S, Benito Y, Brown EL, Lina G, Etienne J, Bowden MG. 2010. The Panton-Valentine leukocidin is a virulence factor in a murine model of necrotizing pneumonia. J. Infect. Dis. 201:967–969 [DOI] [PubMed] [Google Scholar]
- 150. Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. 1996. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274:1859–1866 [DOI] [PubMed] [Google Scholar]
- 151. Hildebrand A, Pohl M, Bhakdi S. 1991. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J. Biol. Chem. 266:17195–17200 [PubMed] [Google Scholar]
- 152. Nilsson IM, Hartford O, Foster T, Tarkowski A. 1999. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect. Immun. 67:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gemmell CG, Goutcher SC, Reid R, Sturrock RD. 1997. Role of certain virulence factors in a murine model of Staphylococcus aureus arthritis. J. Med. Microbiol. 46:208–213 [DOI] [PubMed] [Google Scholar]
- 154. Dajcs JJ, Austin MS, Sloop GD, Moreau JM, Hume EB, Thompson HW, McAleese FM, Foster TJ, O'Callaghan RJ. 2002. Corneal pathogenesis of Staphylococcus aureus strain Newman. Invest. Ophthalmol. Vis. Sci. 43:1109–1115 [PubMed] [Google Scholar]
- 155. Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405–1406 [DOI] [PubMed] [Google Scholar]
- 156. Bramley AJ, Patel AH, O'Reilly M, Foster R, Foster TJ. 1989. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect. Immun. 57:2489–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Callegan MC, Engel LS, Hill JM, O'Callaghan RJ. 1994. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect. Immun. 62:2478–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kernodle DS, Voladri RK, Menzies BE, Hager CC, Edwards KM. 1997. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect. Immun. 65:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Menzies BE, Kernodle DS. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62:1843–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. O'Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, Engel LS, Hill JM. 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 65:1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol. Microbiol. 79:814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. 2010. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634 doi: 10.1371/journal.pone.0011634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gravet A, Colin DA, Keller D, Girardot R, Monteil H, Prevost G. 1998. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 436:202–208 [DOI] [PubMed] [Google Scholar]
- 164. Alonzo F, III, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. 2012. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol. Microbiol. 83:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sugawara N, Tomita T, Kamio Y. 1997. Assembly of Staphylococcus aureus gamma-hemolysin into a pore-forming ring-shaped complex on the surface of human erythrocytes. FEBS Lett. 410:333–337 [DOI] [PubMed] [Google Scholar]
- 166. Yamashita K, Kawai Y, Tanaka Y, Hirano N, Kaneko J, Tomita N, Ohta M, Kamio Y, Yao M, Tanaka I. 2011. Crystal structure of the octameric pore of staphylococcal gamma-hemolysin reveals the beta-barrel pore formation mechanism by two components. Proc. Natl. Acad. Sci. U. S. A. 108:17314–17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Supersac G, Piemont Y, Kubina M, Prevost G, Foster TJ. 1998. Assessment of the role of gamma-toxin in experimental endophthalmitis using a hlg-deficient mutant of Staphylococcus aureus. Microb. Pathog. 24:241–251 [DOI] [PubMed] [Google Scholar]
- 168. Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, Shabb DW, Diep BA, Chambers HF, Otto M, DeLeo FR. 2011. Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 6:e18617 doi: 10.1371/journal.pone.0018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lang S, Palmer M. 2003. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J. Biol. Chem. 278:38167–38173 [DOI] [PubMed] [Google Scholar]
- 170. Jurgens D, Sterzik B, Fehrenbach FJ. 1987. Unspecific binding of group B streptococcal cocytolysin (CAMP factor) to immunoglobulins and its possible role in pathogenicity. J. Exp. Med. 165:720–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Liu GY, Nizet V. 2004. Extracellular virulence factors of group B streptococci. Front. Biosci. 9:1794–1802 [DOI] [PubMed] [Google Scholar]
- 172. Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. 2001. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol. Microbiol. 39:236–247 [DOI] [PubMed] [Google Scholar]
- 173. Gibson RL, Nizet V, Rubens CE. 1999. Group B streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr. Res. 45:626–634 [DOI] [PubMed] [Google Scholar]
- 174. Nizet V, Gibson RL, Chi EY, Framson PE, Hulse M, Rubens CE. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64:3818–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Doran KS, Liu GY, Nizet V. 2003. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Invest. 112:736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hensler ME, Liu GY, Sobczak S, Benirschke K, Nizet V, Heldt GP. 2005. Virulence role of group B Streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J. Infect. Dis. 191:1287–1291 [DOI] [PubMed] [Google Scholar]
- 177. Reiss A, Braun JS, Jager K, Freyer D, Laube G, Buhrer C, Felderhoff-Muser U, Stadelmann C, Nizet V, Weber JR. 2011. Bacterial pore-forming cytolysins induce neuronal damage in a rat model of neonatal meningitis. J. Infect. Dis. 203:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, Nizet V. 2004. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc. Natl. Acad. Sci. U. S. A. 101:14491–14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ring A, Braun JS, Pohl J, Nizet V, Stremmel W, Shenep JL. 2002. Group B streptococcal beta-hemolysin induces mortality and liver injury in experimental sepsis. J. Infect. Dis. 185:1745–1753 [DOI] [PubMed] [Google Scholar]
- 180. Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. 2005. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 121:247–256 [DOI] [PubMed] [Google Scholar]
- 181. Paton JC, Andrew PW, Boulnois GJ, Mitchell TJ. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 47:89–115 [DOI] [PubMed] [Google Scholar]
- 182. Alexander JE, Berry AM, Paton JC, Rubins JB, Andrew PW, Mitchell TJ. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24:167–174 [DOI] [PubMed] [Google Scholar]
- 183. Del Mar Garcia-Suarez M, Florez N, Astudillo A, Vazquez F, Villaverde R, Fabrizio K, Pirofski LA, Mendez FJ. 2007. The role of pneumolysin in mediating lung damage in a lethal pneumococcal pneumonia murine model. Respir. Res. 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Berry AM, Yother J, Briles DE, Hansman D, Paton JC. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Feldman C, Munro NC, Jeffery PK, Mitchell TJ, Andrew PW, Boulnois GJ, Guerreiro D, Rohde JA, Todd HC, Cole PJ. 1991. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am. J. Respir. Cell Mol. Biol. 5:416–423 [DOI] [PubMed] [Google Scholar]
- 186. Sekiya K, Satoh R, Danbara H, Futaesaku Y. 1993. A ring-shaped structure with a crown formed by streptolysin O on the erythrocyte membrane. J. Bacteriol. 175:5953–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, Karin M, Salvesen GS, Nizet V. 2009. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 284:862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Goldmann O, Sastalla I, Wos-Oxley M, Rohde M, Medina E. 2009. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell. Microbiol. 11:138–155 [DOI] [PubMed] [Google Scholar]
- 189. Fontaine MC, Lee JJ, Kehoe MA. 2003. Combined contributions of streptolysin O and streptolysin S to virulence of serotype M5 Streptococcus pyogenes strain Manfredo. Infect. Immun. 71:3857–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2009. Cytolysins augment superantigen penetration of stratified mucosa. J. Immunol. 182:2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sierig G, Cywes C, Wessels MR, Ashbaugh CD. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect. Immun. 71:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ato M, Ikebe T, Kawabata H, Takemori T, Watanabe H. 2008. Incompetence of neutrophils to invasive group A streptococcus is attributed to induction of plural virulence factors by dysfunction of a regulator. PLoS One 3:e3455 doi: 10.1371/journal.pone.0003455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Limbago B, Penumalli V, Weinrick B, Scott JR. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384–6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Carr A, Sledjeski DD, Podbielski A, Boyle MD, Kreikemeyer B. 2001. Similarities between complement-mediated and streptolysin S-mediated hemolysis. J. Biol. Chem. 276:41790–41796 [DOI] [PubMed] [Google Scholar]
- 195. Lin A, Loughman JA, Zinselmeyer BH, Miller MJ, Caparon MG. 2009. Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection. Infect. Immun. 77:5190–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Miyoshi-Akiyama T, Takamatsu D, Koyanagi M, Zhao J, Imanishi K, Uchiyama T. 2005. Cytocidal effect of Streptococcus pyogenes on mouse neutrophils in vivo and the critical role of streptolysin S. J. Infect. Dis. 192:107–116 [DOI] [PubMed] [Google Scholar]
- 197. Jiang M, Babiuk LA, Potter AA. 1996. Cloning, sequencing and expression of the CAMP factor gene of Streptococcus uberis. Microb. Pathog. 20:297–307 [DOI] [PubMed] [Google Scholar]
- 198. Fontaine MC, Perez-Casal J, Song XM, Shelford J, Willson PJ, Potter AA. 2002. Immunisation of dairy cattle with recombinant Streptococcus uberis GapC or a chimeric CAMP antigen confers protection against heterologous bacterial challenge. Vaccine 20:2278–2286 [DOI] [PubMed] [Google Scholar]
- 199. De S, Olson R. 2011. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc. Natl. Acad. Sci. U. S. A. 108:7385–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ikigai H, Akatsuka A, Tsujiyama H, Nakae T, Shimamura T. 1996. Mechanism of membrane damage by El Tor hemolysin of Vibrio cholerae O1. Infect. Immun. 64:2968–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zitzer A, Palmer M, Weller U, Wassenaar T, Biermann C, Tranum-Jensen J, Bhakdi S. 1997. Mode of primary binding to target membranes and pore formation induced by Vibrio cholerae cytolysin (hemolysin). Eur. J. Biochem. 247:209–216 [DOI] [PubMed] [Google Scholar]
- 202. Zitzer A, Walev I, Palmer M, Bhakdi S. 1995. Characterization of Vibrio cholerae El Tor cytolysin as an oligomerizing pore-forming toxin. Med. Microbiol. Immunol. 184:37–44 [DOI] [PubMed] [Google Scholar]
- 203. Bellier A, Chen CS, Kao CY, Cinar HN, Aroian RV. 2009. Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans. PLoS Pathog. 5:e1000689 doi: 10.1371/journal.ppat.1000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Cinar HN, Kothary M, Datta AR, Tall BD, Sprando R, Bilecen K, Yildiz F, McCardell B. 2010. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One 5:e11558 doi: 10.1371/journal.pone.0011558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Saka HA, Bidinost C, Sola C, Carranza P, Collino C, Ortiz S, Echenique JR, Bocco JL. 2008. Vibrio cholerae cytolysin is essential for high enterotoxicity and apoptosis induction produced by a cholera toxin gene-negative V. cholerae non-O1, non-O139 strain. Microb. Pathog. 44:118–128 [DOI] [PubMed] [Google Scholar]
- 206. Debellis L, Diana A, Arcidiacono D, Fiorotto R, Portincasa P, Altomare DF, Spirli C, de Bernard M. 2009. The Vibrio cholerae cytolysin promotes chloride secretion from intact human intestinal mucosa. PLoS One 4:e5074 doi: 10.1371/journal.pone.0005074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Olivier V, Haines GK, III, Tan Y, Satchell KJ. 2007. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect. Immun. 75:5035–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Olivier V, Salzman NH, Satchell KJ. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 75:5043–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Olivier V, Queen J, Satchell KJ. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352 doi: 10.1371/journal.pone.0007352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Haines GK, 3rd, Sayed BA, Rohrer MS, Olivier V, Satchell KJ. 2005. Role of Toll-like receptor 4 in the proinflammatory response to Vibrio cholerae O1 El Tor strains deficient in production of cholera toxin and accessory toxins. Infect. Immun. 73:6157–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Fullner KJ, Mekalanos JJ. 2000. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 19:5315–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Fullner KJ, Boucher JC, Hanes MA, Haines GK, 3rd, Meehan BM, Walchle C, Sansonetti PJ, Mekalanos JJ. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Yanagihara I, Nakahira K, Yamane T, Kaieda S, Mayanagi K, Hamada D, Fukui T, Ohnishi K, Kajiyama S, Shimizu T, Sato M, Ikegami T, Ikeguchi M, Honda T, Hashimoto H. 2010. Structure and functional characterization of Vibrio parahaemolyticus thermostable direct hemolysin. J. Biol. Chem. 285:16267–16274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Takahashi A, Yamamoto C, Kodama T, Yamashita K, Harada N, Nakano M, Honda T, Nakaya Y. 2006. Pore formation of thermostable direct hemolysin secreted from Vibrio parahaemolyticus in lipid bilayers. Int. J. Toxicol. 25:409–418 [DOI] [PubMed] [Google Scholar]
- 215. Hardy SP, Nakano M, Iida T. 2004. Single channel evidence for innate pore-formation by Vibrio parahaemolyticus thermostable direct haemolysin (TDH) in phospholipid bilayers. FEMS Microbiol. Lett. 240:81–85 [DOI] [PubMed] [Google Scholar]
- 216. Honda T, Ni Y, Miwatani T, Adachi T, Kim J. 1992. The thermostable direct hemolysin of Vibrio parahaemolyticus is a pore-forming toxin. Can. J. Microbiol. 38:1175–1180 [DOI] [PubMed] [Google Scholar]
- 217. Hiyoshi H, Kodama T, Iida T, Honda T. 2010. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect. Immun. 78:1772–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Nishibuchi M, Fasano A, Russell RG, Kaper JB. 1992. Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect. Immun. 60:3539–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Raimondi F, Kao JP, Kaper JB, Guandalini S, Fasano A. 1995. Calcium-dependent intestinal chloride secretion by Vibrio parahaemolyticus thermostable direct hemolysin in a rabbit model. Gastroenterology 109:381–386 [DOI] [PubMed] [Google Scholar]
- 220. Buckley JT, Howard SP. 1999. The cytotoxic enterotoxin of Aeromonas hydrophila is aerolysin. Infect. Immun. 67:466–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Boquet P, Ricci V. 2012. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 20:165–174 [DOI] [PubMed] [Google Scholar]
- 222. Hamon MA, Ribet D, Stavru F, Cossart P. 2012. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 20:360–368 [DOI] [PubMed] [Google Scholar]
- 223. Meyer F, Girardot R, Piémont Y, Prévost G, Colin DA. 2009. Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding. Infect. Immun. 77:266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bielecki J, Youngman P, Connelly P, Portnoy DA. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175–176 [DOI] [PubMed] [Google Scholar]
- 225. Kho MF, Bellier A, Balasubramani V, Hu Y, Hsu W, Nielsen-LeRoux C, McGillivray SM, Nizet V, Aroian RV. 2011. The pore-forming protein Cry5B elicits the pathogenicity of Bacillus sp. against Caenorhabditis elegans. PLoS One 6:e29122 doi: 10.1371/journal.pone.0029122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Menestrina G, Serra MD, Prevost G. 2001. Mode of action of beta-barrel pore-forming toxins of the staphylococcal alpha-hemolysin family. Toxicon 39:1661–1672 [DOI] [PubMed] [Google Scholar]
- 227. Iacovache I, van der Goot FG, Pernot L. 2008. Pore formation: an ancient yet complex form of attack. Biochim. Biophys. Acta 1778:1611–1623 [DOI] [PubMed] [Google Scholar]
- 228. Hotze EM, Le HM, Sieber JR, Bruxvoort C, McInerney MJ, Tweten RK. 2013. Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria. Infect. Immun. 81:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Bischofberger M, Gonzalez MR, van der Goot FG. 2009. Membrane injury by pore-forming proteins. Curr. Opin. Cell Biol. 21:589–595 [DOI] [PubMed] [Google Scholar]
- 230. Husmann M, Dersch K, Bobkiewicz W, Beckmann E, Veerachato G, Bhakdi S. 2006. Differential role of p38 mitogen activated protein kinase for cellular recovery from attack by pore-forming S. aureus alpha-toxin or streptolysin O. Biochem. Biophys. Res. Commun. 344:1128–1134 [DOI] [PubMed] [Google Scholar]
- 231. Kao CY, Los FCO, Huffman DL, Wachi S, Kloft N, Husmann M, Karabrahimi V, Schwartz JL, Bellier A, Ha C, Sagong Y, Fan H, Ghosh P, Hsieh M, Hsu CS, Chen L, Aroian RV. 2011. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 7:e1001314 doi: 10.1371/journal.ppat.1001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Metz M, Magerl M, Kuhl NF, Valeva A, Bhakdi S, Maurer M. 2009. Mast cells determine the magnitude of bacterial toxin-induced skin inflammation. Exp. Dermatol. 18:160–166 [DOI] [PubMed] [Google Scholar]
- 233. Alonzo F, III, Kozhaya L, Rawlings SA, Reyes-Robles T, Dumont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. 2013. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Wilke GA, Bubeck Wardenburg J. 2010. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. U. S. A. 107:13473–13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hu W, Ferris SP, Tweten RK, Wu G, Radaeva S, Gao B, Bronson RT, Halperin JA, Qin X. 2008. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat. Med. 14:98–103 [DOI] [PubMed] [Google Scholar]
- 236. Mechaly A, McCluskey AJ, Collier RJ. 2012. Changing the receptor specificity of anthrax toxin. mBio 3:e00088–12 doi: 10.1128/mBio.00088-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Giddings KS, Zhao J, Sims PJ, Tweten RK. 2004. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 11:1173–1178 [DOI] [PubMed] [Google Scholar]
- 238. Aroian R, van der Goot FG. 2007. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr. Opin. Microbiol. 10:57–61 [DOI] [PubMed] [Google Scholar]
- 239. Tilley SJ, Saibil HR. 2006. The mechanism of pore formation by bacterial toxins. Curr. Opin. Struct. Biol. 16:230–236 [DOI] [PubMed] [Google Scholar]
- 240. Ortqvist A, Hedlund J, Kalin M. 2005. Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin. Respir. Crit. Care Med. 26:563–574 [DOI] [PubMed] [Google Scholar]
- 241. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 242. Avery OT, Macleod CM, McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59:591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lappin E, Ferguson AJ. 2009. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 9:281–290 [DOI] [PubMed] [Google Scholar]
- 245. Collis WRF. 1931. Acute rheumatism and haemolytic streptococci. Lancet i:1341–1345 [Google Scholar]
- 246. Burkert T, Watanakunakorn C. 1991. Group A streptococcus endocarditis: report of five cases and review of literature. J. Infect. 23:307–316 [DOI] [PubMed] [Google Scholar]
- 247. O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin. Infect. Dis. 45:853–862 [DOI] [PubMed] [Google Scholar]
- 248. Gase K, Ferretti JJ, Primeaux C, McShan WM. 1999. Identification, cloning, and expression of the CAMP factor gene (cfa) of group A streptococci. Infect. Immun. 67:4725–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bisno AL, Brito MO, Collins CM. 2003. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3:191–200 [DOI] [PubMed] [Google Scholar]
- 250. Maisey HC, Doran KS, Nizet V. 2008. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev. Mol. Med. 10:e27 doi: 10.1017/S1462399408000811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Deutscher M, Lewis M, Zell ER, Taylor TH, Jr, Van Beneden C, Schrag S. 2011. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin. Infect. Dis. 53:114–123 [DOI] [PubMed] [Google Scholar]
- 252. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065 [DOI] [PubMed] [Google Scholar]
- 253. Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ. 2009. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 49:85–92 [DOI] [PubMed] [Google Scholar]
- 254. Rosa-Fraile M, Rodriguez-Granger J, Haidour-Benamin A, Cuerva JM, Sampedro A. 2006. Granadaene: proposed structure of the group B Streptococcus polyenic pigment. Appl. Environ. Microbiol. 72:6367–6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR. 2005. Regulation of virulence by a two-component system in group B streptococcus. J. Bacteriol. 187:1105–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Hensler ME, Quach D, Hsieh CJ, Doran KS, Nizet V. 2008. CAMP factor is not essential for systemic virulence of group B Streptococcus. Microb. Pathog. 44:84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Siqueira JA, Speeg-Schatz C, Freitas FI, Sahel J, Monteil H, Prevost G. 1997. Channel-forming leucotoxins from Staphylococcus aureus cause severe inflammatory reactions in a rabbit eye model. J. Med. Microbiol. 46:486–494 [DOI] [PubMed] [Google Scholar]
- 258. Kobayashi SD, DeLeo FR. 2009. An update on community-associated MRSA virulence. Curr. Opin. Pharmacol. 9:545–551 [DOI] [PubMed] [Google Scholar]
- 259. Joubert O, Voegelin J, Guillet V, Tranier S, Werner S, Colin DA, Serra MD, Keller D, Monteil H, Mourey L, Prevost G. 2007. Distinction between pore assembly by staphylococcal alpha-toxin versus leukotoxins. J. Biomed. Biotechnol. 2007:25935 doi: 10.1155/2007/25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Badiou C, Dumitrescu O, Croze M, Gillet Y, Dohin B, Slayman DH, Allaouchiche B, Etienne J, Vandenesch F, Lina G. 2008. Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin. Microbiol. Infect. 14:1180–1183 [DOI] [PubMed] [Google Scholar]
- 261. Verkaik NJ, Dauwalder O, Antri K, Boubekri I, de Vogel CP, Badiou C, Bes M, Vandenesch F, Tazir M, Hooijkaas H, Verbrugh HA, van Belkum A, Etienne J, Lina G, Ramdani-Bouguessa N, van Wamel WJ. 2010. Immunogenicity of toxins during Staphylococcus aureus infection. Clin. Infect. Dis. 50:61–68 [DOI] [PubMed] [Google Scholar]
- 262. DeLeo FR, Diep BA, Otto M. 2009. Host defense and pathogenesis in Staphylococcus aureus infections. Infect. Dis. Clin. North Am. 23:17–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piemont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759 [DOI] [PubMed] [Google Scholar]
- 264. Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Cooney J, Kienle Z, Foster TJ, O'Toole PW. 1993. The gamma-hemolysin locus of Staphylococcus aureus comprises three linked genes, two of which are identical to the genes for the F and S components of leukocidin. Infect. Immun. 61:768–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Mock M, Fouet A. 2001. Anthrax. Annu. Rev. Microbiol. 55:647–671 [DOI] [PubMed] [Google Scholar]
- 267. Ringertz SH, Hoiby EA, Jensenius M, Maehlen J, Caugant DA, Myklebust A, Fossum K. 2000. Injectional anthrax in a heroin skin-popper. Lancet 356:1574–1575 [DOI] [PubMed] [Google Scholar]
- 268. Koehler TM. 2009. Bacillus anthracis physiology and genetics. Mol. Aspects Med. 30:386–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Smith H. 2000. Discovery of the anthrax toxin: the beginning of in vivo studies on pathogenic bacteria. Trends Microbiol. 8:199–200 [DOI] [PubMed] [Google Scholar]
- 270. Abrami L, Reig N, van der Goot FG. 2005. Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol. 13:72–78 [DOI] [PubMed] [Google Scholar]
- 271. Shannon JG, Ross CL, Koehler TM, Rest RF. 2003. Characterization of anthrolysin O, the Bacillus anthracis cholesterol-dependent cytolysin. Infect. Immun. 71:3183–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Miyata S, Minami J, Tamai E, Matsushita O, Shimamoto S, Okabe A. 2002. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277:39463–39468 [DOI] [PubMed] [Google Scholar]
- 273. Cook TM, Protheroe RT, Handel JM. 2001. Tetanus: a review of the literature. Br. J. Anaesth. 87:477–487 [DOI] [PubMed] [Google Scholar]
- 274. Songer JG. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Tweten RK. 2001. Clostridium perfringens beta toxin and Clostridium septicum alpha toxin: their mechanisms and possible role in pathogenesis. Vet. Microbiol. 82:1–9 [DOI] [PubMed] [Google Scholar]
- 276. Smith LA. 2009. Botulism and vaccines for its prevention. Vaccine 27(Suppl 4):D33–D39 [DOI] [PubMed] [Google Scholar]
- 277. Bohnel H, Gessler F. 2005. Botulinum toxins—cause of botulism and systemic diseases? Vet. Res. Commun. 29:313–345 [DOI] [PubMed] [Google Scholar]
- 278. Brunger AT, Rummel A. 2009. Receptor and substrate interactions of clostridial neurotoxins. Toxicon 54:550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Allerberger F, Wagner M. 2010. Listeriosis: a resurgent foodborne infection. Clin. Microbiol. Infect. 16:16–23 [DOI] [PubMed] [Google Scholar]
- 280. Wollert T, Pasche B, Rochon M, Deppenmeier S, van den Heuvel J, Gruber AD, Heinz DW, Lengeling A, Schubert WD. 2007. Extending the host range of Listeria monocytogenes by rational protein design. Cell 129:891–902 [DOI] [PubMed] [Google Scholar]
- 281. Disson O, Nikitas G, Grayo S, Dussurget O, Cossart P, Lecuit M. 2009. Modeling human listeriosis in natural and genetically engineered animals. Nat. Protoc. 4:799–810 [DOI] [PubMed] [Google Scholar]
- 282. Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Leimeister-Wachter M, Chakraborty T. 1989. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect. Immun. 57:2350–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR., Jr 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. U. S. A. 100:12420–12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Behr MA, Small PM. 1999. A historical and molecular phylogeny of BCG strains. Vaccine 17:915–922 [DOI] [PubMed] [Google Scholar]
- 286. Corbett EL, Marston B, Churchyard GJ, De Cock KM. 2006. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 367:926–937 [DOI] [PubMed] [Google Scholar]
- 287. Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. 2002. Real-time visualization of Mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17:693–702 [DOI] [PubMed] [Google Scholar]
- 288. Duval A, Adehossi E, Parola P. 2009. Mycobacterium marinum infection. BMJ Case Rep. 2009:bcr12.2008.1311 doi: 10.1136/bcr.12.2008.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Grange JM. 2001. Mycobacterium bovis infection in human beings. Tuberculosis 81:71–77 [DOI] [PubMed] [Google Scholar]
- 290. Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J. Infect. Dis. 187:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Maartens G, Wilkinson RJ. 2007. Tuberculosis. Lancet 370:2030–2043 [DOI] [PubMed] [Google Scholar]
- 292. Renshaw PS, Panagiotidou P, Whelan A, Gordon SV, Hewinson RG, Williamson RA, Carr MD. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598–21603 [DOI] [PubMed] [Google Scholar]
- 293. Wards BJ, de Lisle GW, Collins DM. 2000. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber. Lung Dis. 80:185–189 [DOI] [PubMed] [Google Scholar]
- 294. World Health Organization 2008. The top 10 causes of death. Fact sheet no 310. World Health Organization, Geneva, Switzerland [Google Scholar]
- 295. Mueller M, Grauschopf U, Maier T, Glockshuber R, Ban N. 2009. The structure of a cytolytic alpha-helical toxin pore reveals its assembly mechanism. Nature 459:726–730 [DOI] [PubMed] [Google Scholar]
- 296. Wallace AJ, Stillman TJ, Atkins A, Jamieson SJ, Bullough PA, Green J, Artymiuk PJ. 2000. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100:265–276 [DOI] [PubMed] [Google Scholar]
- 297. Bielaszewska M, Karch H. 2005. Consequences of enterohaemorrhagic Escherichia coli infection for the vascular endothelium. Thromb. Haemost. 94:312–318 [DOI] [PubMed] [Google Scholar]
- 298. Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21:26–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Nagy G, Altenhoefer A, Knapp O, Maier E, Dobrindt U, Blum-Oehler G, Benz R, Emody L, Hacker J. 2006. Both alpha-haemolysin determinants contribute to full virulence of uropathogenic Escherichia coli strain 536. Microbes Infect. 8:2006–2012 [DOI] [PubMed] [Google Scholar]
- 300. Kerenyi M, Allison HE, Batai I, Sonnevend A, Emody L, Plaveczky N, Pal T. 2005. Occurrence of hlyA and sheA genes in extraintestinal Escherichia coli strains. J. Clin. Microbiol. 43:2965–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Bauer ME, Welch RA. 1996. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 64:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Schmidt H, Beutin L, Karch H. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Schmidt H, Maier E, Karch H, Benz R. 1996. Pore-forming properties of the plasmid-encoded hemolysin of enterohemorrhagic Escherichia coli O157:H7. Eur. J. Biochem. 241:594–601 [DOI] [PubMed] [Google Scholar]
- 304. Emody L, Kerenyi M, Nagy G. 2003. Virulence factors of uropathogenic Escherichia coli. Int. J. Antimicrob. Agents 22(Suppl 2):29–33 [DOI] [PubMed] [Google Scholar]
- 305. World Health Organization 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 13:117–128 [PubMed] [Google Scholar]
- 306. Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233 [DOI] [PubMed] [Google Scholar]
- 307. Fallarino A, Attridge SR, Manning PA, Focareta T. 2002. Cloning and characterization of a novel haemolysin in Vibrio cholerae O1 that does not directly contribute to the virulence of the organism. Microbiology 148:2181–2189 [DOI] [PubMed] [Google Scholar]
- 308. Weil AA, Arifuzzaman M, Bhuiyan TR, LaRocque RC, Harris AM, Kendall EA, Hossain A, Tarique AA, Sheikh A, Chowdhury F, Khan AI, Murshed F, Parker KC, Banerjee KK, Ryan ET, Harris JB, Qadri F, Calderwood SB. 2009. Memory T-cell responses to Vibrio cholerae O1 infection. Infect. Immun. 77:5090–5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. 2004. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc. Natl. Acad. Sci. U. S. A. 101:10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Ratner AJ, Hippe KR, Aguilar JL, Bender MH, Nelson AL, Weiser JN. 2006. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J. Biol. Chem. 281:12994–12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Gonzalez MR, Bischofberger M, Freche B, Ho S, Parton RG, van der Goot FG. 2011. Pore-forming toxins induce multiple cellular responses promoting survival. Cell. Microbiol. 13:1026–1043 [DOI] [PubMed] [Google Scholar]
- 312. Rampersaud R, Planet PJ, Randis TM, Kulkarni R, Aguilar JL, Lehrer RI, Ratner AJ. 2011. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 193:1034–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Cancino-Rodezno A, Alexander C, Villasenor R, Pacheco S, Porta H, Pauchet Y, Soberon M, Gill SS, Bravo A. 2010. The mitogen-activated protein kinase p38 is involved in insect defense against Cry toxins from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 40:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Ganguly N, Giang PH, Basu SK, Mir FA, Siddiqui I, Sharma P. 2007. Mycobacterium tuberculosis 6-kDa early secreted antigenic target (ESAT-6) protein downregulates lipopolysaccharide induced c-myc expression by modulating the extracellular signal regulated kinases 1/2. BMC Immunol. 8:24 doi: 10.1186/1471-2172-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Aguilar JL, Kulkarni R, Randis TM, Soman S, Kikuchi A, Yin Y, Ratner AJ. 2009. Phosphatase-dependent regulation of epithelial mitogen-activated protein kinase responses to toxin-induced membrane pores. PLoS One 4:e8076 doi: 10.1371/journal.pone.0008076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Stassen M, Muller C, Richter C, Neudorfl C, Hultner L, Bhakdi S, Walev I, Schmitt E. 2003. The streptococcal exotoxin streptolysin O activates mast cells to produce tumor necrosis factor alpha by p38 mitogen-activated protein kinase- and protein kinase C-dependent pathways. Infect. Immun. 71:6171–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–1145 [DOI] [PubMed] [Google Scholar]
- 318. Imre G, Heering J, Takeda AN, Husmann M, Thiede B, Zu Heringdorf DM, Green DR, van der Goot FG, Sinha B, Dotsch V, Rajalingam K. 2012. Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis. EMBO J. 31:2615–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Kloft N, Neukirch C, Bobkiewicz W, Veerachato G, Busch T, von Hoven G, Boller K, Husmann M. 2010. Pro-autophagic signal induction by bacterial pore-forming toxins. Med. Microbiol. Immunol. 199:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Kloft N, Busch T, Neukirch C, Weis S, Boukhallouk F, Bobkiewicz W, Cibis I, Bhakdi S, Husmann M. 2009. Pore-forming toxins activate MAPK p38 by causing loss of cellular potassium. Biochem. Biophys. Res. Commun. 385:503–506 [DOI] [PubMed] [Google Scholar]
- 321. Bischof LJ, Kao CY, Los FC, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. 2008. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 4:e1000176 doi: 10.1371/journal.ppat.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Chen CS, Bellier A, Kao CY, Yang YL, Chen HD, Los FC, Aroian RV. 2010. WWP-1 is a novel modulator of the DAF-2 insulin-like signaling network involved in pore-forming toxin cellular defenses in Caenorhabditis elegans. PLoS One 5:e9494 doi: 10.1371/journal.pone.0009494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Lehrer RI, Jung G, Ruchala P, Wang W, Micewicz ED, Waring AJ, Gillespie EJ, Bradley KA, Ratner AJ, Rest RF, Lu W. 2009. Human alpha-defensins inhibit hemolysis mediated by cholesterol-dependent cytolysins. Infect. Immun. 77:4028–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. 2012. Mitochondrial Ca(2+) and apoptosis. Cell Calcium 52:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. 2008. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. 2010. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 189:1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Corrotte M, Fernandes MC, Tam C, Andrews NW. 2012. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic 13:483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S. 2009. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 583:337–344 [DOI] [PubMed] [Google Scholar]
- 329. Engel F, Blatz R, Kellner J, Palmer M, Weller U, Bhadki S. 1995. Breakdown of the round window membrane permeability barrier evoked by streptolysin O: possible etiologic role in development of sensorineural hearing loss in acute otitis media. Infect. Immun. 63:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Los FCO, Kao CY, Smitham J, McDonald KL, Ha C, Peixoto CA, Aroian RV. 2011. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 9:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Sakurai J, Honda T, Jinguji Y, Arita M, Miwatani T. 1976. Cytotoxic effect of the thermostable direct hemolysin produced by Vibrio parahaemolyticus on FL cells. Infect. Immun. 13:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Diacovich L, Gorvel JP. 2010. Bacterial manipulation of innate immunity to promote infection. Nat. Rev. Microbiol. 8:117–128 [DOI] [PubMed] [Google Scholar]
- 333. Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 334. Pedra JH, Cassel SL, Sutterwala FS. 2009. Sensing pathogens and danger signals by the inflammasome. Curr. Opin. Immunol. 21:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Borish LC, Steinke JW. 2003. Cytokines and chemokines. J. Allergy Clin. Immunol. 111:S460–S475 [DOI] [PubMed] [Google Scholar]
- 336. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813:878–888 [DOI] [PubMed] [Google Scholar]
- 337. Rose-John S, Scheller J, Elson G, Jones SA. 2006. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 80:227–236 [DOI] [PubMed] [Google Scholar]
- 338. Arcidiacono D, Odom S, Frossi B, Rivera J, Paccani SR, Baldari CT, Pucillo C, Montecucco C, de Bernard M. 2008. The Vibrio cholerae cytolysin promotes activation of mast cell (T helper 2) cytokine production. Cell. Microbiol. 10:899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Ma X, Chang W, Zhang C, Zhou X, Yu F. 2012. Staphylococcal Panton-Valentine leukocidin induces pro-inflammatory cytokine production and nuclear factor-kappa B activation in neutrophils. PLoS One 7:e34970 doi: 10.1371/journal.pone.0034970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Tsuchiya K, Kawamura I, Takahashi A, Nomura T, Kohda C, Mitsuyama M. 2005. Listeriolysin O-induced membrane permeation mediates persistent interleukin-6 production in Caco-2 cells during Listeria monocytogenes infection in vitro. Infect. Immun. 73:3869–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Walev I, Vollmer P, Palmer M, Bhakdi S, Rose-John S. 1996. Pore-forming toxins trigger shedding of receptors for interleukin 6 and lipopolysaccharide. Proc. Natl. Acad. Sci. U. S. A. 93:7882–7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Walev I, Tappe D, Gulbins E, Bhakdi S. 2000. Streptolysin O-permeabilized granulocytes shed L-selectin concomitantly with ceramide generation via neutral sphingomyelinase. J. Leukoc. Biol. 68:865–872 [PubMed] [Google Scholar]
- 343. Benton KA, Everson MP, Briles DE. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Benton KA, VanCott JL, Briles DE. 1998. Role of tumor necrosis factor alpha in the host response of mice to bacteremia caused by pneumolysin-deficient Streptococcus pneumoniae. Infect. Immun. 66:839–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 204:937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Ward PD, Turner WH. 1980. Identification of staphylococcal Panton-Valentine leukocidin as a potent dermonecrotic toxin. Infect. Immun. 28:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Zivkovic A, Sharif O, Stich K, Doninger B, Biaggio M, Colinge J, Bilban M, Mesteri I, Hazemi P, Lemmens-Gruber R, Knapp S. 2011. TLR 2 and CD14 mediate innate immunity and lung inflammation to staphylococcal Panton-Valentine leukocidin in vivo. J. Immunol. 186:1608–1617 [DOI] [PubMed] [Google Scholar]
- 348. May AK, Sawyer RG, Gleason T, Whitworth A, Pruett TL. 1996. In vivo cytokine response to Escherichia coli alpha-hemolysin determined with genetically engineered hemolytic and nonhemolytic E. coli variants. Infect. Immun. 64:2167–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Kramer S, Sellge G, Lorentz A, Krueger D, Schemann M, Feilhauer K, Gunzer F, Bischoff SC. 2008. Selective activation of human intestinal mast cells by Escherichia coli hemolysin. J. Immunol. 181:1438–1445 [DOI] [PubMed] [Google Scholar]
- 350. Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. 2000. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405:694–697 [DOI] [PubMed] [Google Scholar]
- 351. van Rossum AM, Lysenko ES, Weiser JN. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Knapp S, Wieland CW, van 't Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. 2004. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 172:3132–3138 [DOI] [PubMed] [Google Scholar]
- 353. Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 73:6479–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 100:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Park JM, Ng VH, Maeda S, Rest RF, Karin M. 2004. Anthrolysin O and other gram-positive cytolysins are Toll-like receptor 4 agonists. J. Exp. Med. 200:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin S, van der Poll T. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6:e1001191 doi: 10.1371/journal.ppat.1001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Dessing MC, Florquin S, Paton JC, van der Poll T. 2008. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell. Microbiol. 10:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Dessing MC, Hirst RA, de Vos AF, van der Poll T. 2009. Role of Toll-like receptors 2 and 4 in pulmonary inflammation and injury induced by pneumolysin in mice. PLoS One 4:e7993 doi: 10.1371/journal.pone.0007993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Rijneveld AW, van den Dobbelsteen GP, Florquin S, Standiford TJ, Speelman P, van Alphen L, van der Poll T. 2002. Roles of interleukin-6 and macrophage inflammatory protein-2 in pneumolysin-induced lung inflammation in mice. J. Infect. Dis. 185:123–126 [DOI] [PubMed] [Google Scholar]
- 361. Bebien M, Hensler ME, Davanture S, Hsu LC, Karin M, Park JM, Alexopoulou L, Liu GY, Nizet V, Lawrence T. 2012. The pore-forming toxin beta hemolysin/cytolysin triggers p38 MAPK-dependent IL-10 production in macrophages and inhibits innate immunity. PLoS Pathog. 8:e1002812 doi: 10.1371/journal.ppat.1002812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Gekara NO, Dietrich N, Lyszkiewicz M, Lienenklaus S, Weiss S. 2009. Signals triggered by a bacterial pore-forming toxin contribute to Toll-like receptor redundancy in gram-positive bacterial recognition. J. Infect. Dis. 199:124–133 [DOI] [PubMed] [Google Scholar]
- 363. Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. 2011. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J. Immunol. 187:434–440 [DOI] [PubMed] [Google Scholar]
- 364. Kafka D, Ling E, Feldman G, Benharroch D, Voronov E, Givon-Lavi N, Iwakura Y, Dagan R, Apte RN, Mizrachi-Nebenzahl Y. 2008. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int. Immunol. 20:1139–1146 [DOI] [PubMed] [Google Scholar]
- 365. Harder J, Franchi L, Munoz-Planillo R, Park JH, Reimer T, Nunez G. 2009. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 183:5823–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205:807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Jounblat R, Kadioglu A, Mitchell TJ, Andrew PW. 2003. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect. Immun. 71:1813–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Quin LR, Moore QC, 3rd, McDaniel LS. 2007. Pneumolysin, PspA, and PspC contribute to pneumococcal evasion of early innate immune responses during bacteremia in mice. Infect. Immun. 75:2067–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Yuste J, Botto M, Paton JC, Holden DW, Brown JS. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 175:1813–1819 [DOI] [PubMed] [Google Scholar]
- 370. Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202:1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Sanders ME, Norcross EW, Moore QC, 3rd, Fratkin J, Thompson H, Marquart ME. 2010. Immunization with pneumolysin protects against both retinal and global damage caused by Streptococcus pneumoniae endophthalmitis. J. Ocul. Pharmacol. Ther. 26:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Denoël P, Philipp MT, Doyle L, Martin D, Carletti G, Poolman JT. 2011. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 29:5495–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Lu J, Wang C, Zhou Z, Zhang Y, Cao T, Shi C, Chen Z, Chen L, Cai C, Fan X. 2011. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin. Dev. Immunol. 2011:6178921 doi: 10.1155/2011/617892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. 2004. CD4–T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect. Immun. 72:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 102:4848–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, Andrew PW. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Mureithi MW, Finn A, Ota MO, Zhang Q, Davenport V, Mitchell TJ, Williams NA, Adegbola RA, Heyderman RS. 2009. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J. Infect. Dis. 200:783–793 [DOI] [PubMed] [Google Scholar]
- 378. Frank KM, Zhou T, Moreno-Vinasco L, Hollett B, Garcia JG, Bubeck Wardenburg J. 2012. Host response signature to Staphylococcus aureus alpha-hemolysin implicates pulmonary TH17 response. Infect. Immun. 80:3161–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Geginat G, Schenk S, Skoberne M, Goebel W, Hof H. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877–1884 [DOI] [PubMed] [Google Scholar]
- 380. Gekara NO, Zietara N, Geffers R, Weiss S. 2010. Listeria monocytogenes induces T cell receptor unresponsiveness through pore-forming toxin listeriolysin O. J. Infect. Dis. 202:1698–1707 [DOI] [PubMed] [Google Scholar]
- 381. Carrero JA, Calderon B, Unanue ER. 2004. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J. Immunol. 172:4866–4874 [DOI] [PubMed] [Google Scholar]
- 382. Carrero JA, Vivanco-Cid H, Unanue ER. 2012. Listeriolysin O is strongly immunogenic independently of its cytotoxic activity. PLoS One 7:e32310 doi: 10.1371/journal.pone.0032310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Hage-Chahine CM, Del Giudice G, Lambert PH, Pechere JC. 1992. Hemolysin-producing Listeria monocytogenes affects the immune response to T-cell-dependent and T-cell-independent antigens. Infect. Immun. 60:1415–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Hara H, Kawamura I, Nomura T, Tominaga T, Tsuchiya K, Mitsuyama M. 2007. Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of listeriolysin O determined by cytolysin gene replacement. Infect. Immun. 75:3791–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Kimoto T, Kawamura I, Kohda C, Nomura T, Tsuchiya K, Ito Y, Watanabe I, Kaku T, Setianingrum E, Mitsuyama M. 2003. Differences in gamma interferon production induced by listeriolysin O and ivanolysin O result in different levels of protective immunity in mice infected with Listeria monocytogenes and Listeria ivanovii. Infect. Immun. 71:2447–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Frehel C, Lety MA, Autret N, Beretti JL, Berche P, Charbit A. 2003. Capacity of ivanolysin O to replace listeriolysin O in phagosomal escape and in vivo survival of Listeria monocytogenes. Microbiology 149:611–620 [DOI] [PubMed] [Google Scholar]
- 387. Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, Schutte H. 2006. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit. Care Med. 34:1947–1954 [DOI] [PubMed] [Google Scholar]
- 388. Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, Oseghale A, Verin AD, Chakraborty T, Matthay MA, Zemskov EA, White R, Block NL, Schally AV. 2012. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc. Natl. Acad. Sci. U. S. A. 109:2084–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Maus UA, Srivastava M, Paton JC, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. 2004. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J. Immunol. 173:1307–1312 [DOI] [PubMed] [Google Scholar]
- 390. Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, Xu H, Bourne P, Ha UH, Ishinaga H, Andalibi A, Feng XH, Zhu H, Huang Y, Zhang W, Weng X, Yan C, Yin Z, Briles DE, Davis RJ, Flavell RA, Li JD. 2007. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity 27:349–360 [DOI] [PubMed] [Google Scholar]
- 391. Kinhikar AG, Verma I, Chandra D, Singh KK, Weldingh K, Andersen P, Hsu T, Jacobs WR, Jr, Laal S. 2010. Potential role for ESAT6 in dissemination of M. tuberculosis via human lung epithelial cells. Mol. Microbiol. 75:92–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Spreer A, Kerstan H, Bottcher T, Gerber J, Siemer A, Zysk G, Mitchell TJ, Eiffert H, Nau R. 2003. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob. Agents Chemother. 47:2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Hirst RA, Gosai B, Rutman A, Guerin CJ, Nicotera P, Andrew PW, O'Callaghan C. 2008. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J. Infect. Dis. 197:744–751 [DOI] [PubMed] [Google Scholar]
- 394. Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661–1669 [DOI] [PubMed] [Google Scholar]
- 395. Wellmer A, Zysk G, Gerber J, Kunst T, Von Mering M, Bunkowski S, Eiffert H, Nau R. 2002. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect. Immun. 70:6504–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Stringaris AK, Geisenhainer J, Bergmann F, Balshusemann C, Lee U, Zysk G, Mitchell TJ, Keller BU, Kuhnt U, Gerber J, Spreer A, Bahr M, Michel U, Nau R. 2002. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol. Dis. 11:355–368 [DOI] [PubMed] [Google Scholar]
- 397. Friedland IR, Paris MM, Hickey S, Shelton S, Olsen K, Paton JC, McCracken GH. 1995. The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J. Infect. Dis. 172:805–809 [DOI] [PubMed] [Google Scholar]
- 398. Hirst RA, Kadioglu A, O'Callaghan C, Andrew PW. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin. Exp. Immunol. 138:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Iliev AI, Djannatian JR, Opazo F, Gerber J, Nau R, Mitchell TJ, Wouters FS. 2009. Rapid microtubule bundling and stabilization by the Streptococcus pneumoniae neurotoxin pneumolysin in a cholesterol-dependent, non-lytic and Src-kinase dependent manner inhibits intracellular trafficking. Mol. Microbiol. 71:461–477 [DOI] [PubMed] [Google Scholar]
- 400. Tsuprun V, Cureoglu S, Schachern PA, Ferrieri P, Briles DE, Paparella MM, Juhn SK. 2008. Role of pneumococcal proteins in sensorineural hearing loss due to otitis media. Otol. Neurotol. 29:1056–1060 [DOI] [PubMed] [Google Scholar]
- 401. Nagahama M, Sakurai J. 1991. Distribution of labeled Clostridium perfringens epsilon toxin in mice. Toxicon 29:211–217 [DOI] [PubMed] [Google Scholar]
- 402. Soler-Jover A, Blasi J, Gomez de Aranda I, Navarro P, Gibert M, Popoff MR, Martin-Satue M. 2004. Effect of epsilon toxin-GFP on MDCK cells and renal tubules in vivo. J. Histochem. Cytochem. 52:931–942 [DOI] [PubMed] [Google Scholar]
- 403. Dorca-Arevalo J, Soler-Jover A, Gibert M, Popoff MR, Martin-Satue M, Blasi J. 2008. Binding of epsilon-toxin from Clostridium perfringens in the nervous system. Vet. Microbiol. 131:14–25 [DOI] [PubMed] [Google Scholar]
- 404. Brell B, Temmesfeld-Wollbruck B, Altzschner I, Frisch E, Schmeck B, Hocke AC, Suttorp N, Hippenstiel S. 2005. Adrenomedullin reduces Staphylococcus aureus alpha-toxin-induced rat ileum microcirculatory damage. Crit. Care Med. 33:819–826 [DOI] [PubMed] [Google Scholar]
- 405. Bishop BL, Lodolce JP, Kolodziej LE, Boone DL, Tang WJ. 2010. The role of anthrolysin O in gut epithelial barrier disruption during Bacillus anthracis infection. Biochem. Biophys. Res. Commun. 394:254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. McClane B. 2010. Clostridium perfringens type C isolates rapidly upregulate their toxin production upon contact with host cells: new insights into virulence? Virulence 1:97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Adamson RH, Ly JC, Fernandez-Miyakawa M, Ochi S, Sakurai J, Uzal F, Curry FE. 2005. Clostridium perfringens epsilon-toxin increases permeability of single perfused microvessels of rat mesentery. Infect. Immun. 73:4879–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Grandel U, Bennemann U, Buerke M, Hattar K, Seeger W, Grimminger F, Sibelius U. 2009. Staphylococcus aureus alpha-toxin and Escherichia coli hemolysin impair cardiac regional perfusion and contractile function by activating myocardial eicosanoid metabolism in isolated rat hearts. Crit. Care Med. 37:2025–2032 [DOI] [PubMed] [Google Scholar]
- 409. Sibelius U, Grandel U, Buerke M, Mueller D, Kiss L, Kraemer HJ, Braun-Dullaeus R, Haberbosch W, Seeger W, Grimminger F. 2000. Staphylococcal alpha-toxin provokes coronary vasoconstriction and loss in myocardial contractility in perfused rat hearts: role of thromboxane generation. Circulation 101:78–85 [DOI] [PubMed] [Google Scholar]
- 410. Nagahama M, Iida H, Sakurai J. 1993. Effect of Clostridium perfringens epsilon toxin on rat isolated aorta. Microbiol. Immunol. 37:447–450 [DOI] [PubMed] [Google Scholar]
- 411. Sakurai J, Nagahama M, Fujii Y. 1983. Effect of Clostridium perfringens epsilon toxin on the cardiovascular system of rats. Infect. Immun. 42:1183–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Sakurai J, Fujii Y, Matsuura M, Endo K. 1981. Pharmacological effect of beta toxin of Clostridium perfringens type C on rats. Microbiol. Immunol. 25:423–432 [DOI] [PubMed] [Google Scholar]
- 413. Nagahama M, Morimitsu S, Kihara A, Akita M, Setsu K, Sakurai J. 2003. Involvement of tachykinin receptors in Clostridium perfringens beta-toxin-induced plasma extravasation. Br. J. Pharmacol. 138:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Stevens DL, Troyer BE, Merrick DT, Mitten JE, Olson RD. 1988. Lethal effects and cardiovascular effects of purified alpha- and theta-toxins from Clostridium perfringens. J. Infect. Dis. 157:272–279 [DOI] [PubMed] [Google Scholar]
- 415. Asmuth DM, Olson RD, Hackett SP, Bryant AE, Tweten RK, Tso JY, Zollman T, Stevens DL. 1995. Effects of Clostridium perfringens recombinant and crude phospholipase C and theta-toxin on rabbit hemodynamic parameters. J. Infect. Dis. 172:1317–1323 [DOI] [PubMed] [Google Scholar]
- 416. Mayer K, Temmesfeld-Wollbruck B, Friedland A, Olschewski H, Reich M, Seeger W, Grimminger AF. 1999. Severe microcirculatory abnormalities elicited by E. coli hemolysin in the rabbit ileum mucosa. Am. J. Respir. Crit. Care Med. 160:1171–1178 [DOI] [PubMed] [Google Scholar]
- 417. Seeger W, Walter H, Suttorp N, Muhly M, Bhakdi S. 1989. Thromboxane-mediated hypertension and vascular leakage evoked by low doses of Escherichia coli hemolysin in rabbit lungs. J. Clin. Invest. 84:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Walmrath D, Ghofrani HA, Rosseau S, Schutte H, Cramer A, Kaddus W, Grimminger F, Bhakdi S, Seeger W. 1994. Endotoxin “priming” potentiates lung vascular abnormalities in response to Escherichia coli hemolysin: an example of synergism between endo- and exotoxin. J. Exp. Med. 180:1437–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Aldick T, Bielaszewska M, Uhlin BE, Humpf HU, Wai SN, Karch H. 2009. Vesicular stabilization and activity augmentation of enterohaemorrhagic Escherichia coli haemolysin. Mol. Microbiol. 71:1496–1508 [DOI] [PubMed] [Google Scholar]
- 420. Bryant AE, Bayer CR, Chen RY, Guth PH, Wallace RJ, Stevens DL. 2005. Vascular dysfunction and ischemic destruction of tissue in Streptococcus pyogenes infection: the role of streptolysin O-induced platelet/neutrophil complexes. J. Infect. Dis. 192:1014–1022 [DOI] [PubMed] [Google Scholar]
- 421. Miclard J, Jaggi M, Sutter E, Wyder M, Grabscheid B, Posthaus H. 2009. Clostridium perfringens beta-toxin targets endothelial cells in necrotizing enteritis in piglets. Vet. Microbiol. 137:320–325 [DOI] [PubMed] [Google Scholar]
- 422. Siegfried L, Kmetova M, Janigova V, Sasinka M, Takacova V. 1995. Serum response of Escherichia coli strains causing dyspepsia and urinary tract infection: relation to alpha-hemolysin production and O type. Infect. Immun. 63:4543–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, Voyich JM. 2012. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 7:e36532 doi: 10.1371/journal.pone.0036532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Birmingham CL, Canadien V, Kaniuk NA, Steinberg BE, Higgins DE, Brumell JH. 2008. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451:350–354 [DOI] [PubMed] [Google Scholar]
- 425. Portnoy DA, Auerbuch V, Glomski IJ. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Singh R, Jamieson A, Cresswell P. 2008. GILT is a critical host factor for Listeria monocytogenes infection. Nature 455:1244–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Wei Z, Schnupf P, Poussin MA, Zenewicz LA, Shen H, Goldfine H. 2005. Characterization of Listeria monocytogenes expressing anthrolysin O and phosphatidylinositol-specific phospholipase C from Bacillus anthracis. Infect. Immun. 73:6639–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Jones S, Portnoy DA. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Tweten RK. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Edelson BT, Cossart P, Unanue ER. 1999. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 163:4087–4090 [PubMed] [Google Scholar]
- 431. Bavdek A, Gekara NO, Priselac D, Gutierrez Aguirre I, Darji A, Chakraborty T, Macek P, Lakey JH, Weiss S, Anderluh G. 2007. Sterol and pH interdependence in the binding, oligomerization, and pore formation of listeriolysin O. Biochemistry 46:4425–4437 [DOI] [PubMed] [Google Scholar]
- 432. Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. 2003. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 198:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 53:1677–1693 [DOI] [PubMed] [Google Scholar]
- 434. Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Abel J, Goldmann O, Ziegler C, Holtje C, Smeltzer MS, Cheung AL, Bruhn D, Rohde M, Medina E. 2011. Staphylococcus aureus evades the extracellular antimicrobial activity of mast cells by promoting its own uptake. J. Innate Immun. 3:495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. 2011. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17:1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. 2012. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J. Infect. Dis. 206:352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Ratner AJ, Lysenko ES, Paul MN, Weiser JN. 2005. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc. Natl. Acad. Sci. U. S. A. 102:3429–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. 2005. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 1:e1 doi: 10.1371/journal.ppat.0010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Lysenko ES, Clarke TB, Shchepetov M, Ratner AJ, Roper DI, Dowson CG, Weiser JN. 2007. Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing. PLoS Pathog. 3:e118 doi: 10.1371/journal.ppat.0030118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A. 2011. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio 2:e00016–11 doi: 10.1128/mBio.00016-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Bricker AL, Carey VJ, Wessels MR. 2005. Role of NADase in virulence in experimental invasive group A streptococcal infection. Infect. Immun. 73:6562–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Madden JC, Ruiz N, Caparon M. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143–152 [DOI] [PubMed] [Google Scholar]
- 444. Magassa N, Chandrasekaran S, Caparon MG. 2010. Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep. 11:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Rose MC, Voynow JA. 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 86:245–278 [DOI] [PubMed] [Google Scholar]
- 446. Ha UH, Lim JH, Kim HJ, Wu W, Jin S, Xu H, Li JD. 2008. MKP1 regulates the induction of MUC5AC mucin by Streptococcus pneumoniae pneumolysin by inhibiting the PAK4-JNK signaling pathway. J. Biol. Chem. 283:30624–30631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Lam GY, Fattouh R, Muise AM, Grinstein S, Higgins DE, Brumell JH. 2011. Listeriolysin O suppresses phospholipase C-mediated activation of the microbicidal NADPH oxidase to promote Listeria monocytogenes infection. Cell Host Microbe 10:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Lizak M, Yarovinsky TO. 2012. Phospholipid scramblase 1 mediates type I interferon-induced protection against staphylococcal alpha-toxin. Cell Host Microbe 11:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Sahu SN, Lewis J, Patel I, Bozdag S, Lee JH, LeClerc JE, Cinar HN. 2012. Genomic analysis of immune response against Vibrio cholerae hemolysin in Caenorhabditis elegans. PLoS One 7:e38200 doi: 10.1371/journal.pone.0038200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Park PW, Pier GB, Hinkes MT, Bernfield M. 2001. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature 411:98–102 [DOI] [PubMed] [Google Scholar]
- 451. Park PW, Pier GB, Preston MJ, Goldberger O, Fitzgerald ML, Bernfield M. 2000. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J. Biol. Chem. 275:3057–3064 [DOI] [PubMed] [Google Scholar]
- 452. Park PW, Foster TJ, Nishi E, Duncan SJ, Klagsbrun M, Chen Y. 2004. Activation of syndecan-1 ectodomain shedding by Staphylococcus aureus alpha-toxin and beta-toxin. J. Biol. Chem. 279:251–258 [DOI] [PubMed] [Google Scholar]
- 453. Valaitis AP. 2008. Bacillus thuringiensis pore-forming toxins trigger massive shedding of GPI-anchored aminopeptidase N from gypsy moth midgut epithelial cells. Insect Biochem. Mol. Biol. 38:611–618 [DOI] [PubMed] [Google Scholar]
- 454. Arenas I, Bravo A, Soberon M, Gomez I. 2010. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 285:12497–12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35 [DOI] [PubMed] [Google Scholar]
- 456. Walker JA, Allen RL, Falmagne P, Johnson MK, Boulnois GJ. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 55:1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Johnson MK. 1977. Cellular location of pneumolysin. FEMS Microbiol. Lett. 2:243–245 [Google Scholar]
- 458. Balachandran P, Hollingshead SK, Paton JC, Briles DE. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Price KE, Camilli A. 2009. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J. Bacteriol. 191:2163–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Price KE, Greene NG, Camilli A. 2012. Export requirements of pneumolysin in Streptococcus pneumoniae. J. Bacteriol. 194:3651–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Stanley P, Packman LC, Koronakis V, Hughes C. 1994. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science 266:1992–1996 [DOI] [PubMed] [Google Scholar]
- 463. Howard SP, Buckley JT. 1985. Activation of the hole-forming toxin aerolysin by extracellular processing. J. Bacteriol. 163:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Sumitomo T, Nakata M, Higashino M, Jin Y, Terao Y, Fujinaga Y, Kawabata S. 2011. Streptolysin S contributes to group A streptococcal translocation across an epithelial barrier. J. Biol. Chem. 286:2750–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Liu T, Milia E, Warburton RR, Hill NS, Gaestel M, Kayyali US. 2012. Anthrax lethal toxin disrupts the endothelial permeability barrier through blocking p38 signaling. J. Cell. Physiol. 227:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. McClane BA, Chakrabarti G. 2004. New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin. Anaerobe 10:107–114 [DOI] [PubMed] [Google Scholar]
- 468. Waalwijk C, MacLaren DM, de Graaff J. 1983. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect. Immun. 42:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. 2009. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin. Microbiol. Infect. 15:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Montgomery CP, Daum RS. 2009. Transcription of inflammatory genes in the lung after infection with community-associated methicillin-resistant Staphylococcus aureus: a role for Panton-Valentine leukocidin? Infect. Immun. 77:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Olsen RJ, Kobayashi SD, Ayeras AA, Ashraf M, Graves SF, Ragasa W, Humbird T, Greaver JL, Cantu C, Swain JL, Jenkins L, Blasdel T, Cagle PT, Gardner DJ, DeLeo FR, Musser JM. 2010. Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am. J. Pathol. 176:1346–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Vandenesch F, Lina G, Henry T. 2012. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Berry AM, Paton JC. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Cowan GJ, Atkins HS, Johnson LK, Titball RW, Mitchell TJ. 2007. Immunisation with anthrolysin O or a genetic toxoid protects against challenge with the toxin but not against Bacillus anthracis. Vaccine 25:7197–7205 [DOI] [PubMed] [Google Scholar]
- 475. Rouzic N, Janvier F, Libert N, Javouhey E, Lina G, Nizou JY, Pasquier P, Stamm D, Brinquin L, Pelletier C, Vandenesch F, Floret D, Etienne J, Gillet Y. 2010. Prompt and successful toxin-targeting treatment of three patients with necrotizing pneumonia due to Staphylococcus aureus strains carrying the Panton-Valentine leukocidin genes. J. Clin. Microbiol. 48:1952–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. 2002. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185:196–203 [DOI] [PubMed] [Google Scholar]
- 477. Moayeri M, Robinson TM, Leppla SH, Karginov VA. 2008. In vivo efficacy of beta-cyclodextrin derivatives against anthrax lethal toxin. Antimicrob. Agents Chemother. 52:2239–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Yannakopoulou K, Jicsinszky L, Aggelidou C, Mourtzis N, Robinson TM, Yohannes A, Nestorovich EM, Bezrukov SM, Karginov VA. 2011. Symmetry requirements for effective blocking of pore-forming toxins: comparative study with alpha-, beta-, and gamma-cyclodextrin derivatives. Antimicrob. Agents Chemother. 55:3594–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Cai C, Che J, Xu L, Guo Q, Kong Y, Fu L, Xu J, Cheng Y, Chen W. 2011. Tumor endothelium marker-8 based decoys exhibit superiority over capillary morphogenesis protein-2 based decoys as anthrax toxin inhibitors. PLoS One 6:e20646 doi: 10.1371/journal.pone.0020646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Singh Y, Khanna H, Chopra AP, Mehra V. 2001. A dominant negative mutant of Bacillus anthracis protective antigen inhibits anthrax toxin action in vivo. J. Biol. Chem. 276:22090–22094 [DOI] [PubMed] [Google Scholar]
- 481. Ideo H, Fukushima K, Gengyo-Ando K, Mitani S, Dejima K, Nomura K, Yamashita K. 2009. A Caenorhabditis elegans glycolipid-binding galectin functions in host defense against bacterial infection. J. Biol. Chem. 284:26493–26501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, Groeneveld AB, Vonk Noordegraaf A, van Hinsbergh VW, van Nieuw Amerongen GP. 2012. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation 126:2728–2738 [DOI] [PubMed] [Google Scholar]
- 483. Hirst RA, Mohammed BJ, Mitchell TJ, Andrew PW, O'Callaghan C. 2004. Streptococcus pneumoniae-induced inhibition of rat ependymal cilia is attenuated by antipneumolysin antibody. Infect. Immun. 72:6694–6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Nakouzi A, Rivera J, Rest RF, Casadevall A. 2008. Passive administration of monoclonal antibodies to anthrolysin O prolong survival in mice lethally infected with Bacillus anthracis. BMC Microbiol. 8:159 doi: 10.1186/1471-2180-8-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Parker MW, Buckley JT, Postma JP, Tucker AD, Leonard K, Pattus F, Tsernoglou D. 1994. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 367:292–295 [DOI] [PubMed] [Google Scholar]
- 486. Madej T, Addess KJ, Fong JH, Geer LY, Geer RC, Lanczycki CJ, Liu C, Lu S, Marchler-Bauer A, Panchenko AR, Chen J, Thiessen PA, Wang Y, Zhang D, Bryant SH. 2012. MMDB: 3D structures and macromolecular interactions. Nucleic Acids Res. 40:D461–D464 [DOI] [PMC free article] [PubMed] [Google Scholar]