Abstract
Cutaneous burn wounds represent a significant public health problem with 500,000 patients per year in the U.S. seeking medical attention. Immediately after skin burn injury, the volume of the wound burn expands due to a cascade of chemical reactions, including lipid peroxidation chain reactions. Based on these chemical reactions, the present paper develops for the first time a three-dimensional mathematical model to quantify the propagation of tissue damage within 12 hours post initial burn. We use the model to investigate the effect of supplemental antioxidant vitamin E for stopping the propagation. We show, for example, that if the production rate of vitamin E is increased, post burn, by five times the natural production in a healthy tissue, then this would slow down the lipid peroxide propagation by at least 50%. Our model is formulated in terms of differential equations, and sensitivity analysis is performed on the parameters to ensure the robustness of the results.
Keywords: burn injury, propagation, lipid peroxidation, antioxidant, mathematical modeling
Introduction
According to the American Burn Association, 500,000 patients per year in the U.S. seek medical attention for burn injuries. Burns are classified as superficial or first-degree burns (epidermal damage only), partial thickness or second-degree burns (dermal injury), full thickness or third-degree burns (destruction also of the subcutaneous layer which contains the hair follicles, sweat glands, and the region where new skin cells are formed), and fourth-degree burns which extends to the underlying tissue of ligaments and muscles. The current clinical care for severely burned patients is intravenous fluid resuscitation, controlling infection, and scar reducing surgical intervention (1). Tissue damage, which can be characterized by the level of lipid peroxide (2), typically increases during the 24 hours after the initial burn. It is currently challenging for burn care providers to estimate the spatial distribution of lipid peroxide in the burned tissue, in order to determine quantitatively the size and depth of the damage.
For a partial thickness burn injury, lipid peroxide is mainly produced through the chain reaction of lipid peroxidation (3–9). The chain reaction is initiated by the excessive reactive oxygen species (ROS), such as the hydroxyl radical (•OH), generated by heat or radiation. The hydroxyl radical combines with a hydrogen atom in a lipid molecule and makes a lipid radical (L•) and water. The lipid radical is very unstable and quickly reacts with oxygen to produce a lipid peroxyl radical (LOO•). The lipid peroxyl radical is also unstable and reacts with free lipid to make lipid peroxide (LOOH) and another free lipid radical. What follows is a chain reaction involving several radical species and the production of lipid peroxide. In this process the initial burn wound propagates and its propagation can be, for practical purposes, traced by the relatively stable lipid peroxide (2, 10, 11). Such propagation may terminate under a number of conditions resulting in neutralization of ROS (12–18). This may happen when antioxidant molecules intercept and terminate lipid peroxidation. One such effective lipid-phase antioxidant is vitamin E (19–22). Vitamin E can be depleted during a burn, and supplementary vitamin E has been indicated as a potential rescue for burn (23–26). The infiltrating leukocytes also produce free radicals that initiate lipid peroxidation, and this normally occurs about 12 hours after the initial injury (27).
Mathematical modeling can be useful to determine the amount of lipid peroxide in the tissue after a burn injury, thus helpful to quantify the extent of damage. Skin burn injury by surface heating or radiation were mathematically described (28–30). These models are formulated in terms of the heat equation in the tissue and do not include the chemical processes that result in the lipid peroxide propagation.
In this paper, we develop, for the first time, a mathematical mechanistic model to quantify the extent of the burn propagation after the initial damage, based on the passive biochemical events that are involved in the lipid peroxidation chain reaction initiated by the burn. Other enzymatic processes are involved in the source and fate of lipid peroxides, for example, through superoxide dismutase, catalase, glutathione peroxidase and metal ions (6). These details are only implicitly included in the rate parameters of the chemical processes. Since we are modeling the propagation of the burn only in the 12 hours from the initial burn, we do not include the infiltrating leukocytes (and their oxidative stress) which occur only at a later time. We show that simulation of the model in the case of comb burn qualitatively agrees with experimental results in (31). We also use the model to examine the relative effect of intervention using vitamin E with different doses on limiting propagation of cutaneous thermal injury. Our simulation results show that if the production rate of vitamin E is increased, post burn, by five times the natural production in a healthy tissue, then this would slow down the lipid peroxide propagation by at least 50%. We performed sensitivity analysis in order to determine to what extend our results depend on uncertainty in the parameters. We identified the parameters which have strong effect on burn propagation, and, at the same time, established robustness of the model predictions within a specified range of the parameters.
Materials and Methods
The lipid peroxidation chain reactions
We consider a partial thickness burn wound as shown in Fig. 1. The thickness of the epidermal layer typically varies from 0.5–1.5 mm and, the thickness of the dermal layer varies from 0.5–3 mm, depending on the location of the burn wound on the human body. When a burn injury occurs, the heat shock initiates a cascade of chemical reactions (3–9). Healthy lipids are converted to lipid peroxide in chemical processes that involve, respectively, hydroxyl radical, lipid alkoxyl radical, and lipid peroxyl radical. These three radicals are also produced by lipid peroxide, thereby enhancing lipid peroxide propagation. This network of chemical reactions is depicted schematically in Fig. 2; the major chemical reactions and reaction rates are given in Table 1. The chemical reactions can be divided into four processes: initiation, propagation, branching, and termination. The propagation and branching are the primary processes responsible for the increase of the size of the burn. It is known that vitamin E breaks up the chain reaction by scavenging the lipid peroxyl radical (6) and by blocking 12-lipoxygenase which oxidizes unsaturated lipid to lipid peroxide (32–34). In this paper, we first model the mechanism by which the wound enlarges, and then model the termination of this process by intervention with vitamin E.
Figure 1.
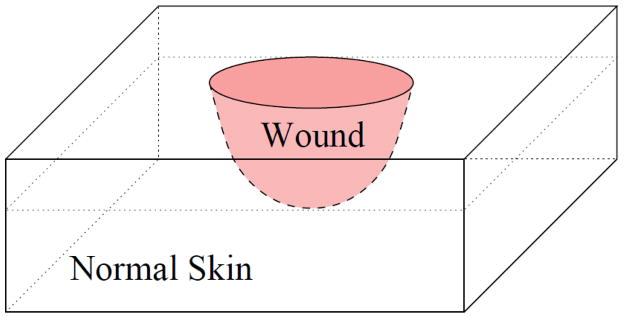
The three-dimensional geometry of a partial thickness burn. The pink region represents the burnt wound which is surrounded by normal skin.
Figure 2.
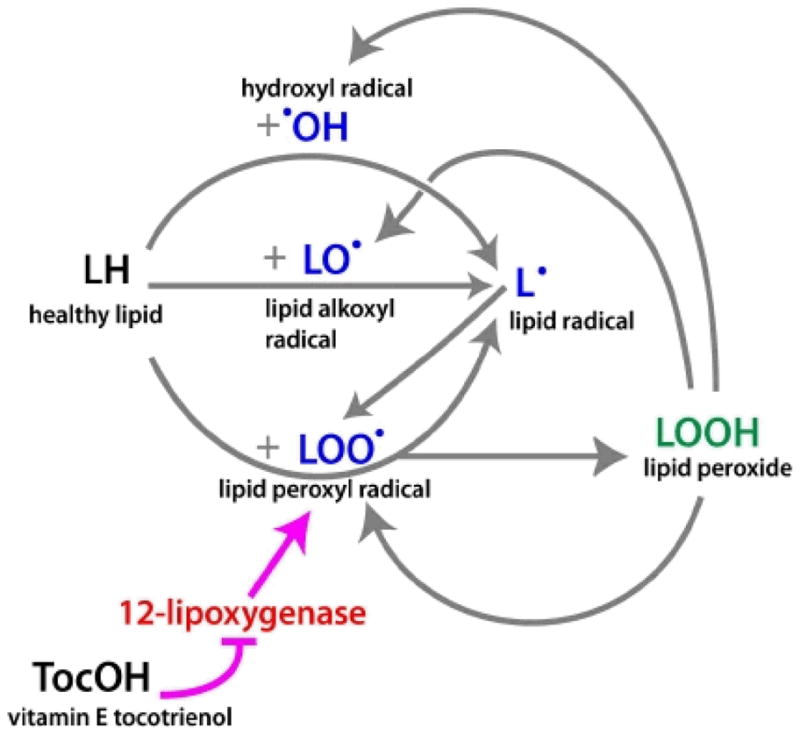
Network of the chemical reactions involved in burn propagation. Pointing arrows represent production of the chemicals, and barred arrow represents inhibition. The magenta colored arrows indicates pathway of the intervention by vitamin E.
Table 1.
The chain reactions and reaction rates
| Role | Reaction | Reaction Rate | Reference |
|---|---|---|---|
|
| |||
| Initiation | (1) LH → L• | Heat or UV light, initial condition | |
| (2) | 5×108 M−1s−1 | (36, 37) | |
|
| |||
| Propagation | (3) | 3.0–4.6×108 M−1s−1 Cellular [O2]= 1.0×10−4 M |
(36) Table 2 (3, 36), (6) pp.4 |
| (4) | 19 M−1s−1 (lipids with 2 double bonds) | (36) Table 2 | |
| (5) | 1.0×10 M−1s−1 | (36) Table 2 | |
|
| |||
| Branching | (6) | 1.5×107 M−1s−1 [Fe2+] = 10−7 M | (6) pp.246 (36) |
| (7) | Slower than above 103 M−1s−1 | (6) pp.246 This work |
|
| (8) | k6/20 | (38) pp. 185, (6) pp. 246, this work | |
|
| |||
| Termination | (9) | 6.6×10 M−1s−1 | (36) Table 2 |
| (10) | 6.6×10 M−1s−1 | (39), (36) pp. 941 | |
| (11) | 6.6×10 M−1s−1 | (39), (36) pp. 941 | |
| (12) | 105 M−1s−1 | (38) pp. 185, this work | |
|
| |||
| Antioxidant | (13) | 106 M−1s−1 | (6) pp. 168 |
| (14) | 2×104 M−1s−1 | λ12/50 | |
| (15) | 5×10−2 M−1s−1 (slo w) | (6) pp. 169 | |
Notations are based on Figure 2, with additional notations: TocO• (vitamin E radical), NRP (non-radical product), Fe2+ (ferrous ion), Fe3+ (ferric ion), OH− (hydroxide ion), and H+ (proton).
Our mathematical model is described in Equations (1) – (8) below. We assume that the burn is instantaneous. The temperature field, which affects the cascade of the chemical reactions, will not be included in our model explicitly; instead, the damage by the high temperature of the burn is reflected by the initial conditions of the chemicals which reflect the damage done by the high temperature of the burn. In particular, reaction (1) occurs instantaneously at time 0, and provides the initial amount of free radicals. Reaction (15) is the pro-oxidation effect the vitamin E. Since it happens on a much slower time scale than the other reactions, we shall neglect it. The species 12-lipoxygenase is only implicitly modeled through the inhibition of lipid peroxyl radicals by vitamin E. Normal lipid molecules diffuse within single cell membranes, but there is no evidence showing that lipid molecules transports from cell to cell, therefore we neglect the diffusion of lipids in the burn tissue. Since the time scale for the propagation of the burn is much smaller than the time scale for remodeling of the damaged vascular system, we do not consider, in our model, the vascular system and immune response.
The mathematical model
Under the above assumptions, we derive, by the law of mass action and diffusion, the model equations (1) – (8). This reaction-diffusion system will be complemented by initial conditions and boundary conditions for each species. Most of the parameters in Equations (1) – (8) are obtained (or calculated) from the literature (Tables 1 and 2), with a few approximated or estimated as described in the Supporting Information (SI). The model involves processes that occur on a huge range of time scales, from nanoseconds (chain reactions) to hours (diffusion), which makes numerical computations of the model very slow. Under the space and time scales considered in burn propagation, we simplified our model by using non-dimensionalization and assuming quasi-steady-state conditions for the five free radicals (see SI). This simplification is justified by a careful numerical comparison of the simplified model and the full model which shows that the solutions are virtually indistinguishable. All our simulations presented in the Results section are performed for the simplified system.
Table 2.
Parameters that do not appear in Table 1 and their references
| Notation | Parameter Value | References and notes |
|---|---|---|
| DLOO• | 1.8×10−5 cm2 h−1 | (40), (36) Table 5 and pp. 927 |
| DLOOH | 1.8×10−5 cm2 h−1 | (40), (36) Table 5 and pp. 927 |
| DTocOH | 1.8×10−2 cm2 h−1 | (41), (36) Table 5 and pp. 927 |
| DTocO• | 1.8×10−2 cm2 h−1 | (41), (36) Table 5 and pp. 927 |
| D•OH | 1.8×10−2 cm2 h−1 | (36) Table 5 and pp. 927 |
| DL• | 1.8×10−5 cm2 h−1 | (40), (36) Table 5 and pp. 927 |
| DLO• | 1.8×10−5 cm2 h−1 | (40), (36) Table 5 and pp. 927 |
| L0 | 2.5×10−2 M | About 10% of cell volume (3, 36) represents the concentration of healthy lipid. |
| A0 | 4×10−4 M | 500 nmol/gm, (specific weight for fat is 0.8 gm/ml) denotes the concentration of vitamin E in healthy skin |
| ka1 | 1.4×10−4 M h−1 | Basal ka1 = μaA0 |
| ka2 | depends on the treatment | |
| μa | ln 2/2 ≈ 0.35 h−1 | half life = 2 hours |
| μs | ln 2/1.9×3600 ≈ 1.313×103 h−1 | half life = 1.9 sec |
| λ15 | 6.6×104 M−1s−1 | =λ11 |
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
Finite difference methods are used to solve the reaction-diffusion system. More specifically, second order central difference is used for spatial discretization, and semi-implicit schemes are used for the time discretization (see SI). Adaptive small time steps are used for accuracy and stability.
Results
Comparison with a brass comb model of burn: 2D simulations
In (31), Singer et al. designed an experimental porcine model to study burn damage. Their model used a 20 mm × 20 mm × 55 mm brass comb with four 10 mm × 20 mm prongs separated by three 5-mm notches that produces four distinctive burn sites separated by three interspaces of unburned skin (refer to Fig. 1 in (31)). The brass comb was preheated in boiling water (100°C) for five minutes and applied without pressure on one side of the back of the pig for a period of 30 seconds, resulting in four full-thickness burns separated by three unburned interspaces (refer to Fig. 2 in (31)). The interspaces are not directly injured, but within hours, they undergo progressive ischemia, and at 24 to 48 hours, they become necrotic.
We compare our model with the experimental results of Singer et al., by comparing the burn propagation along the surface of the skin. In order to establish “proof of concept”’ of our model, we first assumed that all the chemicals does not vary much over the depth of the wound, and therefore simulated the model for geometry of the brass comb in the 2-D case. This assumption can also be thought as averaging all the variables over the depth of the wound, therefore we used the same diffusion rates as in the three dimensional case.
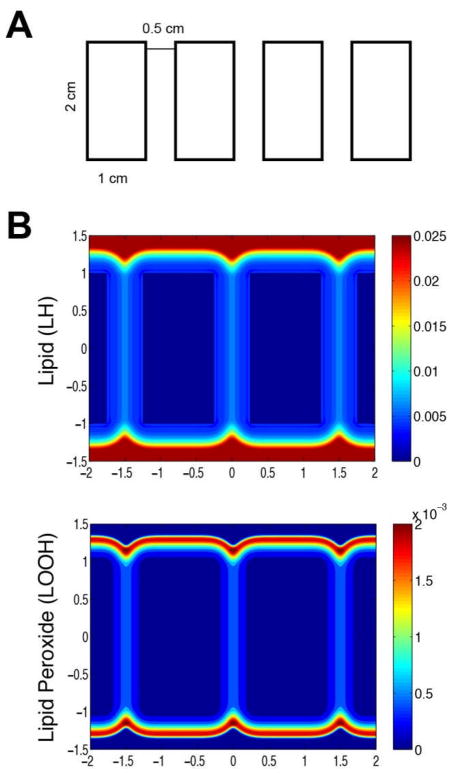
Fig. 3A depicts the 2-D geometry of the brass comb; the initial burn is confined to the four rectangular regions. Fig. 3B shows the numerical solution of the concentrations of lipid and lipid peroxide 12 hours after the initial burn. The parameters used in our simulations are based on Tables 1 and 2, and the simulated physical time is 12 hours. The simulations are performed in a large rectangle (7 cm in the x - direction and 3 cm in the y - direction) which contains all the four small rectangles. We use no-flux boundary conditions for all the species on four boundaries of the rectangular computational domain (−3.5 ≤ x ≤ 3.5, −1.5 ≤ y ≤ 1.5). We assume that initially, in the burn area, [LH] =0, [TocOH] =0, [LOOH] =0, and in the healthy area, [LH]=L0, [TocOH]=A0, [LOOH] =0, with all the other initial species solved by assuming the quasi-steady-state assumptions. The tissue in the interspace between rectangles clearly suffered excessive vascular damage by the burn. We account for this by decreasing the replenish rate of the natural vitamin E, ka1, in the interspace between the rectangles by a factor of 10. Although it is not possible to exactly compare the profiles in Fig. 3B to tissue damage shown in the experiments of Singer et al., the progression of the burn in the simulation and the experiments shows the same pattern.
Figure 3.
Two-dimensional simulation for brass comb model of a burn at 12 hour after the initial heat shock. (A) 2D surface view of the geometry of brass comb burn. (B) Contours of concentrations of lipid (L) and lipid peroxide (P) (zoomed in for viewing the interspace areas), with color bars displaying the levels of these species; the interspace between the original burn area is where the ischemia occurs, and this simulation is using ka1 with values 10 times lower in the interspaces than in other areas.
The effect of vitamin E intervention: 3D simulations
We simulated the burn wound in three-dimensional space, without intervention and with intervention by vitamin E, i.e., ka2 = 0 and ka2 = 5ka1. The goal was to test, by the mathematical model, the efficacy of vitamin E in slowing down the lipid peroxide propagation. For simplicity we assumed that the epidermal layer and the dermal layer are both homogeneous and parallel to the horizontal plane. Although the diffusion rate of the chemical species in the epidermal layer may be smaller than that of the dermal layer, for simplicity, we take them to be the same. We also assume, for simplicity, that the initial wound is a restricted within the pink cube with −0.5 ≤ x ≤ 0.5, −0.5 ≤ y ≤ 0.5, −1 ≤ z ≤ 0, and take the computational domain to be −1 ≤ x ≤ 1, −1 ≤ y ≤ 1, −2 ≤ z ≤ 0, as illustrated in Fig. 4.
Figure 4.
Illustration of the three-dimensional computational domain.
The initial conditions were taken by assuming that the initial damage is proportional to the temperature of the tissue after the burn. More specifically, we assumed that at the initial instant, U = 0 in the burned area, and U = 1 elsewhere, and solved the equation
in the pink region in Figure 4 during the burning, with DU =1cm2h−1, and the boundary conditions
We further define U = 1 outside the burned area, and then take 1-U to represent the temperature distribution immediately after the burn. To incorporate the temperature effect of the initial burn, which has contact only on the surface of the skin z = 0, we take the following initial conditions for the species to be
Where U is the temperature at the end of the burning, i.e., t = 30 sec.
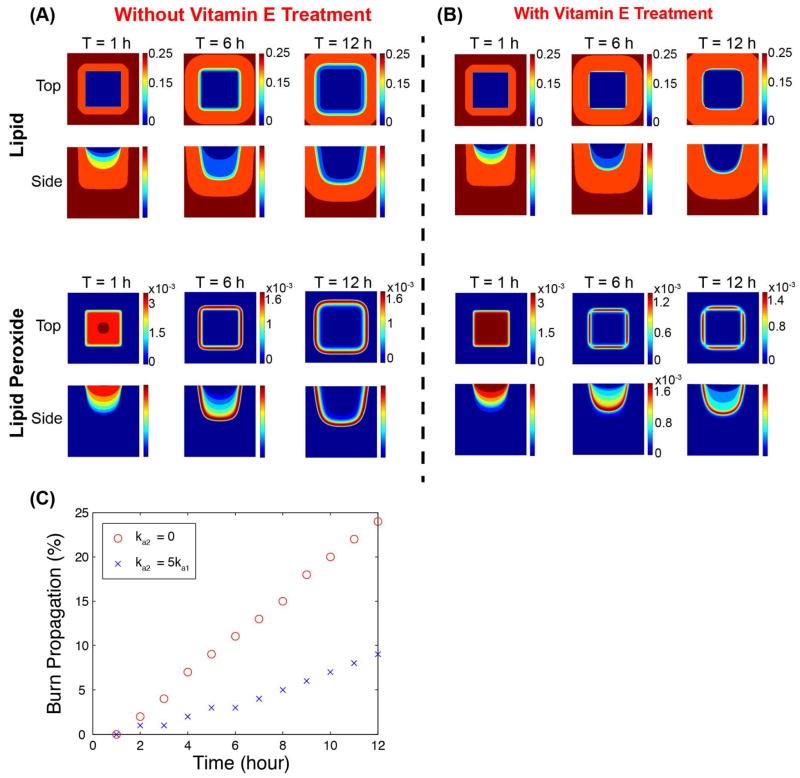
Under the above described three-dimensional scenario, the dynamics of the lipid and lipid peroxide are shown in Figs. 5A and 5B respectively, with both top view and side view at time 1, 6, and 12 hours. The parameters are taken as in Tables 1 and 2 unless otherwise noted. In order to quantitatively analyze the propagation of the burn wound, we tracked the location where the lipid concentration is 90% of its maximum, and use it to quantify the size of the damage in Fig 5C. We note that this criterion gives similar result by using the peak of the lipid peroxide concentration. We found that the progression of damage without vitamin E intervention is rapid, having increased 20 % in the xy-direction and 20 % in xz-direction in 12 hours. However, with vitamin E treatment, the resulting propagation of the burn wound at time 1, 6, 12 hours is reduced to 10%. Fig 5C shows that the size of the wound increases approximately linearly in these 12 hours, with or without vitamin E, and the rate of propagation of the burn wound with vitamin E treatment is about 50% of that without vitamin E treatment. Since we did not take into account different compositions of the skin layers, the propagation speed in horizontal direction and in the vertical direction does not vary. The propagation speed predicted from our simulation is probably more rapid than what has been observed in clinics, because that we did not consider the effect of the heterogeneous complex composition of the skin, which can potentially slow down the diffusion of the chemicals. The propagation may slow down afterwards, as the reparative processes of wound healing may take over.
Figure 5.
3D simulations of the burn wound at 1, 6 and 12 hour after the initial heat shock. (A) Without vitamin E treatment (ka2= 0), distributions lipid (L) and lipid peroxide (P); the upper panels (top view) displays the contours of distributions of those species at z = 0, and the lower panels (side view) displays the contours of distributions of species at y = 0.5. (B) With vitamin E treatment (ka2 =5ka1). Color bars beside the contours indicate the levels of the species, and the scales of the top view and side view are the same unless otherwise indicated. (C) The percentage of burn wound propagation with respect to time are shown for ka2 = 0 and ka2 = 5ka1 respectively. The size of the burn wound with vitamin E treatment is about half of the size of that without vitamin E treatment.
We compared the numerical results with those performed in a larger computational domain (not shown here) and found that the solutions near the initial burn are virtually indistinguishable. This confirms that there is no boundary effect for the computational domain used here.
Sensitivity Analysis
We have performed PRCC sensitivity analysis (35) for the burn propagation at T = 12 in two-dimensional simulations on 19 parameters. The two-dimensional simulations are based on the assumption of homogeneity in the z-direction of the three-dimensional model.
We estimated the expansion of wound along x-axis. The region of the wound is defined as the region where the lipid concentration is less than 90% of that of the healthy tissue. Latin hypercube sampling method was used to sample the parameters. The range of each parameter is chosen as 0.5 to 1.5 times the values listed in Tables 1 and 2. Each range is divided into 1000 intervals of uniform length. Each interval for each parameter is sampled exactly once (without replacement), so that the entire range of each parameter is explored. Table 3 summarizes the PRCC values and p-values for these 19 parameters (see Fig. S1). The significant parameters are those parameters that have p-value less than 0.01. We found that the following parameters DLOOH (diffusion rate of lipid peroxide), μa (degradation rate for Vitamin E), λ4 (reaction rate of lipid peroxyl radial to lipid) are highly positively correlated while the ka1 (growth rate for vitamin E from diet), λ9 (degradation rate for lipid peroxyl radical) are highly negatively correlated. The results are consistent with the intuition that the faster the lipid peroxide diffuses, the faster the burn propagates; and similarly the faster vitamin E degrades, or the slower vitamin E from diet is absorbed, or the slower lipid peroxyl radical degrades, the faster the burn is expected to propagate.
Table 3.
PRCC Sensitivity analysis
| Parameters | PRCC (p-value) |
|---|---|
| DLOOH | 0.91211 (0) |
| DTocOH | −0.11037 (4.7162e-04) |
| ka1 | −0.83987 (4.3089e-267) |
| k3 | −0.025615 (0.41844) |
| k6 | 0.47569 (1.3362e-57) |
| k7 | 0.58658 (1.7463e-93) |
| k8 | 0.19888 (2.2275e-10) |
| μa | 0.74742 (1.9098e-179) |
| μs | −0.037993 (0.22999) |
| λ2 | −0.032165 (0.30957) |
| λ4 | 0.93173 (0) |
| λ5 | 0.025118 (0.42753) |
| λ9 | −0.76215 (1.1801e-190) |
| λ10 | 0.01816 (0.56623) |
| λ11 | 0.016474 (0.60283) |
| λ12 | −0.028103 (0.37467) |
| λ13 | −0.024296 (0.44282) |
| λ14 | −0.04696 (0.13782) |
| λ15 | 0.037622 (0.23457) |
We further investigated the effectiveness of vitamin E treatment against the uncertainties of the parameters (Figure 6). The results show that five times topical vitamin E treatment can slow down the propagation of burn on average by at least 50%, and this prediction is quite robust for a large range of randomly chosen parameters.
Figure 6.
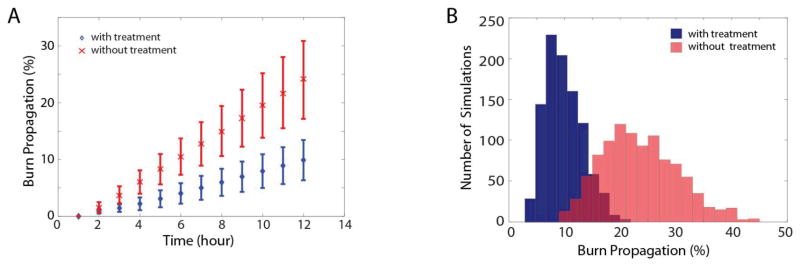
Statistics of 1000 simulations with randomly selected parameters, for burn wound propagation, with and without vitamin E treatment. The parameter ka1 is a random variable in an interval which contains the natural production rate of vitamin E, 1.4×10−4 Mh−1. For the untreated cases, we take ka2 =0, and, for the treated cases, we take ka2=5×1.4×10−4 Mh−1. (A) Time history of the burn propagation. The marks ‘x’ and ‘o’ stand for the mean of the burn propagation over 1000 simulations at each time, and the bars for the standard deviation. (B) Histograms of the statistics at T=12 for treated (blue) and untreated (red) cases.
Discussion
The skin, especially the surface, is packed with lipids. Immediately after skin burn injury has occurred, the tissue damage propagates as a result of a series of chemical reactions of lipid-centered radicals, and this propagation may persists for more than 12 hours. These reactions result in accumulation of lipid peroxide and depletion of antioxidants in the tissue, which cause the tissue deterioration. After 12 hours post burn, infiltrating neutrophils may exaggerate the situation by spitting more ROS to the wound site while they kill bacteria.
This paper develops a novel three-dimensional mathematical description on the propagation of tissue damage within 12 hours after a burn, before neutrophils come in to the wound site. The model is based on the lipid peroxidation chain reactions that take place immediately after thermal wound injury. It is formulated in terms of a system of partial differential equations that incorporate the chemical reactions using mass action kinetics and the diffusion of the chemical species in the tissue surrounding the wound. The various rate parameters are obtained from the experimental literature. The three dimensional formulation is readily applicable to dermal injuries (partial thickness, or second-degree burns). Simulations for comb burns show qualitative agreement with documented experimental measurements.
The mathematical model was used to investigate the effect of treatment with vitamin E. We simulated the wound burn propagation with and without vitamin E treatment, and found that vitamin E treatment can substantially reduce lipid peroxide propagation for partial thickness burn wounds. We have assumed that, to simplify the computation, the tissue surrounding the wound is homogeneous. The heat propagation is incorporated through the initial conditions that reflect the dermal damage caused initially by the burn.
The main interest of this paper is to how tissue damage propagates in the first 12 hours after the initial burn injury. As mentioned, few neutrophils come to the burned area during this period of time. After this initial period, neutrophils spit ROS and exaggerate the oxidative stress. Other reparative processes of wound healing may also be initiated. Therefore in order to get a quantitative understanding of the dynamics of lipid peroxide concentration, one needs to incorporate these factors into the mathematical model, which is left as future work.
Based on the present model, we hypothesize that vitamin E can substantially reduce tissue damage propagation for partial thickness burns immediately after the initial injury. To the extent that this hypothesis is biologically confirmed, and further development of the model include experimental data based on temperature measurements, the model could become a predictive tool for determination of the size and depth of tissue damage, as well as for predicting the results of treatments of cutaneous burn wounds.
Supplementary Material
Acknowledgments
The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. The work is also supported in part by GM069589 and GM077185 from NIGMS to CKS.
References
- 1.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severely burned patients. Ann Surg. 1996;223(1):14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutteridge J. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41(12):1819. [PubMed] [Google Scholar]
- 3.Doktorov AB, Lukzen NN, Pedersen JB. Analysis of lipid peroxidation kinetics. 1. Role of recombination of alkyl and peroxyl radicals. J Phys Chem B. 2008;112(37):11854–61. doi: 10.1021/jp709921m. [DOI] [PubMed] [Google Scholar]
- 4.http://en.wikipedia.org/wiki/Lipid\_peroxidation.
- 5.Latha B, Babu M. The involvement of free radicals in burn injury: a review. Burns. 2001;27(4):309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4. Oxford University Press; 2007. [Google Scholar]
- 7.Logani MK, Davies RE. Lipid oxidation: biologic effects and antioxidants–a review. Lipids. 1980;15(6):485–95. doi: 10.1007/BF02534079. [DOI] [PubMed] [Google Scholar]
- 8.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr. 1985;5:365–90. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 9.Frankel EN. Recent advances in lipid oxidation. J Sci of Food Agric. 1991;54:495–511. [Google Scholar]
- 10.Catalá A. A synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem Biophys Res Commun. 2010;399(3):318–23. doi: 10.1016/j.bbrc.2010.07.087. [DOI] [PubMed] [Google Scholar]
- 11.Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RC, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44(10):1125–71. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 12.Naumov AA, Shatalin YV, Potselueva MM. Effects of a nanocomplex containing antioxidant, lipid, and amino acid on thermal burn wound surface. Bull Exp Biol Med. 2010;149(1):62–6. doi: 10.1007/s10517-010-0876-5. [DOI] [PubMed] [Google Scholar]
- 13.Bekyarova G, Galunska B, Ivanova D, Yankova T. Effect of melatonin on burn-induced gastric mucosal injury in rats. Burns. 2009;35(6):863–8. doi: 10.1016/j.burns.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Bekyarova G, Yankova T. alpha-Tocopherol and reduced glutathione deficiency and decreased deformability of erythrocytes after thermal skin injury. Acta Physiol Pharmacol Bulg. 1998;23(2):55–9. [PubMed] [Google Scholar]
- 15.Bekyarova G, Yankova T, Kozarev I. Suppressive effect of FC-43 perfluorocarbon emulsion on enhanced oxidative haemolysis in the early postburn phase. Burns. 1997;23(2):117–21. doi: 10.1016/s0305-4179(96)00091-5. [DOI] [PubMed] [Google Scholar]
- 16.Bekyarova G, Yankova T, Kozarev I. Combined application of alpha-tocopherol and FC-43 perfluorocarbon emulsion suppresses early postburn lipid peroxidation and improves deformability of erythrocytes. Acta Chir Plast. 1998;40(1):17–21. [PubMed] [Google Scholar]
- 17.Bekyarova G, Yankova T, Kozarev I, Yankov D. Reduced erythrocyte deformability related to activated lipid peroxidation during the early postburn period. Burns. 1996;22(4):291–4. doi: 10.1016/0305-4179(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 18.Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51(5):927–31. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Hortan JW. Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy. Toxicology. 2003;189(1–2):75–88. doi: 10.1016/s0300-483x(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 20.Panin G, Strumia R, Ursini F. Topical alpha-tocopherol acetate in the bulk phase: eight years of experience in skin treatment. Ann N Y Acad Sci. 2004;1031:443–7. doi: 10.1196/annals.1331.069. [DOI] [PubMed] [Google Scholar]
- 21.Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm. 2007;76:1–21. doi: 10.1016/S0083-6729(07)76001-6. [DOI] [PubMed] [Google Scholar]
- 22.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6–17. doi: 10.1016/j.burns.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Traber MG, Leonard SW, Traber DL, Traber LD, Gallagher J, Bobe G, Jeschke MG, Finnerty CC, Herndon D. α-Tocopherol adipose tissue stores are depleted after burn injury in pediatric patients. Am J Clin Nutr. 2010;92(6):1378. doi: 10.3945/ajcn.2010.30017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–61. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–98. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28(5–6):692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 28.Liu KC. Thermal propagation analysis for living tissue with surface heating. Int J Thermal Sci. 2008;47(5):507–13. [Google Scholar]
- 29.Dai W, Wang H, Jordan PM, Mickens RE, Bejan A. A mathematical model for skin burn injury induced by radiation heating. Int J Heat Mass Transfer. 2008;51(23–24):5497– 510. [Google Scholar]
- 30.Ng EYK, Chua LT. Comparison of one- and two-dimensional programmes for predicting the state of skin burns. Burns. 2002;28(1):27–34. doi: 10.1016/s0305-4179(01)00066-3. [DOI] [PubMed] [Google Scholar]
- 31.Singer AJ, McClain SA, Taira BR, Romanov A, Rooney J, Zimmerman T. Validation of a porcine comb burn model. Am J Emerg Med. 2009;27(3):285–8. doi: 10.1016/j.ajem.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Reddanna P, Rao MK, Reddy CC. Inhibition of 5-lipoxygenase by vitamin E. FEBS Lett. 1985;193(1):39–43. doi: 10.1016/0014-5793(85)80075-2. [DOI] [PubMed] [Google Scholar]
- 33.German JB, Kinsella JE. Lipid oxidation in fish tissue. Enzymic initiation via lipoxygenase. J Agric Food Chem. 1985;33:680–3. [Google Scholar]
- 34.http://en.wikipedia.org/wiki/Lipoxygenase.
- 35.Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008:178–96. doi: 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antunes F, Salvador A, Marinho HS, Alves R, Pinto RE. Lipid peroxidation in mitochondrial inner membranes. I. An integrative kinetic model. Free Radic Biol Med. 1996;21(7):917–43. doi: 10.1016/s0891-5849(96)00185-2. [DOI] [PubMed] [Google Scholar]
- 37.Barber DJW, Thomas JK. Reactions of radicals with lecithin bilayers. Radiation Re. 1978;74:51–65. [Google Scholar]
- 38.Belitz HD, Grosch W. Food Chemistry. 2. Springer; 1999. [Google Scholar]
- 39.Barclay LRC, Baskin KA, Locke SJ, Vinqvist MR. Absolute rate constants for lipid peroxidation and inhibition in model biomembranes. Can J Chem. 1989;67:1366–9. [Google Scholar]
- 40.Vaz WLC, Goodsaid-Zalduondo F, Jacobson K. Lateral diffusion of lipids and proteins i Jn bilayer membranes. FEBS Lett. 1984;174(2):199–207. [Google Scholar]
- 41.Aranda F, Coutinho A, Berberan-Santos M, Prieto M, Gomez-Fernandez J. Fluorescence study of the location and dynamics of [alpha]-tocopherol in phospholipid vesicles. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1989;985(1):26–32. [Google Scholar]
- 42.Alexander-North LS, North JA, Kiminyo KP, Buettner GR, Spector AA. Polyunsaturated fatty acids increase lipid radical formation induced by oxidant stress in endothelial cells. J Lipid Res. 1994;35(10):1773–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.