Abstract
Study Objectives:
To assess the interindividual and intraindividual variability in the circadian rhythms of blind individuals with non-24-h disorder and to quantify the influence of environmental time cues in blind subjects lacking entrainment (non-24-h individuals or N-24s).
Design:
An observational study of 21 N-24s (11 females and 10 males, age 9-78 years) who kept a sleep/wake schedule of their choosing. Circadian phase was determined using the melatonin onset (MO) from plasma or saliva samples that were collected every 2 weeks. Melatonin concentrations were measured by radioimmunoassay. A total of 469 MO assessments were conducted over 5,536 days of study. The rate of drift of circadian phase was calculated using a series of MOs (total number of hours the MO drifted divided by the total number of days studied). Stability of the rest/activity rhythm was calculated using chi-squared periodogram analysis of wrist actigraphy data in 19 subjects.
Setting:
Academic medical center.
Participants:
Paid volunteers.
Interventions:
N/A.
Measurements and Results:
Subjects lacked entrainment such that circadian phase drifted an average (± standard deviation) of 0.39 ± 0.29 h later per day; however, there was notable intersubject and intrasubject variability in the rate of drift including relative coordination and periods of transient entrainment during which there was little to no drift in the circadian phase. A regular, reproducible, and significant oscillation in the rate of drift was detected in 14 of the 21 subjects. A significant non-24-h rest/activity rhythm was detected in 18 of 19 subjects. There was a strong correlation (r = 0.793, P = 0.0001) between the non-24-h rest/activity rhythm and the rate of drift of the circadian phase.
Conclusions:
Most N-24s are influenced by unidentified environmental time cues and the non-entrained biological clock in such N-24s is reflected in their rest/activity rhythms. These findings may have diagnostic and treatment implications: this disorder might be diagnosed with actigraphy alone, relative coordination and transient entrainment may result in misdiagnosis and responsiveness to environmental time cues may influence treatment success with oral melatonin.
Citation:
Emens JS; Laurie AL; Songer JB; Lewy AJ. Non-24-hour disorder in blind individuals revisited: variability and the influence of environmental time cues. SLEEP 2013;36(7):1091-1100.
Keywords: Blind, melatonin, circadian, non-24-h disorder
INTRODUCTION
Light is the primary synchronizer of the endogenous circadian pacemaker (biological clock): in most blind individuals lacking conscious light perception, the clock is not synchronized to the 24-h day.1–5 The circadian rhythms of such nonentrained individuals (N-24s) drift to a progressively later, and sometimes earlier, time despite adherence to a regular schedule of light exposure, activity, social and other cues. As a consequence, N-24s suffer from periodic daytime somnolence and nighttime insomnia as the circadian rhythms in alertness and sleep propensity drift in and out of synchrony with the 24-h day.6–8 Most simply put, N-24s are periodically trying to maintain sleep or wakefulness in opposition to their own biological clocks. As such, they represent the quintessential circadian rhythm sleep disorder.9,10 Lockley and colleagues were the first to demonstrate that the nonentrained biological clock was reflected in the sleep and activity of N-24s.11 They demonstrated that the incidence of nonentrained rhythms of sleep (as measured by the timing of sleep onset, sleep offset, naps, or maximal activity) was higher in individuals shown to have nonentrained circadian rhythms of urinary 6-sulfatoxymelatonin.
Such decrements in sleep quality have since been confirmed with polysomnography: N-24s have been shown to have decreased sleep efficiency and increased time awake after sleep onset when they are sleeping out of synchrony with the biological clock as compared to when they are sleeping in synchrony with the biological clock8 and have also been shown to have decreased total sleep time, sleep efficiency and REM sleep when compared to sighted control subjects.12 Evidence for the behavioral consequences of a nonentrained biological clock can also be seen using actigraphy. Lockley and colleagues11 also showed that in seven of 16 N-24s a non-24-h pattern of activity could be detected when actigraphy data were subjected to spectral analysis. We have subsequently shown a correlation between the nonentrained drift in the circadian phase and the non-24-h component of the rest-activity rhythm in 15 of 16 N-24s when wrist actigraphy data was similarly subjected to spectral analysis.13 Recent experiments in animals14 and humans15 raise the possibility that chronic circadian misalignment could also result in adverse metabolic, cognitive, and emotional consequences in N-24s.
Oral administration of low-dose melatonin has proven to be an effective treatment for this circadian rhythm sleep disorder.8,16–21 Indeed, we have demonstrated synchronization of the biological clock (entrainment) using doses of melatonin as low as 0.02 mg.21
For many years it was thought that the biological clock's rate of drift across the 24-h day was constant in untreated N-24s. Over 20 years ago we reported on a cohort of 20 blind subjects of whom 11 were not entrained with an average rate of drift of 33 min per day and we noted that the rate of drift appeared to be “remarkably stable” within individuals.2 However, in that study, as in almost all others, N-24s were typically followed for no more than 6 weeks1–4 or if they were studied for longer periods of time, there may have been many weeks between assessments.5
We have since shown, in a small cohort of N-24s, that the rate of drift in the timing of the biological clock regularly slows and accelerates in a seesaw fashion: the timing of the clock (measured using the melatonin onset) moves rapidly during the daytime (between approximately 08:00 and 20:00) but then slows during the evening and night (between approximately 20:00 and 08:00).22 In one individual the melatonin onset drifted more than 30 min later every day during the daytime but slowed to less than 2 min per day during the night. This oscillation in the rate of drift (termed relative coordination) is well described in the circadian literature and reflects the resetting effects of environmental time cues (zeitgebers) that are strong enough to influence the biological clock but are not strong enough to synchronize it.23,24 It has been hypothesized that a variety of time cues including physical activity,5,25–27 sleep,28–30 and even light (nonvisual photoreception)31,32 might have been responsible. We have further speculated that our ability to treat this disorder with low doses of melatonin may have been aided in some cases by the influence of these weak zeitgebers. However, the prevalence of this phenomenon among N-24s was unknown.
We report here on a relatively large cohort of nonentrained blind individuals who were intensively studied over many months. We found a high prevalence of relative coordination, remarkable intersubject and intrasubject variability in the rate of drift across the 24-h day, periods during which there was little to no drift in circadian phase, and evidence that the non-24-h component of the rest/activity cycle can accurately reflect the drift of the biological clock.
METHODS
Subjects
Subjects were 21 blind individuals lacking conscious light perception (11 women, 10 men; age 8-78 years). These individuals were studied while they kept a sleep/wake schedule of their choosing at home for approximately 1 to 50 months. They were not taking any medications during episodes of plasma or saliva sampling that would interfere with melatonin production nor were they taking oral melatonin, but some subjects did take medications that might influence sleep and wakefulness and that might have impacted the rest/activity cycles we observed (Table 1). All subjects provided written informed consent and both the protocol and consent forms were approved by the Oregon Health & Science University Institutional Review Board.
Table 1.
Demographic and clinical data on nonentrained blind male and female individuals
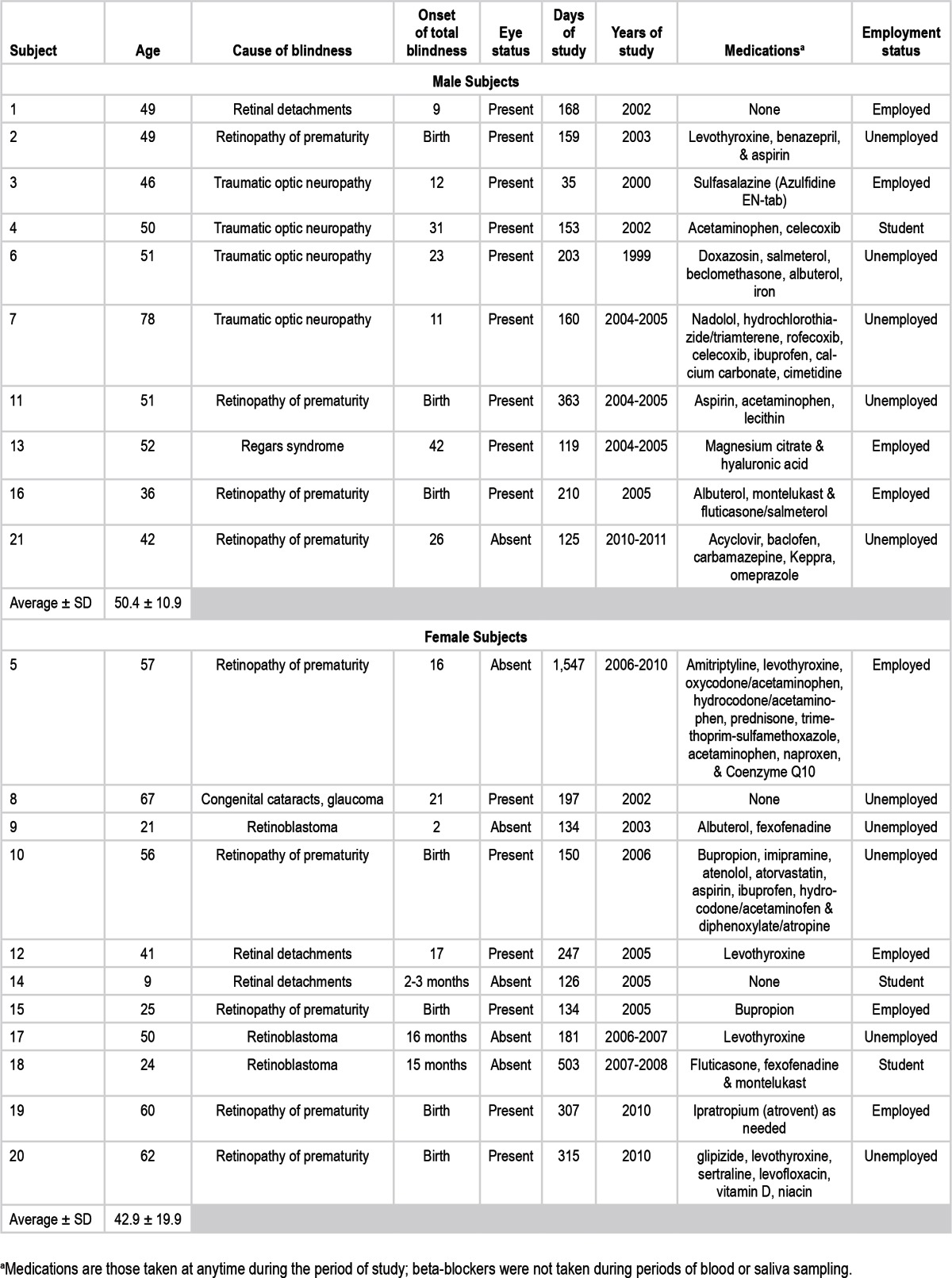
Subjects had complaints of insomnia (8 subjects), excessive daytime fatigue or somnolence (2 subjects) or insomnia plus daytime fatigue or somnolence (8 subjects). Three subjects had no sleep complaints. Subjects were selected for inclusion post hoc if at least one circadian beat cycle (drift of circadian phase across 24 h) of melatonin phase data was available for analysis and if no two successive assessments of phase occurred more than 95% of a circadian beat cycle apart.
Melatonin and Circadian Phase
Plasma or saliva samples were collected every 1-2 h for 14-25 h at the Oregon Health & Science University Clinical and Translational Research Center or at home approximately every 2 weeks (average ± standard deviation [SD] of 12.8 ± 5.8 days) for assessment of circadian phase. No two assessments were more than 95% of a circadian beat cycle apart (drift of circadian phase across 24 h, average of 17.4 ± 25.0% of a circadian beat cycle apart) and subjects were assessed for an average of three circadian beat cycles. Plasma and salivary melatonin concentrations were measured by radioimmunoassay (American Laboratory Products, Windham, NH) and circadian phase was determined using the plasma and salivary melatonin onset (MO) assessed with a 10 or 3 pg/mL threshold, respectively.33,34 The lower limit of sensitivity of this assay is 0.5 pg/mL. These thresholds were chosen to allow comparison to our more recent studies in sighted individuals.35–37 Lighting conditions, sleep schedules, posture, and activity were not controlled during either home or laboratory assessments, although subjects were asked not to exercise during assessments.
Actigraphy
Activity data were gathered using a wrist actigraph (Acti-watch-64 ®, Mini-Mitter Co., Bend, OR). Data were collected using a 30-sec sampling epoch for 70 to 1,519 days. Actigraphy was used to specifically assess rest/activity cycles and not for the assessment of sleep and wakefulness.
Drift of Circadian Phase
The overall rates of drift of circadian phase were calculated using a series of MOs across a complete number of circadian beat cycles (average ± SD of 3.3 ± 3.4 cycles, range of 1-16 cycles) that were determined post hoc (Figure 1). The number of hours drifted from the first MO to the last MO in the series allowed for the calculation of the average rate of drift (e.g., if the MO shifted a total of 50 h later over the course of 100 days of study, then the average drift rate was 0.5 h per day). Individuals were determined to lack entrainment when the 95% confidence intervals for the overall drift rate calculated by linear regression did not overlap 0 h per day. However, linear regression was not used to calculate average drift rates to avoid the distortion resulting from the relative coordination.
Figure 1.
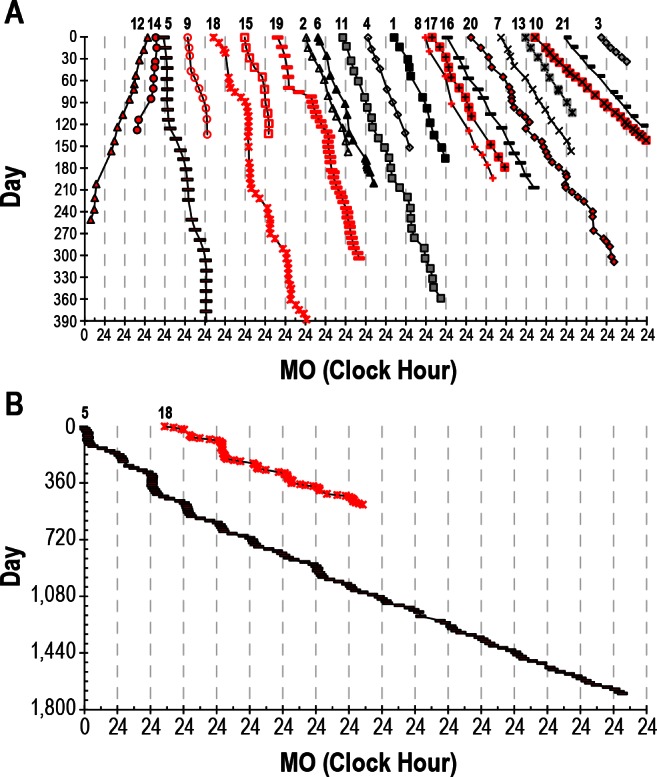
(A) Twenty-one nonentrained blind individuals. Symbols represent an assessment of circadian phase (melatonin onset, MO) assessed using the time that plasma or salivary melatonin levels cross a threshold of 10 or 3 pg/mL, respectively. Women are plotted in red and men in black and gray. Subject numbers are displayed across the top of the figure. Not all data are plotted for Subjects 5 and 18. The data are plotted in a raster format where the day of study is plotted on the y-axis with successive days plotted beneath each other and the clock hour of the MOs are plotted on a repeating 24-h x-axis. The data for each subject are plotted next to each other with overall drift rate generally increasing from left to right so that all of the data can be visualized in one figure. (B) The complete dataset for Subjects 5 and 18.
In order to quantify changes in the MOs' rate of drift across the day we also calculated “two-point drift rates,” which were the slopes between every two successive phase assessments as described previously.22 For example, if an initial MO occurred at 10:00 and after 14 days a second MO occurred 7 h later at 17:00, the two-point drift rate would be 0.5 h per day (the MO drifted 7 h in 14 days, Figure 2A). The two-point drift rates were subsequently referenced to the subjects' average rate of drift by calculating the deviation (DEV) of each two-point drift rate from the subject's average drift rate. In addition, for each pair of consecutive MOs, an average MO was calculated (MOavg). In the above example, the MOavg would be 13:30.
Figure 2.
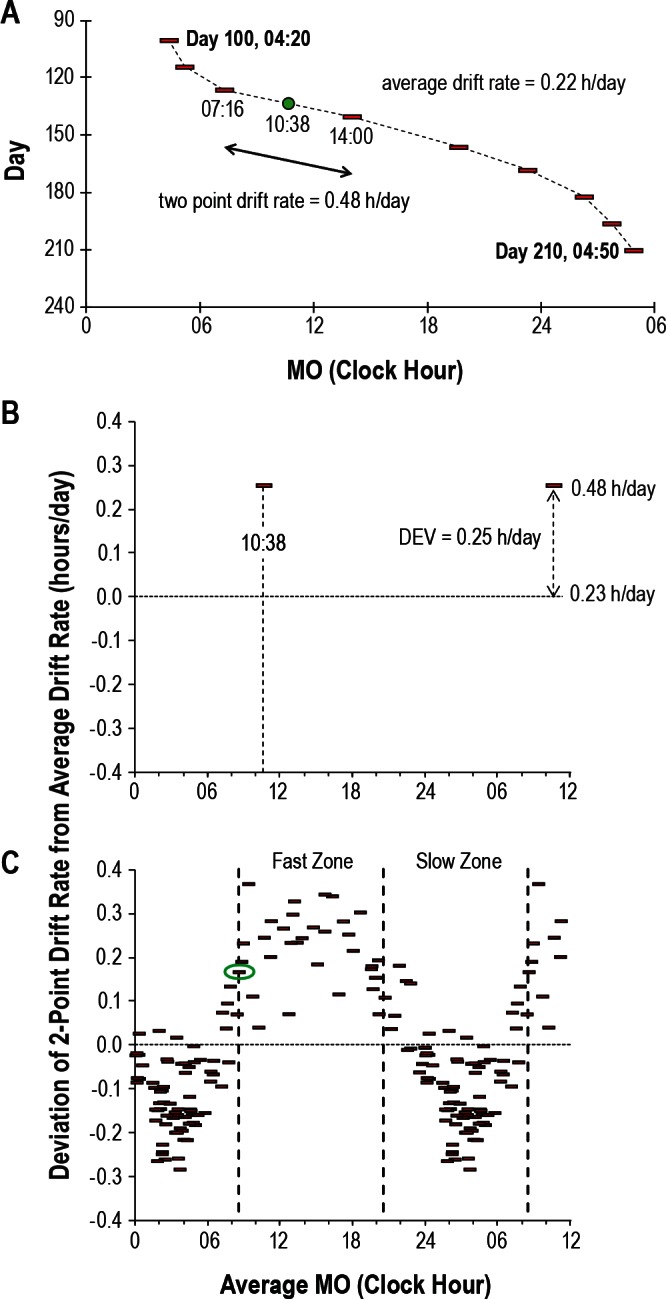
Illustration of the bisection technique for quantification of relative coordination. Data are presented for Subject 5. (A) Representative section of data from study days 100 to 210 with symbols and format as in Figure 1. The average drift rate for this portion of the dataset is 0.22 h/per day (24.5 h over 110 days). This is similar to the 0.23 h/day for all 1,547 days of study). The two-point drift rate between the melatonin onset (MO) occurring at 07:16 and the MO occurring at 14:00 is 0.48 h/day. The average MO (MOavg) between these two is 10:38 (green circle). (B) The average MO from Figure 2A (10:38) is plotted against the corresponding deviation (DEV, in hours) of the two-point drift rate (0.48 h/day) from the average drift rate (0.23 h/day). The data are plotted 1.5 times. (C) The clock hour of each MOavg is plotted against all corresponding DEVs. The data are again plotted 1.5 times. The data point circled in green is the MOavg that resulted in the largest difference in average DEV between two corresponding 12-h zones. The vertical dashed lines denote the boundaries of the zones with fast and slow drift rates.
Stability of the rest/activity rhythm was calculated using a chi-squared periodogram of the actigraphy data gathered during the same period of time using a 1-min block size, test rhythms ranging from 20 to 28 h, and a 0.001 confidence level (Clock-Lab 2.6, Actimetrics; Matlab R2006a, The MathWorks).38 The second highest amplitude rhythm (the highest being 24 h) that was statistically significant was always included in the analysis. In the case of subject 5, significant peaks at 21.00, 22.40, and 25.85 h were also detected in addition to the first and second highest peaks at 24.00 and 24.15 h, respectively. Subject 20 also had multiple significant peaks at 21.00, 21.60, 24.13, 24.22, and 24.42 h in addition to the first and second highest peaks at 24.00 and 23.88 h, respectively.
Assessment of Relative Coordination
As described previously,22 we determined whether the variations in the biological clock's rate of drift across the 24-h day were significant by adapting the bisection technique developed by Kripke and colleagues for the analysis of circadian phase response curves.39 The MOavg was used as the marker of circa-dian phase whereas the DEV was used to measure the variation in drift rate. The bisection test treats each MOavg within a subject's dataset as a potential inflection point (transition) between a fast drift rate zone and a slow drift rate zone (Figures 2B and 2C). The dataset is divided into two 12-h bins on either side of each MOavg. For each 12-h bin, the average DEV is calculated from which the absolute difference (D) in mean DEV between the two bins is determined. D is calculated for each MOavg, so that the number of bin pairs is equivalent to the number of data points. The bin pair and corresponding inflection point with the largest D is designated optimal and is tested for significance using a two-sample Wilcoxon rank-sum (Mann-Whitney U) test of the slow and fast zones. We also determined the amplitude of relative coordination by subtracting the slowest two-point drift rate from the fastest two-point drift rate.
RESULTS
Drift of Circadian Phase
None of the subjects were entrained, and circadian phase (as measured by the MO) had an average overall drift rate (± SD) of 0.39 ± 0.29 h later per day (range of 0.29 h earlier to 0.90 h later per day). There was insufficient statistical power to find a difference in average drift rate between men and women and indeed no difference between women and men was found although there was a strong trend for the female subjects to have slower drift rates (0.29 versus 0.49 h per day in women versus men, respectively, P = 0.057, one sided t-test). There was also no difference in average drift rate between those with and those without eyes (0.43 versus 0.28 h, respectively, P = 0.309). Average drift rate increased with age (r = 0.449, P = 0.041, Figure 3) analogous to our previous report40 but years of blindness did not correlate with average drift rate (r = 0.349, P = 0.121).
Figure 3.
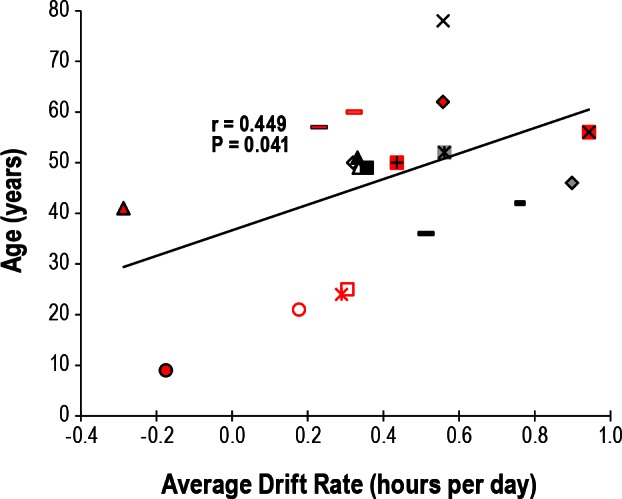
Correlation between age and average drift rate of circadian phase. Symbols are as in Figure 1.
Relative Coordination
Most subjects demonstrated variability in the circadian pacemaker's rate of drift across the 24-h day (Figure 1). In 14 of the 21 subjects, there was a regular slowing and acceleration in the rate of drift that was statistically significant (Table 2, Figure 4). Of the remaining seven subjects, four did not reach statistical significance and three did not have sufficient data to conduct the Wilcoxon rank-sum test (i.e., the optimal inflection point resulted in only one MOavg in one of the 12-h bins). There was considerable intersubject variability in the magnitude of this oscillation: in some subjects the daily rate of drift varied by only a few min whereas in others it varied by over 1 h (Figure 1, Table 2).
Table 2.
Observed circadian drift rate and relative coordination analysis results
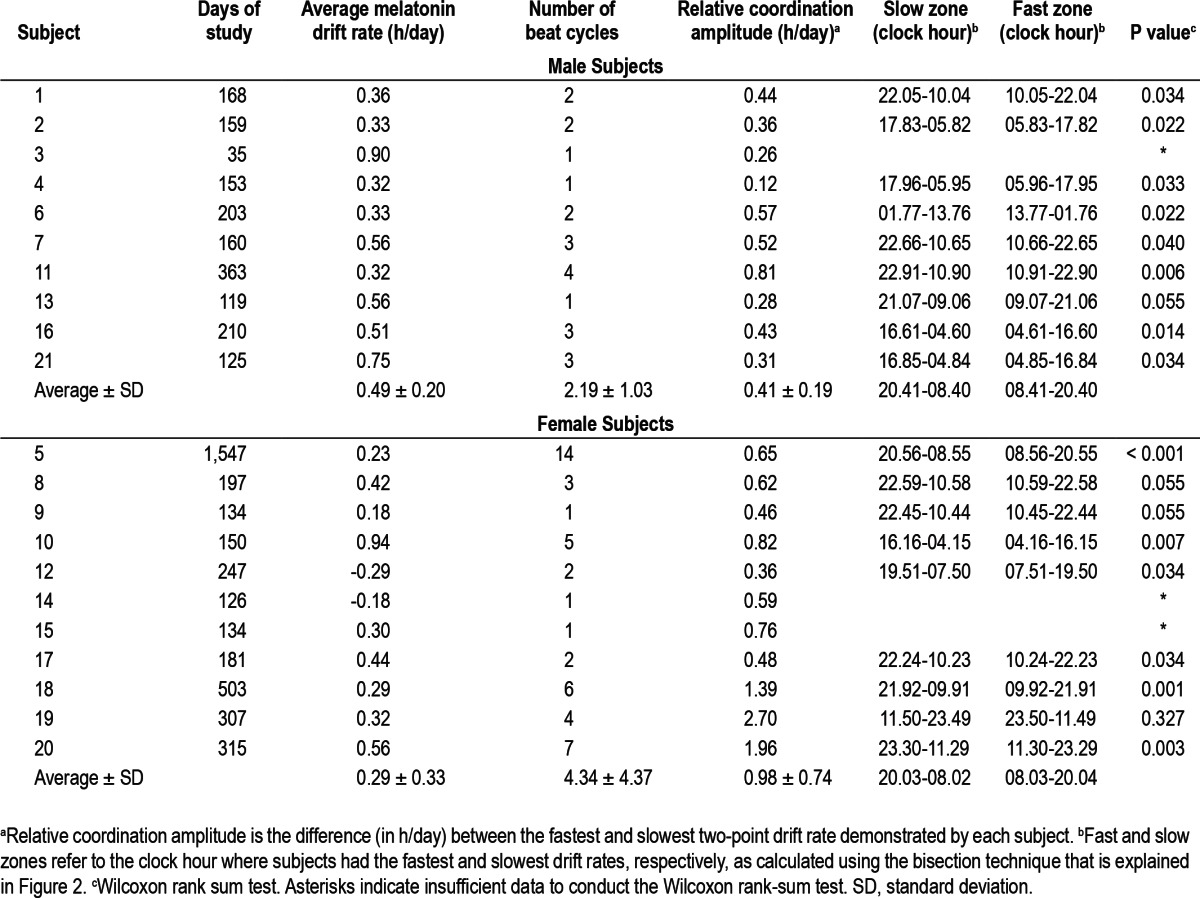
Figure 4.
Plot of drift rate of circadian phase versus clock hour of circadian phase for the 14 subjects who demonstrated statistically significant relative coordination. The deviation of each two-point drift rate from the average drift rate is plotted versus the corresponding average melatonin onset (MOavg) for every subject. The data are plotted 1.5 times.
There was also variability in when the rate of drift periodically accelerated and slowed among those subjects with a significant oscillation in drift rate (Table 2, Figure 4). Although in general the rate of drift was slower (smaller two-point drift rates) when the MO was passing through the early evening and night (approximately 20:00 to 08:00 on average, Table 2 and Figure 4), the earliest clock hour at which the MO's rate of drift began to slow was at approximately 16:00 while the latest was at almost 02:00. Similarly, although the rate of drift tended to be faster when the MO was passing through the morning and afternoon (approximately 08:00 to 20:00 on average, Table 2 and Figure 4), the earliest clock hour at which the MO's rate of drift began to accelerate was at approximately 04:00 while the latest was almost 14:00.
The amplitude of relative coordination was greater in women than in men. The female subjects had an average amplitude of 0.98 h per day versus 0.41 h per day in the men (P = 0.028). A trend for a difference remained when only the 14 subjects with a significant bisection test result were included in the analysis (0.94 h per day versus 0.45 h per day in women versus men, respectively, P = 0.051). Confining the remaining analyses to those subjects with a significant bisection test we found that there was no difference in relative coordination amplitude in those with or without eyes (P = 0.820) and there was no correlation between relative coordination amplitude and either age (P = 0.898) or years of blindness (P = 0.181).
In subject 5, who was studied for the longest duration, the amplitude of relative coordination was found to decrease with time: when the subject's individual beat cycle amplitudes were plotted versus day of study (the corresponding midpoint of each beat cycle) a significant correlation was found (r = 0.648, P = 0.007, Figure 1B). Similarly, average drift rate increased with time (Figure 1B, r = 0.815, P = 0.0001). Her average drift rate increased by over 2 min per day each y on average such that her first and final circadian beat cycles had average drift rates of 0.14 and 0.32 h per day, respectively.
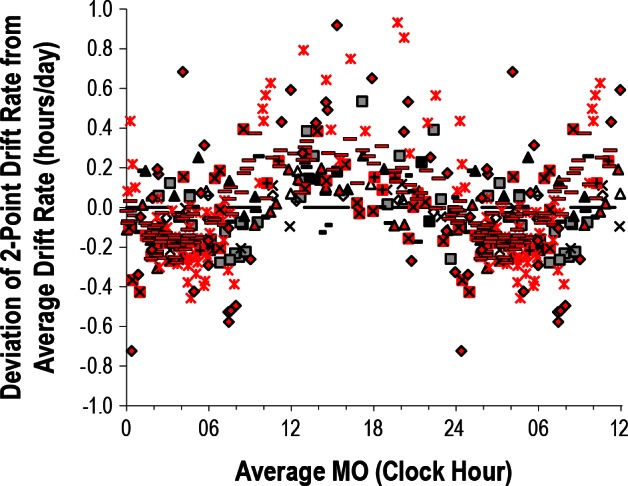
It should also be noted that some subjects had several months during which circadian phase drifted very little and where, for all intents and purposes, they were entrained (Figure 1). Indeed, when we applied our previous definition of entrainment from our treatment studies with oral melatonin (a linear regression drift rate ≤ 0.04 h earlier or later per day with 95% confidence intervals that overlap with 0.00 h per day)21 we found three subjects (5, 14, and 18) who demonstrated such “transient entrainment” for a total of 98, 42, and 71 days, respectively, with an average “entrained” linear regression drift rate of 0.00 ± 0.03 h. Furthermore, later study of subject 15 demonstrated almost 1 year (345 days) of entrainment with a linear regression drift rate of 0.00 ± 0.01 h per day before she lost entrainment with a linear regression drift rate of 0.33 ± 0.06 h per day (Figure 5). There were no known changes in medications, activity, eye status, or hormonal status that precipitated either the spontaneous entrainment or the abrupt resumption of a nonentrained pattern, and the subject drifted at a rate nearly identical to her baseline drift rate of 1.5 years prior.
Figure 5.
Data from subject 15, who was restudied at a later date. Symbols and format are as in Figure 1. The subject demonstrated 345 days of spontaneous transient entrainment (days 809-1,154) during which her average drift rate was only 0.01 h/day (4.1 hours of drift over 345 days, the linear regression drift rate was 0.00 ± 0.01 h/day). She then spontaneously resumed a nonentrained pattern (days 1,168-1,245) with an average drift rate of 0.31 h/day (23.86 h of drift over 77 days, linear regression drift rate of 0.33 ± 0.06 h/day).
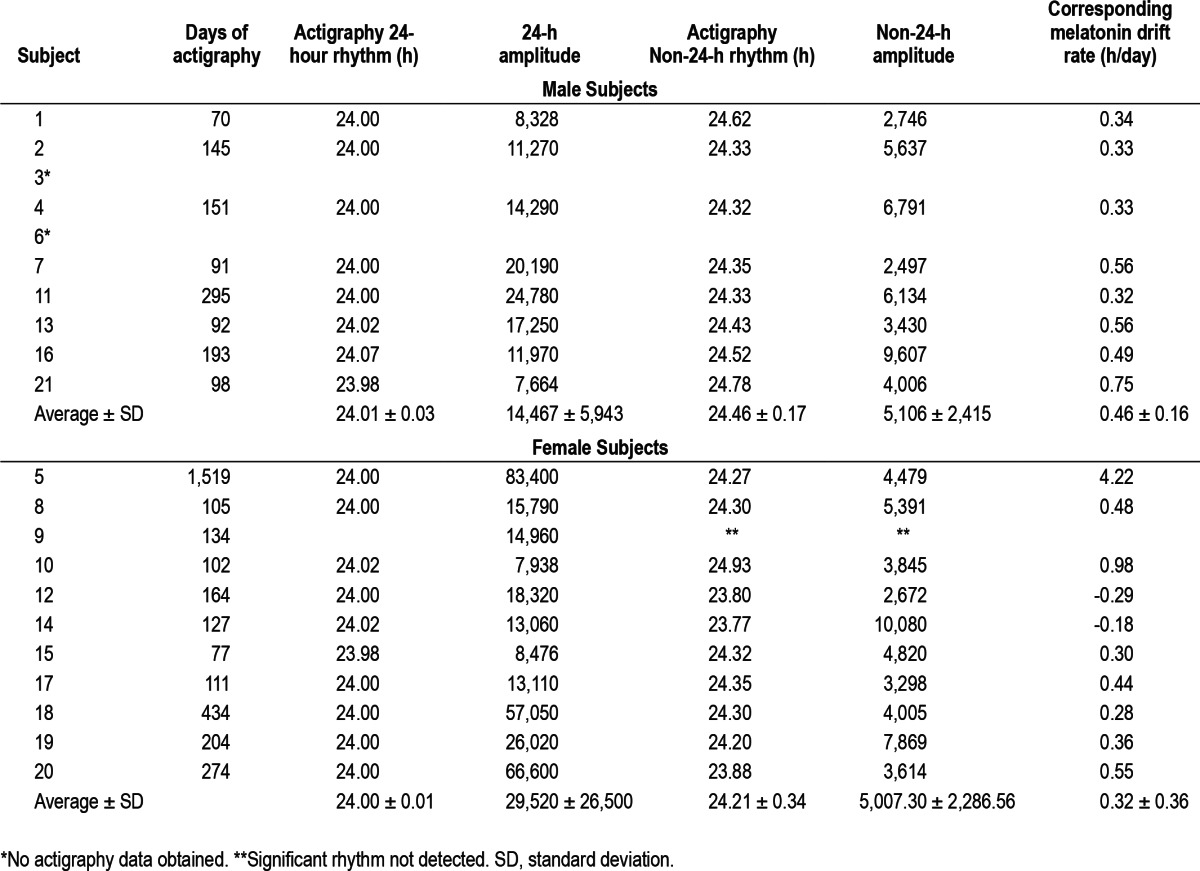
Actigraphy
In addition to a significant 24-h rhythm, a significant non-24-h rest/activity rhythm was detected in 18 of the 19 subjects in whom actigraphy was obtained (not detected in subject 9, Table 3). In all 18 of these subjects the amplitude of the 24-h rest/activity rhythm was significantly greater than that of the non-24-h rhythm (average ± SD of 23,639 ± 22,027 versus 5,051 ± 2,274, P = 0.001, respectively, see Table 3). There was a correlation between the non-24-h rest/activity rhythm and the average drift rate of the melatonin rhythm (r = 0.793, P = 0.0001) with a slope near unity (0.821) in these 18 subjects. In the case of subject 20 the non-24-h actigraphy rhythm (0.12 h earlier per day) was not only quite different from the melatonin drift rate (0.55 h later per day) but was in the opposite direction.
Table 3.
Periodogram analysis of actigraphy data in nonentrained blind individuals
DISCUSSION
We have found that most N-24s are influenced by environmental time cues that result in significant variation both between and within subjects in the rate of drift of the circadian phase and periods of time where the circadian phase may demonstrate little to no drift.
Implications of Relative Coordination
In contrast to our previous study of N-24s,2 we found that circadian phase demonstrates a striking intrasubject variability in the rate of drift across the 24-h day with differences greater than 1.5 h per day within a single individual (Figure 4). In most cases, the drift in circadian phase varied regularly consistent with the phenomenon of relative coordination (Figure 4) whereas a minority of subjects had a less consistent pattern. Among those demonstrating relative coordination there was notable intersubject variability in both the amplitude and timing of relative coordination (Figure 4, Table 2).
The intersubject and intrasubject variability in relative coordination has treatment implications for this circadian rhythm sleep disorder (CRSD). Although oral melatonin is capable of entraining the biological clock and successfully treating N-24s,8,16–21 it is likely that the additional resetting effects of weak time cues account for some of the success of low-dose melatonin treatment.17,18,20,21 Unfortunately, the weak time cues would be expected to entrain N-24s to an abnormally late (or early) time: inspection of Figure 2C and Figure 4 show that the slowest drift rates (for those individuals with a tendency to drift to a later time) occur when the MO occurs after midnight. Therefore, most N-24s are likely to entrain to the weak time cues when their MO is after midnight which is quite delayed. Similarly, Figure 2C and Figure 4 show that the slowest drift rates (for those with a tendency to drift to an earlier time) occur when the MO occurs before 20:00. Therefore, entrainment at the normal time does not maximize the potential “assistance” provided by these weak time cues. In order to determine when and in what direction the weak time cues reset the clock (and therefore know when they might help or deter entrainment) it is necessary to assess circa-dian phase frequently (i.e., at least every 1-2 weeks).
The weak time cues that cause relative coordination appear to cause both phase delays (shifts in the clock to a later hour) and phase advances (shifts in the clock to an earlier hour). Indeed, we observed the same pattern of relative coordination in N-24s regardless of whether circadian phase tended to drift to a progressively later or earlier time. In both groups the MO tended to drift to a later time more rapidly (or to an earlier time more slowly) when the MO occurred during the daytime and tended to drift to a later time more slowly (or to an earlier time more rapidly) when the MO occurred at night and individuals from both groups demonstrated transient entrainment.
Perhaps the most important implication of relative coordination and transient entrainment is in the diagnosis of this disorder. Individuals who demonstrate transient entrainment might easily be misdiagnosed as entrained if circadian phase is not assessed for a sufficient period of time. Inspection of Figure 1 indicates that it may be necessary to assess observed circadian phase for more than 3 months in some cases before a conclusive diagnosis can be made.
Assessment of Drift in Circadian Phase in N-24s
Our estimate of average drift rate of circadian phase in the current sample is likely closer to the population average in N-24s than that of our previous studies2 because we have almost doubled the sample size and have observed the subjects for a relatively long period of time across an integral number of circadian beat cycles (drift of circadian phase, as measured by the MO, across 24 h). It is possible that our current estimate (0.39 ± 0.29 h/day) is shorter than our previous estimate of the average drift rate (0.55 ± 0.31 h/day)2 because the latter did not include individuals who lacked entrainment but had overall drift rates close to 0 h per day (i.e., individuals with significant relative coordination or transient entrainment).
This is not to say that the average drift rates that we observed are equivalent to the “intrinsic” circadian period (i.e., the output of the hypothalamic circadian pacemaker undistorted by the resetting effects of environmental time cues).41,42 The mere presence of relative coordination is evidence that these drift rates were affected by environmental time cues. Therefore, individuals with this disorder cannot be said to be “free-running” in the strictest sense of the word because the drift in circadian phase that we observed does not reflect the “endogenous period” that is seen when an organism is studied “free of environmental time cues.”24 Similarly, such subjects should not be said to have “free-running disorder.” Finally, the fact that in some instances there was little to no drift in circadian phase over many weeks to months clearly has implications for the calculation of the overall rate of drift as discussed in the limitations section below.
Sex Differences
Although the dataset is still relatively small, we found a trend for the female subjects to have greater amplitude of relative coordination than the male subjects. This could be because the women were more responsive to the weak time cues, had greater exposure to the weak time cues, and/or had shorter intrinsic periods that were more within the range of influence of the 24-h cues. In support of the latter possibility was the fact that we found a strong trend for the female subjects to have slower drift rates and that women were found to have shorter circadian periods than men in a large cohort of sighted subjects studied under forced desynchrony.43
The sex difference we found here is consistent with the difference in entrainment status that we previously documented between male and female blind subjects: in a retrospective analysis of 46 totally blind individuals (21 females and 25 males) we found that none of the men were entrained whereas 25% of the women were entrained.44 Similarly, all four of the subjects previously discussed (subjects 5, 14, 15, and 18) who demonstrated transient entrainment were female. Both of these findings are again consistent with the blind women being more sensitive to weak time cues, having greater exposure to such cues, and/or having shorter circadian periods. Further research is warranted to determine the clinical significance of sex effects in this and other CRSDs.
Age Differences
Average drift rate was found to increase with age, similar to our previous report in N-24s.40 For each additional year the rate of drift was 0.008 h per day faster. Such a change in drift rate is small but may have implications for the timing of the biological clock in sighted individuals.43,45 For example, Wright et al.45 found that a 1-h change in circadian period in sighted subjects was associated with as much as a 5.25-h change in the interval between the dim light melatonin onset and habitual sleep time.
Our age findings contrast somewhat with results in sighted individuals studied under conditions of forced desynchrony in which no effect of age on circadian period was found.43,46 It is possible that differences in the proportions or ages of women between the two study populations account for the different age findings.
Medication Differences
The subjects were taking a variety of medications and some of these might have influenced the changes in circadian drift rate we observed (e.g., due to circadian resetting effects of serotonergic medications).47 As noted previously, it is also possible that chronic circadian misalignment could result in adverse metabolic, cardiovascular, endocrine, cognitive, and emotional consequences in N-24s.14,15,36 We therefore speculate that some of the medications taken by this cohort might have been prescribed in response to signs or symptoms that were at least partially related to these individuals' lack of entrainment.
Changes in Relative Coordination With Time
Subject 5 was followed for over 4 years and provides a relatively unique assessment of circadian rhythms in a N-24 over a long period of time.3 As seen in Figure 1B, the magnitude of relative coordination decreased with time. The possible explanations include a change in her intrinsic circadian period, a loss of response to the weak time cues, or a loss of exposure to the weak time cues. Although it is possible that her exposure to the weak time cues decreased gradually over the 4 years of study this seems less likely. A lengthening of her intrinsic period with time could explain the results because a longer intrinsic period should be less influenced by the 24-h weak time cues (further outside the “range of entrainment”). Her average drift rate did indeed significantly increase with time but this could simply reflect the decreased influence of the 24-h weak time cues associated with a corresponding decrease in relative coordination. Although the magnitude of relative coordination changed with time, the timing (clock hour) of accelerations and slowing in the rate of drift were stable across the more than 4 years of study (Figure 4).
Rest/Activity Rhythms
There was a good correlation between the nonentrained endogenous circadian rhythms measured using melatonin and the non-24-h rest/activity cycle measured using actigraphy. This replicates the findings of others demonstrating that the rest/activity cycle of N-24s are significantly influenced by the timing of their circadian pacemakers11 and it suggests that the subjects were, at least to some extent, sleeping according to biological time. This has diagnostic implications because it may be possible using actigraphy alone to determine whether a blind individual is entrained or not. It may also be possible to determine whether circadian phase is drifting to a progressively later time, and therefore evening melatonin administration is required to provide corrective phase advances; or whether circadian phase is drifting to a progressively earlier time, and therefore morning melatonin is required to provide the necessary phase delays. Such an approach would certainly require refinement because two of our subjects showed multiple non-24-h actigraphy rhythms including one individual whose actigraphy rhythm appeared to drift to a progressively earlier time while her average melatonin onset drifted to a later time. It is likely that many weeks to months of data are often necessary to provide an accurate measure of the drift in circadian phase.
It should be noted that in all 18 subjects the amplitude of the 24-h rest/activity rhythm was significantly greater than that of the non-24-h rhythm (Table 3). This indicates that most of the subjects were primarily maintaining a 24-h rest/activity schedule despite their lack of entrainment to the 24-h day. Therefore it is unlikely that measurement of sleep timing alone will be able to accurately diagnose N-24s (e.g., visual inspection of sleep diary data is likely to have a high false negative rate). A physiological assessment of entrainment status is necessary for accurate diagnosis of this CRSD whether it be directly through serial determinations of circadian phase or possibly indirectly via spectral analysis of longitudinal actigraphy data.
Limitations
Although this was a relatively large cohort of N-24s who were studied over very long periods of time, the sample size was still small. Firm conclusions regarding the influence of such variables as sex, age, duration of blindness, and medications on relative coordination and circadian drift rate should not be made until larger sample sizes are investigated using a multivariate analysis where all of these variables can be controlled for simultaneously.
It could also be argued that even an overall average drift rate in circadian phase cannot be calculated in the presence of relative coordination or that, at the very least, periods of transient entrainment should be excluded from the analysis. However, we do not think it is possible to edit the data in such a way as to remove the influence of the time cues that were causing the relative coordination because we were not measuring either the strength or timing of those cues and indeed have not even positively identified them.
Instead we attempted to provide a very gross measure of the average rate of drift of circadian phase while also quantifying the intrasubject variability in this rate of drift. We think such a measure is still clinically useful because it tells a clinician how long, on average, it takes circadian phase to drift 24-h in an N-24. In this way, our methodology for calculating average drift rate is analogous to calculating the average number of miles traveled per day in a cross-country trip: such calculations are useful in determining how long it will take to make the journey but obscure any variability in the distance traveled each day or any periods of little to no travel.
CONCLUSIONS
There is significant heterogeneity in the physiological presentations of non-24-h disorder in the blind. This variability occurs both between and within N-24s and this likely reflects differences in their exposure or response to environmental time cues. Notably, some individuals demonstrate periods of transient entrainment where the disorder may appear to remit for lengthy periods of time.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Emens is the recipient of an Investigator Initiated grant from Forest Labs. Dr. Lewy is coinventor on several process patents owned by OHSU and currently not licensed to any company. Dr. Lewy is a consultant for Servier and has consulted in the past for Takeda. The remaining authors do not have any disclosures. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
R01 EY018312-09A1, R01 HD42125, and R01 AG21826 (AJL); Sleep Research Society Foundation Gillin Award, K23RR017636, and NARSAD Young Investigator Award (JSE); and MO1 RR000334 and UL1 RR024120.
REFERENCES
- 1.Lewy AJ, Newsome DA. Different types of melatonin circadian secretory rhythms in some blind subjects. J Clin Endocrinol Metab. 1983;56:1103–7. doi: 10.1210/jcem-56-6-1103. [DOI] [PubMed] [Google Scholar]
- 2.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–34. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 3.Klein T, Martens H, Dijk DJ, Kronauer RE, Seely EW, Czeisler CA. Circadian sleep regulation in the absence of light perception: chronic non-24-hour circadian rhythm sleep disorder in a blind man with a regular 24-hour sleep-wake schedule. Sleep. 1993;16:333–43. doi: 10.1093/sleep/16.4.333. [DOI] [PubMed] [Google Scholar]
- 4.Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82:3763–70. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- 5.Klerman EB, Rimmer DW, Dijk D, Kronauer RE, Rizzo JF, III, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;43:R991–6. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 6.Lockley SW, Skene DJ, Butler LJ, Arendt J. Sleep and the activity rhythms are related to circadian phase in the blind. Sleep. 1999;22:616–23. doi: 10.1093/sleep/22.5.616. [DOI] [PubMed] [Google Scholar]
- 7.Leger D, Guilleminault C, Defrance R, Domont A, Paillard M. Prevalence of sleep/wake disorders in persons with blindness. CliniSci. 1999;97:193–9. [PubMed] [Google Scholar]
- 8.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–7. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 9.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. Sleep. 2007;30:1460–83. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockley SW, Skene DJ, Butler LJ, Arendt J. Sleep and activity rhythms are related to circadian phase in the blind. Sleep. 1999;22:616–23. doi: 10.1093/sleep/22.5.616. [DOI] [PubMed] [Google Scholar]
- 12.Leger D, Guilleminault C, Santos C, Paillard M. Sleep/wake cycles in the dark: sleep recorded by polysomnography in 26 totally blind subjects compared to controls. Clin Neurophysiol. 2002;113:1607–14. doi: 10.1016/s1388-2457(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 13.Emens J, Lewy AJ, Laurie AL, Songer JB. Rest-activity cycle and melatonin rhythm in blind free-runners have similar periods. J Biol Rhythms. 2010;25:381–4. doi: 10.1177/0748730410379080. [DOI] [PubMed] [Google Scholar]
- 14.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657–62. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 17.Lewy AJ, Bauer VK, Hasler BP, Kendall AR, Pires LN, Sack RL. Capturing the circadian rhythms of free-running blind people with 0.5 mg melatonin. Brain Res. 2001;918:96–100. doi: 10.1016/s0006-8993(01)02964-x. [DOI] [PubMed] [Google Scholar]
- 18.Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19:649–58. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- 19.Hack LM, Lockley SW, Arendt J, Skene DJ. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J Biol Rhythms. 2003;18:420–9. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 20.Lewy AJ, Emens JS, Bernert RA, Lefler BJ. Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circa-dian phase of treatment initiation: clinical implications. J Biol Rhythms. 2004;19:68–75. doi: 10.1177/0748730403259670. [DOI] [PubMed] [Google Scholar]
- 21.Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22:1093–106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- 22.Emens JS, Lewy AJ, Lefler BJ, Sack RL. Relative coordination to unknown “weak zeitgebers” in free-running blind individuals. J Biol Rhythms. 2005;20:159–67. doi: 10.1177/0748730404273294. [DOI] [PubMed] [Google Scholar]
- 23.Wever RA. Light effects on human circadian rhythms. A review of recent Andechs experiments. J Biol Rhythms. 1989;4:161–86. [PubMed] [Google Scholar]
- 24.Moore-Ede M, Sulzman FM, Fuller CA. The clocks that time us. Cambridge, MA: Harvard University Press; 1982. [Google Scholar]
- 25.Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1077–84. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- 26.Buxton OM, Lee CW, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R714–24. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 27.Klerman EB. Non-photic effects on the circadian system: results from experiments in blind and sighted individuals. In: Honma K, Honma S, editors. Zeitgebers, entrainment and masking of the circadian system. Sapporo: Hokkaido University Press; 2001. pp. 155–69. [Google Scholar]
- 28.Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18:170–8. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- 29.Gordijn M, Beersma D, Korte HJ, Van Den Hoofdakker RH. Effects of light exposure and sleep displacement on dim light melatonin onset. J Sleep Res. 1999;8:163–74. doi: 10.1046/j.1365-2869.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoban TM, Lewy AJ, Sack RL, Singer CM. The effects of shifting sleep two hours within a fixed photoperiod. J Neural Trans. 1991;85:61–8. doi: 10.1007/BF01244658. [DOI] [PubMed] [Google Scholar]
- 31.Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 32.Klerman EB, Shanahan TL, Brotman DJ, et al. Photic Resetting of the Human Circadian Pacemaker in the Absence of Conscious Vision. J Biol Rhythms. 2002;17:548–55. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- 33.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 34.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 35.Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol Int. 2009;26:474–93. doi: 10.1080/07420520902821077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emens JS, Lewy AJ, Kinzie MJ, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Emens J, Lewy AJ, Rough JN, Songer JB. Sub-clinical dysphoria correlates with phase-delayed circadian misalignment in healthy individuals. Sleep. 2009;32:A355. [Google Scholar]
- 38.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theoret Biol. 1978;72:131–60. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 39.Kripke DF, Clopton P, Marler MR, Youngstedt SD, Elliot JA. PRC bisection tests. Chronobiol Int. 2003;20:1117–23. doi: 10.1081/cbi-120025535. [DOI] [PubMed] [Google Scholar]
- 40.Kendall AR, Lewy AJ, Sack RL. Effects of aging on the intrinsic circa-dian period of totally blind humans. J Biol Rhythms. 2001;16:87–95. doi: 10.1177/074873040101600110. [DOI] [PubMed] [Google Scholar]
- 41.Campbell S. Is there an intrinsic period of the circadian clock? Science. 2000;288:1174. [PubMed] [Google Scholar]
- 42.Czeisler CA, Dijk DJ, Kronauer RE, et al. Is there an intrinsic period of the circadian clock? Response. Science. 2000;288:1174–5. [PubMed] [Google Scholar]
- 43.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci. 2011;108:15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewy A, Yuhas K, Emens J, et al. Are the circadian rhythms of blind adult males less sensitive to social cues than females? Sleep. 2007:30A63. [Google Scholar]
- 45.Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–77. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 47.Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Ann Med. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]


