Abstract
OBJECTIVES
An increasing prevalence of pediatric asthma has led to increasing burdens of critical illness in children with severe acute asthma exacerbations, often leading to respiratory distress, progressive hypoxia, and respiratory failure. We review the definitions, epidemiology, pathophysiology, and clinical manifestations of severe acute asthma, with a view to developing an evidence-based, stepwise approach for escalating therapy in these patients.
METHODS
Subject headings related to asthma, status asthmaticus, critical asthma, and drug therapy were used in a MEDLINE search (1980–2012), supplemented by a manual search of personal files, references cited in the reviewed articles, and treatment algorithms developed within Le Bonheur Children's Hospital.
RESULTS
Patients with asthma require continuous monitoring of their cardiorespiratory status via noninvasive or invasive devices, with serial clinical examinations, objective scoring of asthma severity (using an objective pediatric asthma score), and appropriate diagnostic tests. All patients are treated with β-agonists, ipratropium, and steroids (intravenous preferable over oral preparations). Patients with worsening clinical status should be progressively treated with continuous β-agonists, intravenous magnesium, helium-oxygen mixtures, intravenous terbutaline and/or aminophylline, coupled with high-flow oxygen and non-invasive ventilation to limit the work of breathing, hypoxemia, and possibly hypercarbia. Sedation with low-dose ketamine (with or without benzodiazepines) infusions may allow better toleration of non-invasive ventilation and may also prepare the patient for tracheal intubation and mechanical ventilation, if indicated by a worsening clinical status.
CONCLUSIONS
Severe asthma can be a devastating illness in children, but most patients can be managed by using serial objective assessments and the stepwise clinical approach outlined herein. Following multidisciplinary education and training, this approach was successfully implemented in a tertiary-care, metropolitan children's hospital.
INDEX TERMS: asthma, child, critical care, infant, respiratory failure, status asthmaticus
INTRODUCTION
Asthma is one of the most common chronic diseases in childhood, with increasing prevalence in the past 3 decades. The severity of asthma can range from mild to severe; however, despite the degree of severity all patients are at risk for developing an acute severe asthma exacerbation. This review is focused on the treatment of acute asthma exacerbation in the pediatric intensive care setting with the application of a stepwise approach according to the severity and progression of the exacerbation, calculated with a pediatric asthma score1 that has been implemented in our institution, Le Bonheur Children's Hospital, Pediatric Intensive Care.
DEFINITION
Severe acute asthma is defined as a condition in which children with acute asthma exacerbation and respiratory distress do not respond to bronchodilators and who either have not received corticosteroids as an outpatient or continue to experience respiratory distress despite outpatient treatment. The severity of these episodes varies from mild to severe and each episode has the potential to progress to respiratory failure.2 Severe acute asthma is currently the most common medical emergency in children and is responsible for nearly half a million admissions to the pediatric intensive care unit (PICU) each year.1,3,4
Different terms have been described for severe acute asthma exacerbation, which include status asthmaticus, near-fatal asthma, sudden asphyxic asthma, and acute fatal asthma. Status asthmaticus refers to an acute asthma exacerbation in which bronchial obstruction is severe and continues to worsen or not improve despite the institution of adequate standard therapy, leading to respiratory failure. Near-fatal asthma was described as an asthma exacerbation of sudden onset that rapidly progresses to hypercapnia and hypoxemia, leading to respiratory arrest. The near-fatal attribute of the exacerbation was due to the severe asphyxia rather than cardiac arrhythmias.5,6 Sudden asphyxic asthma was characterized in a group of young patients presenting with rapid decompensation in less than 3 hours after onset of symptoms, severe hypercapnia, silent chest, and high incidence of respiratory arrest.7 Acute fatal asthma was reported when respiratory failure and death occurred within minutes of the exacerbation onset.8
EPIDEMIOLOGY
Recently, the National Center of Health Statistics reported that the asthma prevalence for the year 2009 in the United States was 8.2%, affecting 24.6 million people of all ages.9 When discriminated by age, 17.5 million adults and 7.1 million children (0–17 years) were affected. The asthma exacerbation prevalence, defined as the proportion of the affected population with at least 1 attack in the previous year, was 4.2% of a total of 12.8 million people, of which 4.0 million were children.
Asthma prevalence increased from 2001 to 2009 at an annual rate of 1.2%. However, asthma exacerbation prevalence did not increase significantly during 1997–2009 (3.9%–4.3%). This trend could be explained by the distribution of national asthma guidelines focusing on preventive treatment as well as more effective clinical management.4,9–11
Age group, sex, race, ethnicity and socioeconomic factors play a major role in asthma prevalence, health care use, and mortality.12–14 Among patients aged 0 to 17 years, males have higher prevalence than females: 11.3% and 7.9%, respectively. Also, children have higher current asthma prevalence: 9.6% as compared to 7.7% in adults. Compared with the white population, the prevalence is higher among African Americans and lower for Asians.15 Compared with non-Hispanic whites and non-Hispanic blacks, the current asthma prevalence is higher among Puerto Ricans and lower among Mexican ethnic groups.16,17 The population with a family income below the federal poverty level has higher asthma prevalence than the population with incomes near or above the poverty threshold.9 In the 2009 American Community Survey, 14.3% of the United States population had incomes below their respective poverty thresholds, providing a population at risk for the environmental factors associated with increased prevalence of asthma.12
Children aged 5 to 17 years missed 10.5 million school days owing to asthma, and activity limitations were reported in 5.5% as well. There were 1.75 million total emergency department (ED) visits, including 0.64 million for children aged 0 to 17 years. Total asthma-related hospitalizations were 456,000, of which 157, 000 were children. During 2007, there were 3447 deaths due to asthma, and 185 were for children aged 0 to 17 years. Asthma deaths for children and adults younger than 35 years for that year was 3.4 per million.9,19
PATHOPHYSIOLOGY
Asthma involves inflammation and edema of the bronchial mucosa, increased mucus production with airway plugging, and bronchospasm.20,21 These factors produce increased airway resistance, leading to increased work of breathing. As the degree of airway obstruction progresses, expiration becomes active and inspiration starts before termination of the previous expiration, resulting in air trapping and hyperinflation. Areas of obstruction and premature airway closure cause ventilation/perfusion mismatch,22 leading to hypoxemia.23
During a severe asthma exacerbation, the marked changes in lung volume and pleural pressures have a significant impact on cardiopulmonary interactions. Dynamic hyperinflation with progressive increased lung volumes stretches the pulmonary vasculature, increasing pulmonary vascular resistance and right ventricular after-load. Pulmonary vasoconstriction, secondary to hypoxia and acidosis, further contributes to the increase in right ventricular afterload.24 The high negative pulmonary pressures generated during inspiration in spontaneously breathing patients causes an increased left ventricular afterload25 and decreased cardiac output with exaggerated decrease in systolic blood pressure during inspiration.26 A decrease in systolic blood pressure by more than 10 mm Hg during inspiration is termed pulsus paradoxus.27 The negative intrapleural pressure generated favors transcapillary fluid edema to the alveolar space.28
There appear to be 2 subsets of children who die from severe acute asthma, perhaps representing some differences in their pathophysiology.29 Some children with fatal asthma have a long history of poorly controlled severe asthma, often with a previous history of respiratory failure (type 1, or slow onset-late arrival). This pattern of fatal asthma, responsible for most asthma-related deaths, is generally considered preventable, with death occurring owing to acute respiratory failure and asphyxia, or from complications associated with mechanical ventilation.6,30–35 Pathologic examination in these cases demonstrates extensive bronchial mucus plugging, edema, and eosinophilic infiltrations of the airways.
Alternatively, some children present with a history of mild asthma, or even without a prior history of asthma. These patients experience a sudden onset of bronchospasm and rapidly progress to cardiac arrest and death (type 2, or fast-onset, asthma).7,34,36,37 If recognized and managed early, these children respond faster to β-agonists and mechanical ventilatory support than children with type 1 fatal asthma.38 Pathologic examination of these cases shows empty airways devoid of mucus plugging with a greater proportion of neutrophils and eosinophils.37,39
Robertson et al40 reviewed 51 pediatric deaths from asthma in Australia between 1986 and 1999, and found that nearly one-third of these children were judged to have mild asthma with no prior hospitalizations. In the final acute exacerbation, 63% of these children experienced a sudden onset and collapse within minutes of the beginning of symptoms, and 75% died before reaching a hospital. Only 25% of these children had an acute progression of chronic, poorly controlled asthma that resulted in eventual death. These authors concluded that 39% of these deaths would have been preventable with earlier recognition and intervention.
During a 6-year period at The Hospital for Sick Children in Toronto, 89 children were admitted to the PICU for severe acute asthma. Three children died in the PICU from hypoxic ischemic encephalopathy following out-of-hospital cardiac arrest.41 Kravis and Kolski42 reported a case series of 13 deaths secondary to asthma. Only 1 child died after admission to the hospital. Similarly, nearly 50% of asthmatic children in another study died before reaching the hospital, with the time from the onset of symptoms to death being less than 1 hour in 21%, and less than 2 hours in 50% of these cases, respectively.43,44 Thus, these case series underscore the need for early recognition of children at risk for type 2 fast-onset, sudden asphyxial asthma.
CLINICAL MANIFESTATIONS
Children with severe acute asthma commonly present with tachypnea, increased work of breathing, use of accessory muscles, nasal flaring, diaphoresis, and anxiety. They may also present obtunded, in respiratory failure, or cardiopulmonary arrest.
Wheezing is a common clinical finding in patients with acute asthma exacerbation, which is generated by turbulent airflow in the intrathoracic obstructed airways. The degree of wheezing correlates poorly with the severity of disease,45 because wheezes are heard only in the presence of airflow. The presence of silent chest due to limited airflow is an ominous sign of impeding respiratory failure. Wheezing is predominantly expiratory owing to the dynamic compression of the airways, but also can be biphasic and usually is symmetrical; an asymmetrical distribution should alert a clinician for the presence of atelectasis, pneumothorax, or foreign body.
Evaluating disease severity and response to therapy objectively are most important. A number of authors46–48 have developed clinical asthma scores. Clinical scores have been shown to correlate with the need for hospitalization and prolonged bronchodilator therapy, and with the severity of an exacerbation.49 However, they are not as effective at predicting disease progression and prolonged hospitalization.50,51 The implementation of an inpatient clinical pathway correlated with the pediatric asthma score in hospitalized asthmatic children could lead to a decrease in length of stay and a reduction in total cost with overall improving quality of care.1 Because early recognition is important for improving outcomes, various authors13,52–54 have attempted to define the risk factors for fatal asthma. These may include, among other factors:
- History of previous asthma exacerbation with:
- Severe, rapid progression of symptoms
- Respiratory failure requiring endotracheal intubation or ventilatory support
- Seizures or loss of consciousness
- PICU admission
Denial or failure to perceive the severity of illness
Non-compliance with controller medications or asthma care plan
Lack of social supports or safety network (e.g., dysfunctional family, poverty)
Associated psychiatric disorder, for example, depression
African American and Hispanic children
CHEST RADIOGRAPHY
Chest radiography is not routinely indicated in a child with previous history of asthma.55,56 Potential indications for chest radiography may include clinical suspicion for pneumothorax, atelectasis, foreign body aspiration, or after endotracheal intubation.
ARTERIAL BLOOD GAS
Arterial blood gas measurement provides objective information on gas exchange. Early in the course of asthma, hypoxemia and hypocapnia are found due to ventilation/perfusion mismatch and hyperventilation.57 As the airflow obstruction progresses, PaCO2 measurement returns to normal values, though in a tachypneic and hyper-ventilating child, a normal PaCO2 value should be interpreted as a sign of early muscle fatigue.58 Sicker patients often have a mixed respiratory and metabolic acidosis.59 Lactic acidosis reflects a combination of excess production from respiratory muscles, tissue hypoxia (due to hypoxemia and decreased cardiac output), and dehydration (due to decreased intake and increased insensible losses).60 The decision to intubate a child with severe acute asthma should be based on the child's clinical status and not simply the arterial blood gas values.61
TREATMENT
Monitoring
Patients admitted to the PICU require intravenous access, as well as continuous monitoring of their cardiorespiratory status, including noninvasive blood pressure and oxygen saturations (SpO2). Those with respiratory failure requiring mechanical ventilation should undergo the placement of central venous, arterial, and urinary bladder catheters. These are often the sickest patients in the PICU and require expert nursing care, monitoring of fluid intake and output, and skin care to prevent decubitus ulcers, as well as frequent clinical examination.
The Pediatric Asthma Score (PAS)1 has been adopted by Le Bonheur Children's Hospital for the assessment of patients with asthma exacerbation (Table 1). During patient assessment a respiratory therapist will document the PAS every 2 hours while the patient is receiving continuous albuterol treatment. Advancement in therapy to the next level will be performed when there is worsening of the PAS, using the stepwise approach (Figure).
Table 1.
Pediatric Intensive Care Unit Pediatric Asthma Score1
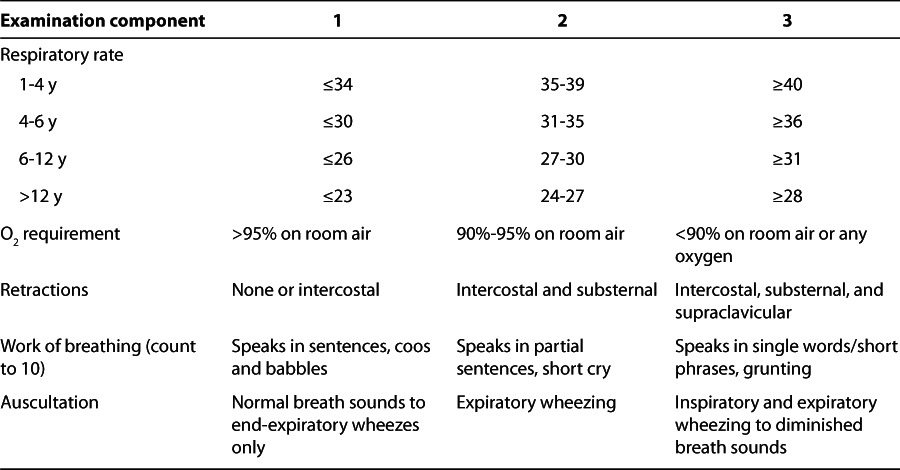
Figure.
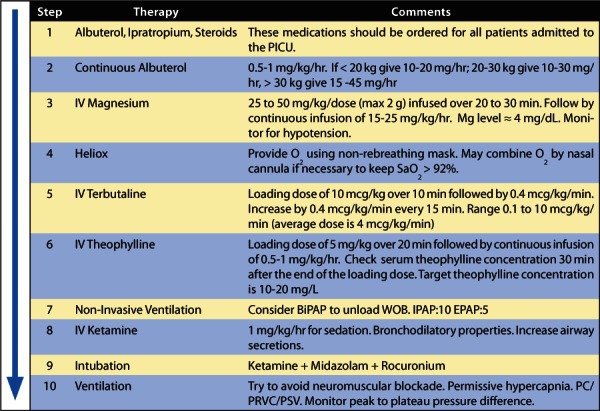
Severe acute asthma stepwise approach for escalating therapy.
BiPAP, bilevel positive airway pressure; EPAP, Expiratory Positive Airway Pressure; IPAP, Inspiratory Positive Airway Pressure; Mg, Magnesium; O2, oxygen; PC, Pressure Controlled Ventilation; PRVC, Pressure Regulated Volume Control; PSV, Pressure Support Ventilation; SaO2, saturation level of oxygen; WOB, work of breathing
Oxygen
Children with severe acute asthma possibly will have ventilation/perfusion mismatch23 as an effect of mucus plugging and atelectasis, causing hypoxemia. Treatment with β-agonists may aggravate hypoxemia by increasing cardiac output and eliminating the compensatory hypoxic pulmonary vasoconstriction.61,62 Oxygen should be used as carrier gas for intermittent or continuous nebulization63 and to keep oxygen saturation above 92%. There is no evidence that oxygen suppresses respiratory drive in children with severe asthma.64
Fluids
Patients with severe acute asthma are often dehydrated because of poor oral intake and increased insensible fluid losses. Appropriate fluid resuscitation and maintenance fluids are indicated. The key is to avoid overhydration because of the increased risk of transpulmonary edema in children with severe asthma associated with large fluctuations in intrathoracic pressures. The use of half normal saline or isotonic solution in dextrose is preferred in the pediatric population.
Steroids
Corticosteroids are the first line of treatment for severe acute asthma, because of the inflammatory process.65 Steroids control airway inflammation through a number of mechanisms,66 such as reducing the number and activation of lymphocytes, eosinophils, mast cells, and macrophages; suppressing the production of cytokines, tumor necrosis factor-α, granulocyte-macrophage colony-stimulating factor, adhesion molecules, and inducible enzymes, including nitric oxide synthase and cyclooxygenase-2.67 Mucous production is decreased, and inflammatory cell infiltration and activation are reduced.68,69 In children with severe acute asthma, systemic corticosteroids are indicated and in the intensive care unit (ICU) setting the intravenous route is preferred.70 Methylprednisolone is a widely used agent because of its limited mineralocorticoid effect.71 The previous National Asthma Education and Prevention Program (NAEPP), still in use by some centers, recommended an initial methylprednisolone loading dose of 2 mg/kg followed by 0.5 to 1 mg/kg every 6 hours.72 Nevertheless, the new dose recommendation for asthma exacerbations by the current NAEPP (2007) review is 1 to 2 mg/kg/day (maximum 60 mg/day) in 2 divided doses for children younger than 12 years, and 40 to 80 mg/day for children older than 12 years and adults given either oral or intravenous prednisolone or methylprednisolone, respectively.71–75 The effect starts within 1 to 3 hours and peaks at 4 to 8 hours.76 Duration of treatment depends on the severity of the asthma exacerbation. Adverse drug reactions related to the use of high-dose steroids have been described and include hyperglycemia, hypertension, and acute psychosis.77 Allergic reactions have been described with the use of methylprednisolone.78 The incidence of muscle weakness and myopathy is increased, especially when aminosteroid neuromuscular-blocking agents are used in combination with steroids in mechanically ventilated patients.79
β-Agonists
β-Agonists are sympathomimetic agents that cause bronchodilatation due to bronchial smooth muscle relaxation by activating β2-adrenergic receptors, which increase intracellular cyclic adenosine monophosphate (cAMP) concentrations within smooth muscles. The β2-adrenoceptor coupling to adenylate cyclase is affected via a trimetric Gs protein with α, β, and γ subunits.80 The mechanism involved in airway smooth muscle cell relaxation is cAMP release, which catalyzes the activation of protein kinase A (PKA). PKA, in turn, phosphorylates regulatory and cytoskeletal proteins involved in the control of muscle tone. cAMP inhibits the release of calcium ion from intracellular stores and reduces the membrane calcium entry and its intracellular sequestration, leading to airway smooth muscle relaxation.80,81 Alternative, cAMP independent pathways involving the activation of membrane maxi-potassium channels through Gi to the MAP kinase system have also been described.82
The most reported adverse drug reactions with β-agonists are tachycardia, tremors, and nausea. Cardiovascular effects include diastolic hypotension,83 arrhythmias, and prolonged QTc interval with hypokalemia.84 Hypokalemia is the result of intracellular potassium shifting from an increased number of sodium-potassium pumps.85
Albuterol
Albuterol is a 50:50 racemic mixture of R-albuterol and S-albuterol. The R-enantiomer is pharmacologically active, whereas the S-enantiomer is considered inactive and has a longer elimination half-life. Levalbuterol is the pure R-enantiomer as a preservative-free solution. In comparison trials the use of equivalent doses of levalbuterol was not superior to albuterol.85,86 Albuterol remains the drug of choice for treatment of severe acute asthma in the ICU owing to its lower cost and similar efficacy.
Continuous β2-agonist nebulization is considered to be superior87 or at least equivalent88 to intermittent treatment89 for severe asthma and was not associated with severe cardiotoxicity.90–93 Transient increase in creatinine phosphokinase without evidence of cardiotoxicity during continuous albuterol nebulization has been reported.94 Continuous nebulization offers more comfort to patients and is more cost-effective.95 From the amount of drug that can be delivered per hour of nebulized treatment, approximately 25% of the dose will reach the lungs.96 The usual dose for continuous albuterol nebulization ranges from 0.15 to 0.5 mg/kg/h. Larger doses of continuous albuterol nebulization for near-fatal asthma have been used,97 although we generally do not exceed maximum rates of 30 mg/h. In our institution we use conventional continuous-output nebulizer devices with oxygen.
Terbutaline
Intravenous β2-agonist should be considered in patients who are not improving with continuous albuterol nebulization,98 probably owing to decreased respiratory flow and tidal volume resulting in a reduction of drug delivery to the small airways. Terbutaline is considered the drug of choice in the United States, although intravenous albuterol and other β2-agonists are also available elsewhere.
Subcutaneous terbutaline is preferred because there are fewer adverse drug reactions when compared to epinephrine.99 Subcutaneous terbutaline sulfate has been recommended for hospitalized children or adolescents older than 12 years with asthma exacerbation, at a dose of 0.25 mg every 20 minutes for a total of 3 doses,55 as well as for children 12 years of age or younger at a dose of 0.01 mg/kg (maximum dose, 0.25 mg) every 20 minutes for a total of 3 doses, repeated every 2 to 6 hours as needed.55 To accelerate the therapeutic effect, an intravenous loading dose of 2 to 10 mcg/kg infused for 10 minutes is recommended, followed by a continuous infusion of 0.1 to 10 mcg/kg/min.83 Because of differences in drug metabolism and clinical effect among patients, dose adjustment should be assessed at regular intervals. Usually the starting dose is 0.4 mcg/kg/min; it is titrated to achieve the desired clinical effect with increments of 0.2 to 0.4 mcg/kg/min every 15 to 30 minutes depending on the patient's clinical response and on adverse drug reactions.100 Monitoring the patient for the side effects of tachycardia, arrhythmias, diastolic hypotension, hypokalemia, or (rarely) myocardial ischemia is more important in patients receiving continuous infusions of β-adrenergic agonists.
Ipratropium
Ipratropium bromide is a quaternary ammonium atropine derivative that does not cross the blood-brain barrier, precluding the manifestation of central anticholinergic effects. Ipratropium can produce bronchodilatation by inhibition of cholinergic-mediated bronchospasm, occurring without the inhibition of mucociliary clearance.101 The parasympatholytic effect is produced by blocking acetylcholine interaction with the muscarinic receptors on bronchial smooth muscle cells and reducing intracellular cyclic guanosine monophosphate concentrations that impair bronchoconstriction. Nebulized anticholinergic agents are considered an important adjunct in the treatment of moderate to severe asthma exacerbation in the ED setting.102–104 After 1 dose of steroids, the use of ipratropium with the second and third albuterol doses was associated with clinical improvement and decreased hospital admission rates,105 compared with albuterol and corticosteroids alone.106,107 Nebulized ipratropium, in 0.25 to 0.50 mg doses, can be used every 20 minutes during the first hour, followed by the same dose range every 6 hours.108–110 Systemic effects are usually minimal; nevertheless, mydriasis and blurred vision have been reported.111,112 Although there is no significant apparent benefit with the addition of multiple doses of ipratropium to an albuterol and steroid regimen in hospitalized pediatric patients,113,114 there is a need for specific data in the PICU population. With the high safety profile and documented beneficial effects in the ED setting,115 we recommend its use every 6 hours in the critically ill patient owing to its potential advantages, despite not being recommended by the current National Heart, Lung, and Blood Institute asthma guidelines, until further data are obtained.55
Magnesium Sulfate
Magnesium is a calcium antagonist that causes smooth muscle relaxation as a result of the inhibition of calcium uptake.116 With respect to asthmatic patients, mechanisms such as the inhibitory action on smooth muscle contraction,117 histamine release from mast cells,118 acetylcholine release from nerve terminals,119 and sedative action65,120 may contribute to its therapeutic effects.
From current data, magnesium sulfate is likely to improve bronchospasm and consequent clinical symptoms and reduce hospitalization in children with moderate to severe acute asthma when added to classic therapy.121 Some studies122–124 have shown clinical improvement in patients with severe asthma receiving intravenous magnesium infusion.
The usual dose of magnesium sulfate in children with severe acute asthma is 25 to 50 mg/kg/dose (maximum 2 g), infused for 20 to 30 minutes.125–127 A larger loading dose of magnesium sulfate has been recommended128 to achieve serum magnesium concentrations between 3 to 5 mg/dL. For the treatment of acute asthma exacerbation refractory to conventional therapies, an initial bolus dose of 50 mg/kg (maximum dose, 2 g) infused for 20 to 30 minutes is recommended, followed by continuous infusion dependent on the patient's weight. Children weighing less than 30 kg may receive an infusion of 25 mg/kg/h and children weighing more than 30 kg may receive 20 mg/kg/h, although infusion rates must not exceed 2 g/h in any patient.129 Titration to the desired clinical effect should be based on serum magnesium concentrations and tolerability. Adverse drug reactions, such as nausea, flushing, somnolence, vision changes, muscle weakness, and hypotension, were reported with magnesium concentrations above 9 mg/dL.130 Severe adverse reactions such as respiratory depression and arrhythmias occurred with concentrations above 12 mg/dL.36
Methylxanthines
Methylxanthines are formed by the methylation of xanthines, such as theophylline. The combination of theophylline and ethylenediamine generates a water-soluble salt, aminophylline. The proposed mechanism for bronchodilatation involves the non-selective inhibition of phosphodiesterase isoenzymes,131 in particular, the inhibition of phosphodiesterase-IV, which reduces the intracellular degradation of cAMP.132 Other mechanisms include increased respiratory drive and diaphragmatic contractility,133 stimulation of endogenous catecholamine release,134 prostaglandin antagonism,135 and inhibition of afferent neuronal activity.136 In addition, theophylline is known to have anti-inflammatory and immunomodulatory effects, although the contributions of these mechanisms to its therapeutic effects in children with asthma have not been studied.137,138
The therapeutic range for theophylline is narrow, 10 to 20 mcg/mL, and overlaps with toxicity concentrations, which occurs above 15 mcg/mL.139 We recommend titration of dosing to a peak concentration of 10 to 15 mcg/mL. However, at a concentration above 5 mcg/mL bronchodilatory and anti-inflammatory effects were detected.140 The theophylline dose is 80% of the aminophylline dose. A loading intravenous dose, 5mg/kg of theophylline or 6 mg/kg of aminophylline, given during 20 minutes is needed to achieve a therapeutic concentration.97 Assuming an average volume of distribution, 1 mg/kg of theophylline or 1.25 mg/kg of aminophylline increases serum concentration by 2 mcg/mL. After the loading dose, a continuous infusion should be started. The drug clearance is decreased in neonates and infants, and, therefore, infusion rates are based on patient age. Recommended doses for infants younger than 6 months are 0.5 mg/kg/h; for infants 6 months to 1 year, 0.85 to 1 mg/kg/h; for children 1 to 9 years, 1 mg/kg/h; and for children older than 9 years, 0.75 mg/kg/h.141 These recommended doses are for patients with normal cardiac and liver functions. Serum drug concentrations should be obtained 30 to 60 minutes after the loading dose is finished, and again at 12 hours after the beginning of the continuous infusion, to check for steady-state concentrations, and then every 12 to 24 hours or when toxicity is suspected. Theophylline clearance possibly will be decreased in patients with acute pulmonary edema, congestive heart failure, cor pulmonale, fever, hepatic disease, hypothyroidism, sepsis with multiorgan failure, neonates, infants younger than 3 months with decreased renal function, children younger than 1 year, and patients after cessation of smoking or substantial second-hand smoke exposure. Many drugs decrease theophylline clearance. The most common are erythromycin, cimetidine, and oral contraceptives. Azithromycin has no effect on theophylline clearance because of the absence of interactions with cytochrome P450.142
Theophylline should be used for children with severe acute asthma exacerbation with impeding respiratory failure, or those on mechanical ventilation who are already getting other bronchodilator or anti-inflammatory therapies.143,144
Helium-Oxygen Mixture (Heliox)
Helium is a low-density gas that, when used in a mixture with oxygen, reduces turbulent airflow, enhancing laminar flow and in consequence reducing airflow resistance.145 Limited evidence exists for the efficacy of helium-oxygen mixtures (i.e., heliox), typically 70%:30% or 79%:21%, in children with severe acute asthma. It has been demonstrated that heliox promotes a greater delivery and percentage of particle retention from nebulized albuterol.146 Furthermore, a superior clinical improvement was associated with heliox use than with oxygen-driven continuous albuterol in children with moderate to severe asthma.147 Nevertheless, other studies148,149 failed to show a decrease in length of hospital stay or a significant clinical improvement. Heliox reduces peak airway pressures when used in patients who require mechanical ventilation, presumably by allowing hyperinflated alveoli to decompress during expiration and reducing the functional residual capacity of patients with asthma.150
The existing evidence does not provide support for the routine use of helium-oxygen mixtures to all ED patients with acute asthma. However, new evidence suggests certain beneficial effects in patients with more severe airway obstruction.151 Given the relatively small patient numbers and few studies, there is still a role for heliox trials in refractory severe acute asthma.152,153
Ketamine
Ketamine, a non-competitive N-methyl-D-aspartate receptor antagonist, is used routinely as an anesthetic, analgesic, and sedative owing to its wide therapeutic index and favorable hemodynamic effects.154,155 The bronchodilatory effects of ketamine were noted with its early use,156 although a randomized, double-blind, placebo-controlled trial of intravenous ketamine in acute asthma showed no beneficial effects in adult patients.157 Despite the current lack of high-level evidence, some studies have reported beneficial clinical effects from ketamine use in children presenting to the ED with acute asthma.158,159 In critically ill children with asthma, a loading dose of ketamine (2 mg/kg) followed by continuous infusions (20–60 mcg/kg/min) significantly improved the PaO2/FiO2 ratio in all patients, the dynamic compliance and PaCO2, and peak inspiratory pressures in mechanically ventilated patients.160 The mean duration of ketamine infusion in this study was 40 ± 31 hours and no significant side effects were noted. Ketamine infusions have been used in patients with near-fatal asthma, in combination with other bronchodilator therapies.161
Bilevel Positive Airway Pressure
Noninvasive positive pressure ventilation (NPPV) in addition to conventional therapy showed clinical improvement and correction of gas exchange abnormalities in children and adults with asthma.162–164 NPPV was well tolerated in children, including patients as young as 1 year.164,165 Utilization of NPPV in younger patients can be challenging. However, under strict monitoring, the use of a small dose of benzodiazepines or propofol with or without a low-dose ketamine infusion or dexmedetomidine may facilitate the tolerance for NPPV. Typically recommended settings include an inspiratory positive airway pressure of 10 cm H2O, an expiratory positive airway pressure of 5 cm H2O, with or without a low back-up ventilation rate. In patients with severe asthma, a low level of continuous positive airway pressure may reduce the premature airway closure point, reducing intrinsic end expiratory pressure and subsequently the inspiratory workload.166,167 In addition, the use of NPPV may well improve the delivery of aerosolized albuterol to poorly ventilated areas.168
In conclusion, acute asthma exacerbation treatment in the pediatric population continues to require our proficiency to promptly intervene with a systematic and aggressive methodology. A stepwise approach for the management of acute asthma exacerbation is shown in the Figure. The use of this progressive treatment guideline is suggested for patients admitted to the PICU who are not responding to steroids and β2-agonists and need further therapy, based on their clinical status and asthma score.
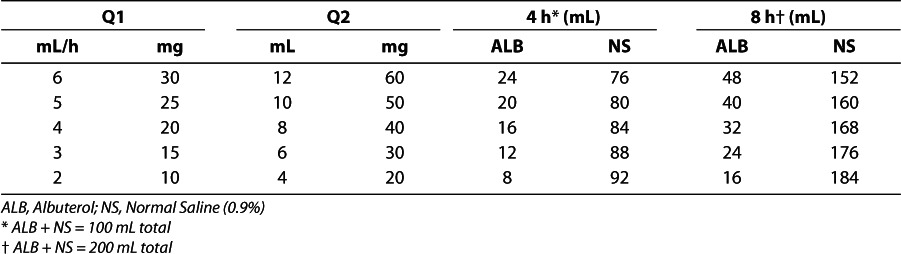
At Le Bonheur Children's Hospital ICU, we use different doses of continuous albuterol per hour, based on the patient's age and weight, keeping the dose per kilogram roughly the same in each group. The guidelines and clinical pathway for treatment with and weaning from continuous albuterol are described in the Figure and Appendix. Table 2 provides practical guidelines for the dosage and mixing concentrations of the albuterol solutions used at Le Bonheur Children's Hospital.
Table 2.
Albuterol Dosage (0.5% Albuterol, 5 mg/mL) and Mixing Proportions for Continuous Albuterol
ABBREVIATIONS
- cAMP
cyclic adenosine monophosphate
- ED
emergency department
- FiO2
fraction of inspired oxygen
- Heliox
helium-oxygen mixtures
- ICU
intensive care unit
- NAEPP
National Asthma Education and Prevention Program
- NPPV
noninvasive positive pressure ventilation
- PaCO2
partial pressure of carbon dioxide in the arterial blood
- PaO2
partial pressure of oxygen in arterial blood
- PAS
Pediatric Asthma Score
- PICU
pediatric intensive care unit
- PKA
protein kinase A
- SpO2
oxygen saturation
APPENDIX
The Pediatric Asthma Score1 (PAS) has been adopted by Le Bonheur Children's Hospital for the assessment of patients with asthma exacerbation (Table 1). A respiratory therapist documents the PAS every 2 hours while the patient is receiving continuous albuterol treatment. Weaning can be done every 2 hours by lowering the dose by 5 mg/h for PAS scores ≤8. Advancement can be made to the next level only when there is worsening of the PAS. Patients should remain on continuous albuterol therapy until terbutaline is discontinued. Atrovent 0.25 to 0.5 mg will be administered every 6 hours.115 Peak flow measurements will be instituted along with the PAS assessment for children older than 5 years.
Guidelines for Patients <20 kg:
Begin continuous albuterol treatment at 15 mg/h or per physician order. Duration = 8 hours.
Mix subsequent continuous solutions for a duration of 4 hours.
Continue at 15 mg/h or at present strength of solution for PAS assessment scores >8.
Begin and continue to wean albuterol by 5 mg/h for PAS assessment scores ≤8.
Guidelines for Patients 20–30 kg:
Begin continuous albuterol treatment at 25 mg/h or per physician order. Duration = 8 hours.
Mix subsequent continuous solutions for a duration of 4 hours.
Continue at 25 mg/h or at present strength of solution for PAS assessment scores >8.
Begin and continue to wean albuterol by 5 mg/h for PAS assessment scores ≤8.
Guidelines for Patients >30 kg:
Begin continuous albuterol treatment at 30 mg/h or per physician order. Duration = 8 hours.
Mix subsequent continuous solutions for a duration of 4 hours.
Begin and continue to wean albuterol by 5 mg/h for PAS assessment scores ≤8.
Continue at 30 mg/h or present milligram per hour delivery for PAS assessment scores >8.
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Kelly CS, Andersen CL, Pestian JP. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509–516. doi: 10.1016/S1081-1206(10)62514-8. et al. [DOI] [PubMed] [Google Scholar]
- 2.Kaza V, Bandi V, Guntupalli KK. Acute severe asthma: recent advances. Curr Opinion Pulmon Med. 2007;13(1):1–7. doi: 10.1097/MCP.0b013e328011a91c. [DOI] [PubMed] [Google Scholar]
- 3.Birken CS, Parkin PC, Macarthur C. Asthma severity scores for preschoolers displayed weaknesses in reliability, validity, and responsiveness. J Clin Epidemiol. 2004;57(11):1177–1181. doi: 10.1016/j.jclinepi.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Homa DM, Akinbami LJ. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. et al. [PubMed] [Google Scholar]
- 5.Molfino NA, Nannini LJ, Martelli AN. Respiratory arrest in near-fatal asthma. N Engl J Med. 1991;324(5):285–288. doi: 10.1056/NEJM199101313240502. et al. [DOI] [PubMed] [Google Scholar]
- 6.Molfino NA, Slutsky AS. Near-fatal asthma. Eur Respir J. 1994;7(5):981–990. [PubMed] [Google Scholar]
- 7.Wasserfallen JB, Schaller MD, Feihl F. Sudden asphyxic asthma: a distinct entity? Am Rev Respir Dis. 1990;142(1):108–111. doi: 10.1164/ajrccm/142.1.108. et al. [DOI] [PubMed] [Google Scholar]
- 8.Robin ED, Lewiston N. Unexpected, unexplained sudden death in young asthmatic subjects. Chest. 1989;96(4):790–793. doi: 10.1378/chest.96.4.790. [DOI] [PubMed] [Google Scholar]
- 9.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Rep. 2011;(32):1–14. [PubMed] [Google Scholar]
- 10.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;(381):1–24. [PubMed] [Google Scholar]
- 11.Akinbami LJ, Schoendorf KC, Parker J. US childhood asthma prevalence estimates: the impact of the 1997 National Health Interview Survey redesign. Am J Epidemiol. 2003;158(2):99–104. doi: 10.1093/aje/kwg109. [DOI] [PubMed] [Google Scholar]
- 12.Akinbami LJ, Moorman JE, Garbe PL. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. et al. [DOI] [PubMed] [Google Scholar]
- 13.Moorman JE, Rudd RA, Johnson CA. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56(8):1–54. et al. [PubMed] [Google Scholar]
- 14.Schatz M, Camargo CA., Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91(6):553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- 15.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115(5):1254–1260. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- 16.Boudreaux ED, Emond SD, Clark S. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics. 2003;111(5 Pt1):e615–e621. doi: 10.1542/peds.111.5.e615. et al. [DOI] [PubMed] [Google Scholar]
- 17.Lara M, Akinbami L, Flores G. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117(1):43–53. doi: 10.1542/peds.2004-1714. et al. [DOI] [PubMed] [Google Scholar]
- 18.Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002;2(5):382–387. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 20.Hays SR, Fahy JV. The role of mucus in fatal asthma. Am J Med. 2003;115(1):68–69. doi: 10.1016/s0002-9343(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 21.Kuyper LM, Pare PD, Hogg JC. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115(1):6–11. doi: 10.1016/s0002-9343(03)00241-9. et al. [DOI] [PubMed] [Google Scholar]
- 22.Roca J, Ramis L, Rodriguez-Roisin R. Serial relationships between ventilation-perfusion inequality and spirometry in acute severe asthma requiring hospitalization. Am Rev Respir Dis. 1988;137(5):1055–1061. doi: 10.1164/ajrccm/137.5.1055. et al. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Roisin R, Ballester E, Roca J. Mechanisms of hypoxemia in patients with status asthmaticus requiring mechanical ventilation. Am Rev Respir Dis. 1989;139(3):732–739. doi: 10.1164/ajrccm/139.3.732. et al. [DOI] [PubMed] [Google Scholar]
- 24.Dawson CA, Grimm DJ, Linehan JH. Lung inflation and longitudinal distribution of pulmonary vascular resistance during hypoxia. J Appl Physiol. 1979;47(3):532–536. doi: 10.1152/jappl.1979.47.3.532. [DOI] [PubMed] [Google Scholar]
- 25.Buda AJ, Pinsky MR, Ingels NB., Jr Effect of intrathoracic pressure on left ventricular performance. New Engl J Med. 1979;301(9):453–459. doi: 10.1056/NEJM197908303010901. et al. [DOI] [PubMed] [Google Scholar]
- 26.Rebuck AS, Pengelly LD. Development of pulsus paradoxus in the presence of airways obstruction. New Engl J Med. 1973;288(2):66–69. doi: 10.1056/NEJM197301112880203. [DOI] [PubMed] [Google Scholar]
- 27.Jardin F, Farcot JC, Boisante L. Mechanism of paradoxic pulse in bronchial asthma. Circulation. 1982;66(4):887–894. doi: 10.1161/01.cir.66.4.887. et al. [DOI] [PubMed] [Google Scholar]
- 28.Stalcup SA, Mellins RB. Mechanical forces producing pulmonary edema in acute asthma. New Engl J Med. 1977;297(11):592–596. doi: 10.1056/NEJM197709152971107. [DOI] [PubMed] [Google Scholar]
- 29.Papiris S, Kotanidou A, Malagari K. Clinical review: severe asthma. Crit Care. 2002;6(1):30–44. doi: 10.1186/cc1451. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.British Thoracic Association. Death from asthma in two regions of England. Br Med J (Clin Res Ed) 1982;285(6350):1251–1255. [PMC free article] [PubMed] [Google Scholar]
- 31.Benatar SR. Fatal asthma. N Engl J Med. 1986;314(7):423–429. doi: 10.1056/NEJM198602133140706. [DOI] [PubMed] [Google Scholar]
- 32.McFadden ER., Jr. Fatal and near-fatal asthma. N Engl J Med. 1991;324(6):409–411. doi: 10.1056/NEJM199102073240609. [DOI] [PubMed] [Google Scholar]
- 33.McFadden ER, Jr, Warren EL. Observations on asthma mortality. Ann Intern Med. 1997;127(2):142–147. doi: 10.7326/0003-4819-127-2-199707150-00009. [DOI] [PubMed] [Google Scholar]
- 34.Strunk RC. Identification of the fatality-prone subject with asthma. J Allergy Clin Immunol. 1989;83(2 Pt 1):477–485. doi: 10.1016/0091-6749(89)90137-1. [DOI] [PubMed] [Google Scholar]
- 35.Strunk RC. Death due to asthma: new insights into sudden unexpected deaths, but the focus remains on prevention. Am Rev Respir Dis. 1993;148(3):550–552. doi: 10.1164/ajrccm/148.3.550. [DOI] [PubMed] [Google Scholar]
- 36.DeNicola LK, Monem GF, Gayle MO. Treatment of critical status asthmaticus in children. Pediatr Clin North Am. 1994;41(6):1293–1324. doi: 10.1016/s0031-3955(16)38874-5. et al. [DOI] [PubMed] [Google Scholar]
- 37.Sur S, Crotty TB, Kephart GM. Sudden-onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148(3):713–719. doi: 10.1164/ajrccm/148.3.713. et al. [DOI] [PubMed] [Google Scholar]
- 38.Maffei FA, van der Jagt EW, Powers KS. Duration of mechanical ventilation in life-threatening pediatric asthma: description of an acute asphyxial subgroup. Pediatrics. 2004;114(3):762–767. doi: 10.1542/peds.2004-0294. et al. [DOI] [PubMed] [Google Scholar]
- 39.Reid LM. The presence or absence of bronchial mucus in fatal asthma. J Allergy Clin Immunol. 1987;80:415–416. doi: 10.1016/0091-6749(87)90064-9. [DOI] [PubMed] [Google Scholar]
- 40.Robertson CF, Rubinfeld AR, Bowes G. Pediatric asthma deaths in Victoria: the mild are at risk. Pediatr Pulmonol. 1992;13(2):95–100. doi: 10.1002/ppul.1950130207. [DOI] [PubMed] [Google Scholar]
- 41.Stein R, Canny GJ, Bohn DJ. Severe acute asthma in a pediatric intensive care unit: six years' experience. Pediatrics. 1989;83(6):1023–1028. et al. [PubMed] [Google Scholar]
- 42.Kravis LP, Kolski GB. Unexpected death in childhood asthma: a review of 13 deaths in ambulatory patients. Am J Dis Child. 1985;139(6):558–563. doi: 10.1001/archpedi.1985.02140080028026. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher HJ, Ibrahim SA, Speight N. Survey of asthma deaths in the Northern region, 1970–85. Arch Dis Child. 1990;65(2):163–167. doi: 10.1136/adc.65.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui T, Baba M. Death from asthma in children. Acta Paediatr Jpn. 1990;32(2):205–208. doi: 10.1111/j.1442-200x.1990.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 45.McFadden ER, Jr, Kiser R, DeGroot WJ. Acute bronchial asthma: relations between clinical and physiologic manifestations. New Engl J Med. 1973;288(5):221–225. doi: 10.1056/NEJM197302012880501. [DOI] [PubMed] [Google Scholar]
- 46.Becker AB, Nelson NA, Simons FE. The pulmonary index: assessment of a clinical score for asthma. Am J Dis Child. 1984;138(6):574–576. doi: 10.1001/archpedi.1984.02140440058015. [DOI] [PubMed] [Google Scholar]
- 47.DiGiulio GA, Kercsmar CM, Krug SE. Hospital treatment of asthma: lack of benefit from theophylline given in addition to nebulized albuterol and intravenously administered corticosteroid. J Pediatr. 1993;122(3):464–469. doi: 10.1016/s0022-3476(05)83442-0. et al. [DOI] [PubMed] [Google Scholar]
- 48.Wood DW, Downes JJ, Lecks HI. A clinical scoring system for the diagnosis of respiratory failure: preliminary report on childhood status asthmaticus. Am J Dis Child. 1972;123(3):227–228. doi: 10.1001/archpedi.1972.02110090097011. [DOI] [PubMed] [Google Scholar]
- 49.Keogh KA, Macarthur C, Parkin PC. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273–277. doi: 10.1067/mpd.2001.116282. et al. [DOI] [PubMed] [Google Scholar]
- 50.Baker MD. Pitfalls in the use of clinical asthma scoring. Am J Dis Child. 1988;142(2):183–185. doi: 10.1001/archpedi.1988.02150020085035. [DOI] [PubMed] [Google Scholar]
- 51.van der Windt DA, Nagelkerke AF, Bouter LM. Clinical scores for acute asthma in pre-school children: a review of the literature. J Clin Epidemiol. 1994;47(6):635–646. doi: 10.1016/0895-4356(94)90211-9. et al. [DOI] [PubMed] [Google Scholar]
- 52.Restrepo RD, Peters J. Near-fatal asthma: recognition and management. Curr Opin Pulm Med. 2008;14(1):13–23. doi: 10.1097/MCP.0b013e3282f1982d. [DOI] [PubMed] [Google Scholar]
- 53.Romagnoli M, Caramori G, Braccioni F. Near-fatal asthma phenotype in the ENFUMOSA Cohort. Clin Exp Allergy. 2007;37(4):552–557. doi: 10.1111/j.1365-2222.2007.02683.x. et al. [DOI] [PubMed] [Google Scholar]
- 54.Turner MO, Noertjojo K, Vedal S. Risk factors for near-fatal asthma: a case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1804–1809. doi: 10.1164/ajrccm.157.6.9708092. et al. [DOI] [PubMed] [Google Scholar]
- 55.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 56.Brooks LJ, Cloutier MM, Afshani E. Significance of roentgenographic abnormalities in children hospitalized for asthma. Chest. 1982;82(3):315–318. doi: 10.1378/chest.82.3.315. [DOI] [PubMed] [Google Scholar]
- 57.McFadden ER, Jr, Lyons HA. Arterial-blood gas tension in asthma. New Engl J Med. 1968;278(19):1027–1032. doi: 10.1056/NEJM196805092781901. [DOI] [PubMed] [Google Scholar]
- 58.Weiss EB, Faling LJ. Clinical significance of PaCO2 during status asthma: the cross-over point. Ann Allergy. 1968;26(10):545–551. [PubMed] [Google Scholar]
- 59.Mountain RD, Heffner JE, Brackett NC., Jr Acid-base disturbances in acute asthma. Chest. 1990;98(3):651–655. doi: 10.1378/chest.98.3.651. et al. [DOI] [PubMed] [Google Scholar]
- 60.Appel D, Rubenstein R, Schrager K. Lactic acidosis in severe asthma. Am J Med. 1983;75(4):580–584. doi: 10.1016/0002-9343(83)90436-9. et al. [DOI] [PubMed] [Google Scholar]
- 61.Qureshi F. Management of children with acute asthma in the emergency department. Pediatr Emerg Care. 1999;15(3):206–214. doi: 10.1097/00006565-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Connett G, Lenney W. Prolonged hypoxaemia after nebulised salbutamol. Thorax. 1993;48(5):574–575. doi: 10.1136/thx.48.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gleeson JG, Green S, Price JF. Air or oxygen as driving gas for nebulised salbutamol. Arch Dis Child. 1988;63(8):900–904. doi: 10.1136/adc.63.8.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiff M. Control of breathing in asthma. Clin Chest Med. 1980;1(1):85–89. [PubMed] [Google Scholar]
- 65.Warner JO, Naspitz CK. Third International Pediatric Consensus statement on the management of childhood asthma: International Pediatric Asthma Consensus Group. Pediatr Pulmonol. 1998;25(1):1–17. doi: 10.1002/(sici)1099-0496(199801)25:1<1::aid-ppul1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 66.Barnes PJ. Effect of corticosteroids on airway hyperresponsiveness. Am Rev Respir Dis. 1990;141(2 Pt 2):S70–76. [PubMed] [Google Scholar]
- 67.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14(12):436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 68.Dunlap NE, Fulmer JD. Corticosteroid therapy in asthma. Clin Chest Med. 1984;5(4):669–683. [PubMed] [Google Scholar]
- 69.Dworski R, Fitzgerald GA, Oates JA. Effect of oral prednisone on airway inflammatory mediators in atopic asthma. Am J Respir Crit Care Med. 1994;149(4 Pt 1):953–959. doi: 10.1164/ajrccm.149.4.8143061. et al. [DOI] [PubMed] [Google Scholar]
- 70.Rowe BH, Spooner C, Ducharme FM. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. doi: 10.1002/14651858.CD002178. et al. [DOI] [PubMed] [Google Scholar]
- 71.Younger RE, Gerber PS, Herrod HG. Intravenous methylprednisolone efficacy in status asthmaticus of childhood. Pediatrics. 1987;80(2):225–230. et al. [PubMed] [Google Scholar]
- 72.Ressel GW. NAEPP updates guidelines for the diagnosis and management of asthma. Am Fam Physician. 2003;68(1):169–170. [PubMed] [Google Scholar]
- 73.Barnett PL, Caputo GL, Baskin M. Intravenous versus oral corticosteroids in the management of acute asthma in children. Ann Emerg Med. 1997;29(2):212–217. doi: 10.1016/s0196-0644(97)70270-1. et al. [DOI] [PubMed] [Google Scholar]
- 74.Smith M, Iqbal S, Elliott TM. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003;(2):CD002886. doi: 10.1002/14651858.CD002886. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urbano FL. Review of the NAEPP 2007 Expert Panel Report (EPR-3) on Asthma Diagnosis and Treatment Guidelines. J Manag Care Pharm. 2008;14(1):41–49. doi: 10.18553/jmcp.2008.14.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chipps BE, Murphy KR. Assessment and treatment of acute asthma in children. J Pediatr. 2005;147(3):288–294. doi: 10.1016/j.jpeds.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 77.Klein-Gitelman MS, Pachman LM. Intravenous corticosteroids: adverse reactions are more variable than expected in children. J Rheumatol. 1998;25(10):1995–2002. [PubMed] [Google Scholar]
- 78.Kamm GL, Hagmeyer KO. Allergic-type reactions to corticosteroids. Ann Pharmacother. 1999;33(4):451–460. doi: 10.1345/aph.18276. [DOI] [PubMed] [Google Scholar]
- 79.Leatherman JW, Fluegel WL, David WS. Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med. 1996;153(5):1686–1690. doi: 10.1164/ajrccm.153.5.8630621. et al. [DOI] [PubMed] [Google Scholar]
- 80.Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med. 1998;158(5 Pt 3):S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- 81.Johnson M. Pharmacology of long-acting beta-agonists. Ann Allergy Asthma Immunol. 1995;75(2):177–179. [PubMed] [Google Scholar]
- 82.Cook SJ, Small RC, Berry JL. Beta-adrenoceptor subtypes and the opening of plasmalemmal K(+)-channels in trachealis muscle: electrophysiological and mechanical studies in guinea-pig tissue. Br J Pharmacol. 1993;109(4):1140–1148. doi: 10.1111/j.1476-5381.1993.tb13741.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stephanopoulos DE, Monge R, Schell KH. Continuous intravenous terbutaline for pediatric status asthmaticus. Crit Care Med. 1998;26(10):1744–1748. doi: 10.1097/00003246-199810000-00033. et al. [DOI] [PubMed] [Google Scholar]
- 84.Haalboom JR, Deenstra M, Struyvenberg A. Hypokalaemia induced by inhalation of fenoterol. Lancet. 1985;1(8438):1125–1127. doi: 10.1016/s0140-6736(85)92432-8. [DOI] [PubMed] [Google Scholar]
- 85.Tveskov C, Djurhuus MS, Klitgaard NA. Potassium and magnesium distribution, ECG changes, and ventricular ectopic beats during beta 2-adrenergic stimulation with terbutaline in healthy subjects. Chest. 1994;106(6):1654–1659. doi: 10.1378/chest.106.6.1654. et al. [DOI] [PubMed] [Google Scholar]
- 86.Qureshi F, Zaritsky A, Welch C. Clinical efficacy of racemic albuterol versus levalbuterol for the treatment of acute pediatric asthma. Ann Emerg Med. 2005;46(1):29–36. doi: 10.1016/j.annemergmed.2005.02.001. et al. [DOI] [PubMed] [Google Scholar]
- 87.Moler FW, Hurwitz ME, Custer JR. Improvement in clinical asthma score and PaCO2 in children with severe asthma treated with continuously nebulized terbutaline. J Allergy Clin Immunol. 1988;81(6):1101–1109. doi: 10.1016/0091-6749(88)90876-7. [DOI] [PubMed] [Google Scholar]
- 88.Rodrigo GJ, Rodrigo C. Continuous vs intermittent beta-agonists in the treatment of acute adult asthma: a systematic review with meta-analysis. Chest. 2002;122(1):160–165. doi: 10.1378/chest.122.1.160. [DOI] [PubMed] [Google Scholar]
- 89.Schuh S, Parkin P, Rajan A. High-versus low-dose, frequently administered, nebulized albuterol in children with severe, acute asthma. Pediatrics. 1989;83(4):513–518. et al. [PubMed] [Google Scholar]
- 90.Craig VL, Bigos D, Brilli RJ. Efficacy and safety of continuous albuterol nebulization in children with severe status asthmaticus. Pediatr Emerg Care. 1996;12(1):1–5. [PubMed] [Google Scholar]
- 91.Montgomery VL, Eid NS. Low-dose beta-agonist continuous nebulization therapy for status asthmaticus in children. J Asthma. 1994;31(3):201–207. doi: 10.3109/02770909409044827. [DOI] [PubMed] [Google Scholar]
- 92.Papo MC, Frank J, Thompson AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Crit Care Med. 1993;21(10):1479–1486. doi: 10.1097/00003246-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 93.Portnoy J, Nadel G, Amado M. Continuous nebulization for status asthmaticus. Ann Allergy. 1992;69(1):71–79. et al. [PubMed] [Google Scholar]
- 94.Katz RW, Kelly HW, Crowley MR. Safety of continuous nebulized albuterol for bronchospasm in infants and children. Pediatrics. 1993;92(5):666–669. et al. [PubMed] [Google Scholar]
- 95.Ackerman AD. Continuous nebulization of inhaled beta-agonists for status asthmaticus in children: a cost-effective therapeutic advance? Crit Care Med. 1993;21(10):1422–1424. doi: 10.1097/00003246-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Raabe OG, Wong TM, Wong GB. Continuous nebulization therapy for asthma with aerosols of beta2 agonists. Ann Allergy Asthma Immunol. 1998;80(6):499–508. doi: 10.1016/S1081-1206(10)63074-8. et al. [DOI] [PubMed] [Google Scholar]
- 97.Werner HA. Status asthmaticus in children: a review. Chest. 2001;119(6):1913–1929. doi: 10.1378/chest.119.6.1913. [DOI] [PubMed] [Google Scholar]
- 98.Chiang VW, Burns JP, Rifai N. Cardiac toxicity of intravenous terbutaline for the treatment of severe asthma in children: a prospective assessment. J Pediatr. 2000;137(1):73–77. doi: 10.1067/mpd.2000.106567. et al. [DOI] [PubMed] [Google Scholar]
- 99.Simons FE, Gillies JD. Dose response of subcutaneous terbutaline and epinephrine in children with acute asthma. Am J Dis Child. 1981;135(3):214–217. doi: 10.1001/archpedi.1981.02130270006004. [DOI] [PubMed] [Google Scholar]
- 100.Fuglsang G, Pedersen S, Borgstrom L. Dose-response relationships of intravenously administered terbutaline in children with asthma. J Pediatr. 1989;114(2):315–320. doi: 10.1016/s0022-3476(89)80805-4. [DOI] [PubMed] [Google Scholar]
- 101.Gross NJ. Ipratropium bromide. New Engl J Med. 1988;319(8):486–494. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- 102.Plotnick LH, Ducharme FM. Should inhaled anticholinergics be added to beta2 agonists for treating acute childhood and adolescent asthma: a systematic review. Br Med J. 1998;317(7164):971–977. doi: 10.1136/bmj.317.7164.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plotnick LH, Ducharme FM. Combined inhaled anticholinergics and beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2000;(4):CD000060. doi: 10.1002/14651858.CD000060. [DOI] [PubMed] [Google Scholar]
- 104.Plotnick LH, Ducharme FM. Acute asthma in children and adolescents: should inhaled anticholinergics be added to beta(2)-agonists? Am J Respir Med. 2003;2(2):109–115. doi: 10.1007/BF03256642. [DOI] [PubMed] [Google Scholar]
- 105.Zaritsky A, Qureshi F. Ipratropium does indeed reduce admissions to hospital with severe asthma. Br Med J. 1999;318(7185):738. doi: 10.1136/bmj.318.7185.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qureshi F, Pestian J, Davis P. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New Engl J Med. 1998;339(15):1030–1035. doi: 10.1056/NEJM199810083391503. et al. [DOI] [PubMed] [Google Scholar]
- 107.Qureshi F, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severely asthmatic children. Ann Emerg Med. 1997;29(2):205–211. doi: 10.1016/s0196-0644(97)70269-5. [DOI] [PubMed] [Google Scholar]
- 108.Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta-analysis. Thorax. 2005;60(9):740–746. doi: 10.1136/thx.2005.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodrigo GJ, Rodrigo C. First-line therapy for adult patients with acute asthma receiving a multiple-dose protocol of ipratropium bromide plus albuterol in the emergency department. Am J Respir Crit Care Med. 2000;161(6):1862–1868. doi: 10.1164/ajrccm.161.6.9908115. [DOI] [PubMed] [Google Scholar]
- 110.Rodrigo GJ, Rodrigo C. The role of anti-cholinergics in acute asthma treatment: an evidence-based evaluation. Chest. 2002;121(6):1977–1987. doi: 10.1378/chest.121.6.1977. [DOI] [PubMed] [Google Scholar]
- 111.Kizer KM, Bess DT, Bedford NK. Blurred vision from ipratropium bromide inhalation. Am J Health Sys Pharm. 1999;56(9):914. doi: 10.1093/ajhp/56.9.914a. [DOI] [PubMed] [Google Scholar]
- 112.Woelfle J, Zielen S, Lentze MJ. Unilateral fixed dilated pupil in an infant after inhalation of nebulized ipratropium bromide. J Pediatr. 2000;136(3):423–424. [PubMed] [Google Scholar]
- 113.Craven D, Kercsmar CM, Myers TR. Ipratropium bromide plus nebulized albuterol for the treatment of hospitalized children with acute asthma. J Pediatr. 2001;138(1):51–58. doi: 10.1067/mpd.2001.110120. et al. [DOI] [PubMed] [Google Scholar]
- 114.Goggin N, Macarthur C, Parkin PC. Randomized trial of the addition of ipratropium bromide to albuterol and corticosteroid therapy in children hospitalized because of an acute asthma exacerbation. Arch Pediatr Adoles Med. 2001;155(12):1329–1334. doi: 10.1001/archpedi.155.12.1329. [DOI] [PubMed] [Google Scholar]
- 115.Iramain R, Lopez-Herce J, Coronel J. Inhaled salbutamol plus ipratropium in moderate and severe asthma crises in children. J Asthma. 2011;48(3):298–303. doi: 10.3109/02770903.2011.555037. et al. [DOI] [PubMed] [Google Scholar]
- 116.Lindeman KS, Hirshman CA, Freed AN. Effect of magnesium sulfate on bronchoconstriction in the lung periphery. J Appl Physiol. 1989;66(6):2527–2532. doi: 10.1152/jappl.1989.66.6.2527. [DOI] [PubMed] [Google Scholar]
- 117.Altura BM, Altura BT, Carella A. Magnesium deficiency-induced spasms of umbilical vessels: relation to preeclampsia, hypertension, growth retardation. Science. 1983;221(4608):376–378. doi: 10.1126/science.6867714. [DOI] [PubMed] [Google Scholar]
- 118.Bois P. Effect of magnesium deficiency on mast cells and urinary histamine in rats. Br J Exper Pathol. 1963;44:151–155. [PMC free article] [PubMed] [Google Scholar]
- 119.Del Castillo J, Engbaek L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wacker WE, Vallee BL. Magnesium metabolism. New Engl J Med. 1958;259(10):475–482. doi: 10.1056/NEJM195809042591005. [DOI] [PubMed] [Google Scholar]
- 121.Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74–77. doi: 10.1136/adc.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dib JG, Engstrom FM, Sisca TS. Intravenous magnesium sulfate treatment in a child with status asthmaticus. Am J Health Syst Pharm. 1999;56(10):997–1000. doi: 10.1093/ajhp/56.10.997. et al. [DOI] [PubMed] [Google Scholar]
- 123.Rowe BH, Bretzlaff JA, Bourdon C. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;(2):CD001490. doi: 10.1002/14651858.CD001490. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Silverman RA, Osborn H, Runge J. IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. 2002;122(2):489–497. doi: 10.1378/chest.122.2.489. et al. [DOI] [PubMed] [Google Scholar]
- 125.Ciarallo L, Brousseau D, Reinert S. Higher-dose intravenous magnesium therapy for children with moderate to severe acute asthma. Arch Pediatr Adoles Med. 2000;154(10):979–983. doi: 10.1001/archpedi.154.10.979. [DOI] [PubMed] [Google Scholar]
- 126.Ciarallo L, Sauer AH, Shannon MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr. 1996;129(6):809–814. doi: 10.1016/s0022-3476(96)70023-9. [DOI] [PubMed] [Google Scholar]
- 127.Gurkan F, Haspolat K, Bosnak M. Intravenous magnesium sulphate in the management of moderate to severe acute asthmatic children nonresponding to conventional therapy. Eur J Emerg Med. 1999;6(3):201–205. doi: 10.1097/00063110-199909000-00005. et al. [DOI] [PubMed] [Google Scholar]
- 128.Sydow M, Crozier TA, Zielmann S. High-dose intravenous magnesium sulfate in the management of life-threatening status asthmaticus. Intensive Care Med. 1993;19(8):467–471. doi: 10.1007/BF01711089. et al. [DOI] [PubMed] [Google Scholar]
- 129.Glover ML, Machado C, Totapally BR. Magnesium sulfate administered via continuous intravenous infusion in pediatric patients with refractory wheezing. J Crit Care. 2002;17(4):255–258. doi: 10.1053/jcrc.2002.36759. [DOI] [PubMed] [Google Scholar]
- 130.Okayama H, Aikawa T, Okayama M. Bronchodilating effect of intravenous magnesium sulfate in bronchial asthma. JAMA. 1987;257(8):1076–1078. et al. [PubMed] [Google Scholar]
- 131.Aubier M, Barnes PJ. Theophylline and phosphodiesterase inhibitors. Eur Respir J. 1995;8(3):347–348. doi: 10.1183/09031936.95.08030347. [DOI] [PubMed] [Google Scholar]
- 132.Nicholson CD, Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes—their potential utility in the therapy of asthma. Pulm Pharmacol. 1994;7(1):1–17. doi: 10.1006/pulp.1994.1001. [DOI] [PubMed] [Google Scholar]
- 133.Aubier M, De Troyer A, Sampson M. Aminophylline improves diaphragmatic contractility. New Engl J Med. 1981;305(5):249–252. doi: 10.1056/NEJM198107303050503. et al. [DOI] [PubMed] [Google Scholar]
- 134.Higbee MD, Kumar M, Galant SP. Stimulation of endogenous catecholamine release by theophylline: a proposed additional mechanism of action for theophylline effects. J Allergy Clin Immunol. 1982;70(5):377–382. doi: 10.1016/0091-6749(82)90028-8. [DOI] [PubMed] [Google Scholar]
- 135.Horrobin DF, Manku MS, Franks DJ. Methyl xanthine phosphodiesterase inhibitors behave as prostaglandin antagonists in a perfused rat mesenteric artery preparation. Prostaglandins. 1977;13(1):33–40. doi: 10.1016/0090-6980(77)90040-5. et al. [DOI] [PubMed] [Google Scholar]
- 136.Barlinski J, Lockhart A, Frossard N. Modulation by theophylline and enprofylline of the excitatory non-cholinergic transmission in guinea-pig bronchi. Eur Respir J. 1992;5(10):1201–1205. [PubMed] [Google Scholar]
- 137.Kidney J, Dominguez M, Taylor PM. Immunomodulation by theophylline in asthma: demonstration by withdrawal of therapy. Am J Rrespir Criti Care Med. 1995;151(6):1907–1914. doi: 10.1164/ajrccm.151.6.7767539. et al. [DOI] [PubMed] [Google Scholar]
- 138.Spina D. Theophylline and PDE4 inhibitors in asthma. Curr Opin Pulm Med. 2003;9(1):57–64. doi: 10.1097/00063198-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 139.Weinberger M, Hendeles L. Theophylline in asthma. New Engl J Med. 1996;334(21):1380–1388. doi: 10.1056/NEJM199605233342107. [DOI] [PubMed] [Google Scholar]
- 140.Sullivan P, Bekir S, Jaffar Z. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343(8904):1006–1008. doi: 10.1016/s0140-6736(94)90127-9. et al. [DOI] [PubMed] [Google Scholar]
- 141.Weinberger M, Hendeles L, Ahrens R. Clinical pharmacology of drugs used for asthma. Pediatr Clin North Am. 1981;28(1):47–75. doi: 10.1016/s0031-3955(16)33962-1. [DOI] [PubMed] [Google Scholar]
- 142.Nahata M. Drug interactions with azithromycin and the macrolides: an overview. J Antimicrobial Chemother. 1996;37(suppl C):133–142. doi: 10.1093/jac/37.suppl_c.133. [DOI] [PubMed] [Google Scholar]
- 143.Ream RS, Loftis LL, Albers GM. Efficacy of IV theophylline in children with severe status asthmaticus. Chest. 2001;119(5):1480–1488. doi: 10.1378/chest.119.5.1480. et al. [DOI] [PubMed] [Google Scholar]
- 144.Wheeler DS, Jacobs BR, Kenreigh CA. Theophylline versus terbutaline in treating critically ill children with status asthmaticus: a prospective, randomized, controlled trial. Pediatr Crit Care Med. 2005;6(2):142–147. doi: 10.1097/01.PCC.0000154943.24151.58. et al. [DOI] [PubMed] [Google Scholar]
- 145.Gupta VK, Cheifetz IM. Heliox administration in the pediatric intensive care unit: an evidence-based review. Pediatr Crit Care Med. 2005;6(2):204–211. doi: 10.1097/01.PCC.0000154946.62733.94. [DOI] [PubMed] [Google Scholar]
- 146.Hess DR, Acosta FL, Ritz RH. The effect of heliox on nebulizer function using a beta-agonist bronchodilator. Chest. 1999;115(1):184–189. doi: 10.1378/chest.115.1.184. et al. [DOI] [PubMed] [Google Scholar]
- 147.Kim IK, Phrampus E, Venkataraman S. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: a randomized, controlled trial. Pediatrics. 2005;116(5):1127–1133. doi: 10.1542/peds.2004-2136. et al. [DOI] [PubMed] [Google Scholar]
- 148.Bigham MT, Jacobs BR, Monaco MA. Helium/oxygen-driven albuterol nebulization in the management of children with status asthmaticus: a randomized, placebo-controlled trial. Pediatr Crit Care Med. 2010;11(3):356–361. et al. [PubMed] [Google Scholar]
- 149.Carter ER, Webb CR, Moffitt DR. Evaluation of heliox in children hospitalized with acute severe asthma: a randomized crossover trial. Chest. 1996;109(5):1256–1261. doi: 10.1378/chest.109.5.1256. [DOI] [PubMed] [Google Scholar]
- 150.Abd-Allah SA, Rogers MS, Terry M. Helium-oxygen therapy for pediatric acute severe asthma requiring mechanical ventilation. Pediatr Crit Care Med. 2003;4(3):353–357. doi: 10.1097/01.PCC.0000074267.11280.78. et al. [DOI] [PubMed] [Google Scholar]
- 151.Rodrigo G, Pollack C, Rodrigo C. Heliox for nonintubated acute asthma patients. Cochrane Database Syst Rev. 2006;(4):CD002884. doi: 10.1002/14651858.CD002884.pub2. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carroll CL. Heliox for children with acute asthma: has the sun set on this therapy? Pediatr Crit Care Med. 2010;11(3):428–429. doi: 10.1097/PCC.0b013e3181ce6d19. [DOI] [PubMed] [Google Scholar]
- 153.Tobias JD, Garrett JS. Therapeutic options for severe, refractory status asthmaticus: inhalational anaesthetic agents, extracorporeal membrane oxygenation and helium/oxygen ventilation. Paediatr Anaesth. 1997;7(1):47–57. doi: 10.1046/j.1460-9592.1997.d01-33.x. [DOI] [PubMed] [Google Scholar]
- 154.Cohen SP, Liao W, Gupta A. Ketamine in pain management. Adv Psychosom Med. 2011;30:139–161. doi: 10.1159/000324071. et al. [DOI] [PubMed] [Google Scholar]
- 155.Subramaniam K, Subramaniam B, Stein-brook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004;99(2):482–495. doi: 10.1213/01.ANE.0000118109.12855.07. [DOI] [PubMed] [Google Scholar]
- 156.Park GR, Manara AR, Mendel L. Ketamine infusion: its use as a sedative, inotrope and bronchodilator in a critically ill patient. Anaesthesia. 1987;42(9):980–983. doi: 10.1111/j.1365-2044.1987.tb05370.x. et al. [DOI] [PubMed] [Google Scholar]
- 157.Howton JC, Rose J, Duffy S. Randomized, double-blind, placebo-controlled trial of intravenous ketamine in acute asthma. Ann Emerg Med. 1996;27(2):170–175. doi: 10.1016/s0196-0644(96)70319-0. et al. [DOI] [PubMed] [Google Scholar]
- 158.Petrillo TM, Fortenberry JD, Linzer JF. Emergency department use of ketamine in pediatric status asthmaticus. J Asthma. 2001;38(8):657–664. doi: 10.1081/jas-100107543. et al. [DOI] [PubMed] [Google Scholar]
- 159.Priestley SJ, Taylor J, McAdam CM. Ketamine sedation for children in the emergency department. Emerg Med (Fremantle) 2001;13(1):82–90. doi: 10.1046/j.1442-2026.2001.00184.x. et al. [DOI] [PubMed] [Google Scholar]
- 160.Youssef-Ahmed MZ, Silver P, Nimkoff L. Continuous infusion of ketamine in mechanically ventilated children with refractory bronchospasm. Intensive Care Med. 1996;22(9):972–976. doi: 10.1007/BF02044126. et al. [DOI] [PubMed] [Google Scholar]
- 161.Mazzeo AT, Spada A, Pratico C. Hypercapnia: what is the limit in paediatric patients: a case of near-fatal asthma successfully treated by multipharmacological approach. Paediatr Anaesth. 2004;14(7):596–603. doi: 10.1111/j.1460-9592.2004.01260.x. et al. [DOI] [PubMed] [Google Scholar]
- 162.Meduri GU, Cook TR, Turner RE. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996;110(3):767–774. doi: 10.1378/chest.110.3.767. et al. [DOI] [PubMed] [Google Scholar]
- 163.Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest. 2003;123(4):1018–1025. doi: 10.1378/chest.123.4.1018. [DOI] [PubMed] [Google Scholar]
- 164.Thill PJ, McGuire JK, Baden HP. Noninvasive positive-pressure ventilation in children with lower airway obstruction. Pediatr Crit Care Med. 2004;5(4):337–342. doi: 10.1097/01.pcc.0000128670.36435.83. et al. [DOI] [PubMed] [Google Scholar]
- 165.Carroll CL, Schramm CM. Noninvasive positive pressure ventilation for the treatment of status asthmaticus in children. Ann Allergy Asthma Immunol. 2006;96(3):454–459. doi: 10.1016/S1081-1206(10)60913-1. [DOI] [PubMed] [Google Scholar]
- 166.Cassart M, Pettiaux N, Gevenois PA. Effect of chronic hyperinflation on diaphragm length and surface area. Am J Resp Crit Care Med. 1997;156(2 Pt 1):504–508. doi: 10.1164/ajrccm.156.2.9612089. et al. [DOI] [PubMed] [Google Scholar]
- 167.Smith TC, Marini JJ. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol. 1988;65(4):1488–1499. doi: 10.1152/jappl.1988.65.4.1488. [DOI] [PubMed] [Google Scholar]
- 168.Pollack CV, Jr, Fleisch KB, Dowsey K. Treatment of acute bronchospasm with beta-adrenergic agonist aerosols delivered by a nasal bilevel positive airway pressure circuit. Ann Emerg Med. 1995;26(5):552–557. doi: 10.1016/s0196-0644(95)70003-x. [DOI] [PubMed] [Google Scholar]


