Abstract
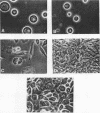
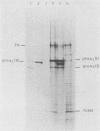
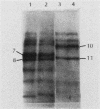
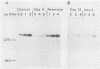
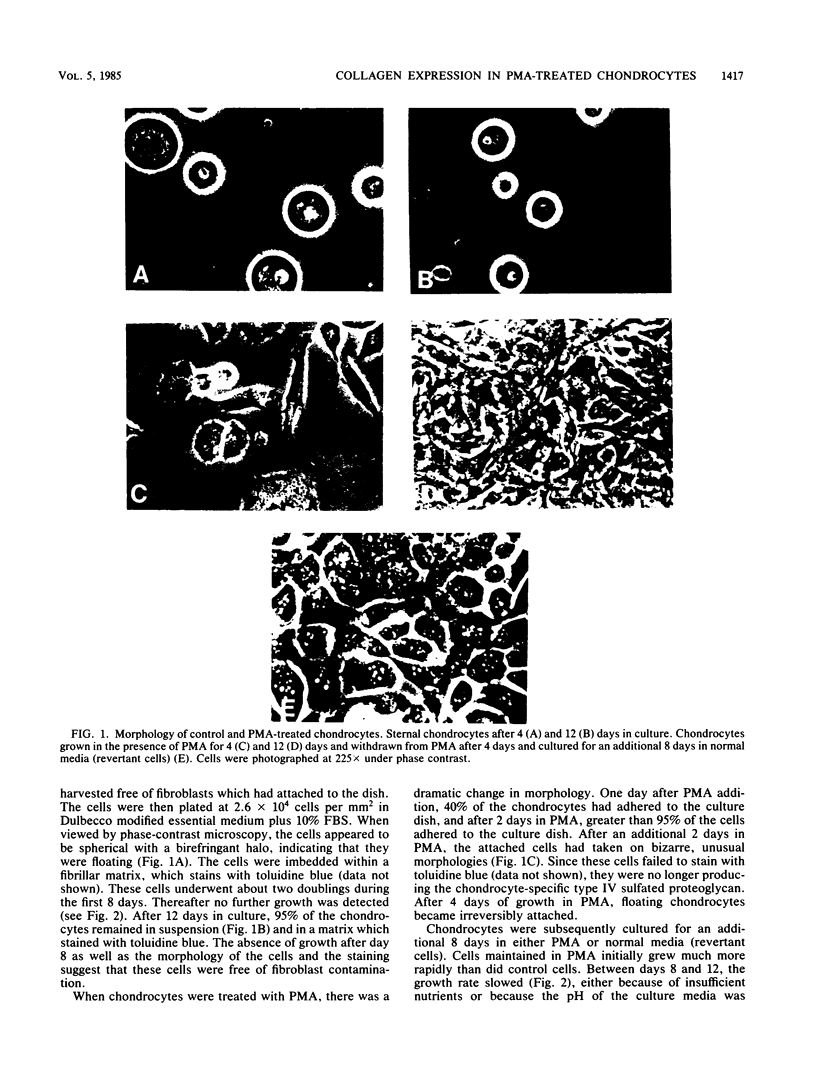
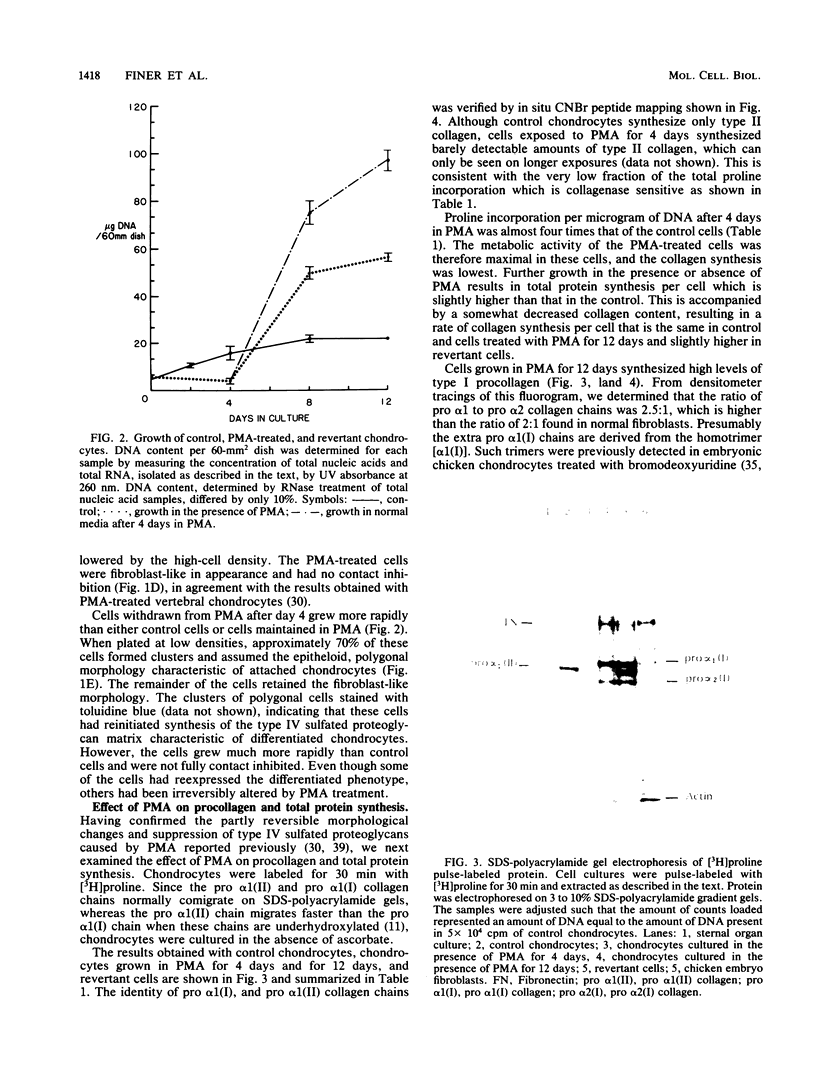
Growth of embryonic chicken sternal chondrocytes in the presence of phorbol-12-myristate-13-acetate (PMA), a potent tumor promoter, resulted in a dramatic morphological change from spherical floating cells to adherent fibroblastic cells. This morphological change was accompanied by a quantitative switch from synthesis of cartilage-specific type II procollagen to type I procollagen. Type II procollagen mRNA levels decreased 10-fold in PMA-treated cells. Activation of type I collagen genes led to the accumulation of type I procollagen mRNA levels comparable to those of type II mRNA in these cells. However, only type I procollagen mRNA was translated. In addition to gene activation, unprocessed pro alpha 1(I) transcripts present at low levels in control chondrocytes were processed to mature mRNA species. Redifferentiation of PMA-treated chondrocytes was possible if cells were removed from PMA after the morphological change and cessation of type II procollagen synthesis but before detectable amounts of type I procollagen were synthesized. Production of type I collagen thus marks a late phase of chondrocyte "dedifferentiation" from which reversion is no longer possible. Redifferentiated cell populations contained 24-fold more pro alpha 1(II) collagen mRNA than pro alpha 1(I) collagen mRNA, but the rates of procollagen synthesis were comparable. This suggests that the PMA-mediated dedifferentiation of chondrocytes as well as their redifferentiation is under both transcriptional and posttranscriptional regulation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells, V. The effect of 5-bromodeoxyuridine on cloned chondrocytes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1144–1151. doi: 10.1073/pnas.59.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Adams S. L., Boettiger D., Focht R. J., Holtzer H., Pacifici M. Regulation of the synthesis of extracellular matrix components in chondroblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1982 Sep;30(2):373–384. doi: 10.1016/0092-8674(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Banerji S. S., Theodorakis N. G., Morimoto R. I. Heat shock-induced translational control of HSP70 and globin synthesis in chicken reticulocytes. Mol Cell Biol. 1984 Nov;4(11):2437–2448. doi: 10.1128/mcb.4.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978 Dec;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. The progeny of rabbit articular chondrocytes synthesize collagen types I and III and type I trimer, but not type II. Verifications by cyanogen bromide peptide analysis. Biochemistry. 1977 Mar 8;16(5):865–872. doi: 10.1021/bi00624a009. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chacko S., Abbott J., Holtzer S., Holtzer H. The loss of phenotypic traits by differentiated cells. VI. Behavior of the progeny of a single chondrocyte. J Exp Med. 1969 Aug 1;130(2):417–442. doi: 10.1084/jem.130.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S., Grant M. E., Jackson D. S. Translation of type II procollagen mRNA and hydroxylation of the cell-free product. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1025–1031. doi: 10.1016/0006-291x(79)91982-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleman A. W., Coleman J. R., Kankel D., Werner I. The reversible control of animal cell differentiation by the thymidine analog, 5-bromodeoxyuridine. Exp Cell Res. 1970 Feb;59(2):319–328. doi: 10.1016/0014-4827(70)90606-3. [DOI] [PubMed] [Google Scholar]
- De Luca S., Lohmander L. S., Nilsson B., Hascall V. C., Caplan A. I. Proteoglycans from chick limb bud chondrocyte cultures. Keratan sulfate and oligosaccharides which contain mannose and sialic acid. J Biol Chem. 1980 Jul 10;255(13):6077–6083. [PubMed] [Google Scholar]
- Dessau W., Sasse J., Timpl R., Jilek F., von der Mark K. Synthesis and extracellular deposition of fibronectin in chondrocyte cultures. Response to the removal of extracellular cartilage matrix. J Cell Biol. 1978 Nov;79(2 Pt 1):342–355. doi: 10.1083/jcb.79.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., von der Mark H., von der Mark K., Fischer S. Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J Embryol Exp Morphol. 1980 Jun;57:51–60. [PubMed] [Google Scholar]
- Gerstenfeld L. C., Crawford D. R., Boedtker H., Doty P. Expression of type I and III collagen genes during differentiation of embryonic chicken myoblasts in culture. Mol Cell Biol. 1984 Aug;4(8):1483–1492. doi: 10.1128/mcb.4.8.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Finer M. H., Boedtker H. Altered beta-actin gene expression in phorbol myristate acetate-treated chondrocytes and fibroblasts. Mol Cell Biol. 1985 Jun;5(6):1425–1433. doi: 10.1128/mcb.5.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld L., Beldekas J. C., Franzblau C., Sonenshein G. E. Cell-free translation of calf type III collagen. Effect of magnesium on ribosome movement during elongation. J Biol Chem. 1983 Oct 10;258(19):12058–12063. [PubMed] [Google Scholar]
- Goetinck P. F., Pennypacker J. P., Royal P. D. Proteochondroitin sulfate synthesis and chondrogenic expression. Exp Cell Res. 1974 Aug;87(2):241–248. doi: 10.1016/0014-4827(74)90476-5. [DOI] [PubMed] [Google Scholar]
- Gonnerman W. A., Goral A. B., Franzblau C. Use of soluble, collagenous peptides from medium of chick calvaria cultures as substrate for prolyl hydroxylase. Anal Biochem. 1980 Feb;102(1):8–12. doi: 10.1016/0003-2697(80)90308-5. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Abbott J., Lash J., Holtzer S. THE LOSS OF PHENOTYPIC TRAITS BY DIFFERENTIATED CELLS IN VITRO, I. DEDIFFERENTIATION OF CARTILAGE CELLS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K., Martin G. R., Yamada Y. Isolation and characterization of a cDNA clone for the amino-terminal portion of the pro-alpha 1(II) chain of cartilage collagen. J Biol Chem. 1984 Nov 25;259(22):13668–13673. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Boedtker H. Construction and characterization of pro alpha 1 collagen complementary deoxyribonucleic acid clones. Biochemistry. 1979 Jul 10;18(14):3146–3152. doi: 10.2196/47873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Crkvenjakov R., Boedtker H., Doty P. Construction and characterization of a 2.5-kilobase procollagen clone. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5417–5421. doi: 10.1073/pnas.75.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer T. F., Toole B. P., Trelstad R. L. Temporal and spatial transitions in collagen types during embryonic chick limb development. Dev Biol. 1973 Dec;35(2):232–239. doi: 10.1016/0012-1606(73)90020-1. [DOI] [PubMed] [Google Scholar]
- Lowe M. E., Pacifici M., Holtzer H. Effects of phorbol-12-myristate-13-acetate on the phenotypic program of cultured chondroblasts and fibroblasts. Cancer Res. 1978 Aug;38(8):2350–2356. [PubMed] [Google Scholar]
- Lukens L. N., Frischauf A. M., Pawlowski P. J., Brierley G. T., Lehrach H. Construction and characterization of type II collagen complementary deoxyribonucleic acid clones. Nucleic Acids Res. 1983 Sep 10;11(17):6021–6039. doi: 10.1093/nar/11.17.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons B. L., Schwarz R. I. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984 Mar 12;12(5):2569–2579. doi: 10.1093/nar/12.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4511–4515. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Holtzer H. Effects of a tumor-promoting agent on chondrogenesis. Am J Anat. 1977 Sep;150(1):207–212. doi: 10.1002/aja.1001500116. [DOI] [PubMed] [Google Scholar]
- Pawlowski P. J., Brierley G. T., Lukens L. N. Changes in the type II and type I collagen messenger RNA population during growth of chondrocytes in 5-bromo-2-deoxyuridine. J Biol Chem. 1981 Aug 10;256(15):7695–7698. [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Rowe L. B., Schwarz R. I. Role of procollagen mRNA levels in controlling the rate of procollagen synthesis. Mol Cell Biol. 1983 Feb;3(2):241–249. doi: 10.1128/mcb.3.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Schwarz R., Colarusso L., Doty P. Maintenance of differentiation in primary cultures of avian tendon cells. Exp Cell Res. 1976 Oct 1;102(1):63–71. doi: 10.1016/0014-4827(76)90299-8. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tate V. E., Finer M. H., Boedtker H., Doty P. Chick pro alpha 2 (I) collagen gene: exon location and coding potential for the prepropeptide. Nucleic Acids Res. 1983 Jan 11;11(1):91–104. doi: 10.1093/nar/11.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]
- Yamada Y., Mudryj M., de Crombrugghe B. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J Biol Chem. 1983 Dec 25;258(24):14914–14919. [PubMed] [Google Scholar]
- Young M. F., Vogeli G., Nunez A. M., Fernandez M. P., Sullivan M., Sobel M. E. Isolation of cDNA and genomic DNA clones encoding type II collagen. Nucleic Acids Res. 1984 May 25;12(10):4207–4228. doi: 10.1093/nar/12.10.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K. Immunological studies on collagen type transition in chondrogenesis. Curr Top Dev Biol. 1980;14(Pt 2):199–225. doi: 10.1016/s0070-2153(08)60195-7. [DOI] [PubMed] [Google Scholar]
- von der Mark K., von der Mark H. Immunological and biochemical studies of collagen type transition during in vitro chrondrogenesis of chick limb mesodermal cells. J Cell Biol. 1977 Jun;73(3):736–747. doi: 10.1083/jcb.73.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]