Abstract
Study Objectives:
Examine sleep restriction's effects on weight gain, daily caloric intake, and meal timing.
Design:
Repeated-measures experiments assessing body weight at admittance and discharge in all subjects (N = 225) and caloric intake and meal timing across days following 2 baseline nights, 5 sleep restriction nights and 2 recovery nights or across days following control condition nights in a subset of subjects (n = 37).
Setting:
Controlled laboratory environment.
Participants:
Two hundred twenty-five healthy adults aged 22-50 y (n = 198 sleep-restricted subjects; n = 31 with caloric intake data; n = 27 control subjects; n = 6 with caloric intake data).
Interventions:
Approximately 8-to-1 randomization to an experimental condition (including five consecutive nights of 4 h time in bed [TIB]/night, 04:00-08:00) or to a control condition (all nights 10 h TIB/night, 22:00-08:00).
Measurements and Results:
Sleep-restricted subjects gained more weight (0.97 ± 1.4 kg) than control subjects (0.11 ± 1.9 kg; d = 0.51, P = 0.007). Among sleep-restricted subjects, African Americans gained more weight than Caucasians (d = 0.37, P = 0.003) and males gained more weight than females (d = 0.38, P = 0.004). Sleep-restricted subjects consumed extra calories (130.0 ± 43.0% of daily caloric requirement) during days with a delayed bedtime (04:00) compared with control subjects who did not consume extra calories (100.6 ± 11.4%; d = 0.94, P = 0.003) during corresponding days. In sleep-restricted subjects, increased daily caloric intake was due to more meals and the consumption of 552.9 ± 265.8 additional calories between 22:00-03:59. The percentage of calories derived from fat was greater during late-night hours (22:00-03:59, 33.0 ± 0.08%) compared to daytime (08:00-14:59, 28.2 ± 0.05%) and evening hours (15:00-21:59, 29.4 ± 0.06%; Ps < 0.05).
Conclusions:
In the largest, most diverse healthy sample studied to date under controlled laboratory conditions, sleep restriction promoted weight gain. Chronically sleep-restricted adults with late bedtimes may be more susceptible to weight gain due to greater daily caloric intake and the consumption of calories during late-night hours.
Citation:
Spaeth AM; Dinges DF; Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. SLEEP 2013;36(7):981-990.
Keywords: Caloric intake, gender, late-night eating, macronutrients, meal timing, race, sleep restriction
INTRODUCTION
The 2004-2007 National Health Interview Survey revealed that approximately 28.3% of adults report sleeping 6 h or less per night,1 and other studies have indicated that the prevalence of short sleepers (adults who report an average of ≤ 6 h of sleep within a 24-h period) has significantly increased in recent decades.1,2 Reported associations between short sleep duration and energy homeostasis suggest the former may be a risk factor for a higher body mass index (BMI)3–5 and prospective cohort studies have identified short sleep duration as a predictor of greater weight gain.6–9 This relationship may be stronger for men than for women.10,11 However, no controlled laboratory studies have experimentally examined the effect of sleep restriction (SR) on weight gain in a large, diverse sample of men and women.
Laboratory studies have elucidated possible behavioral and physiological mechanisms underlying the relationship between sleep duration and weight gain. Spiegel and colleagues12 found that subjects undergoing 2 nights of SR (4 h time in bed [TIB]/night) with controlled energy intake via an intravenous glucose infusion exhibited increased levels of ghrelin (an orexigenic hormone released from the stomach) and decreased levels of leptin (an anorexigenic hormone released from adipocytes). These neuroendocrine changes were accompanied by significant increases in self-reported ratings of hunger and appetite, specifically for foods with high carbohydrate content.12 Other experiments have also shown that sleep loss increased ghrelin levels and decreased leptin in calorically-restricted humans.13,14 By contrast, in laboratory studies using ad libitum food access, which mimics a more natural environment, sleep loss was associated with either no change in ghrelin or leptin or an increase in leptin levels.15–20 Such ad libitum experiments, however, demonstrated that sleep loss was associated with increased caloric intake.15,21–23 These investigations collectively indicate that humans compensate (or over-compensate) for sleep loss by increasing energy intake.
Sleep restriction also affects macronutrient intake and meal frequency. Adolescents who habitually slept less than 8 h per night consumed a higher proportion of calories from fat and a lower proportion of calories from carbohydrates and protein than those who slept 8 h or more per night.24 In adults, SR has been associated with increased craving for foods high in carbohydrate content,12 greater consumption of calories from carbohydrates16 or fats,22 and increased caloric intake derived from snacks.16,25
In addition to daily caloric intake, meal timing is an important contributor to weight gain.26,27 Baron and colleagues28 examined differences in sleep, eating, and weight between “normal sleepers” (sleep midpoint < 05:30) and “late sleepers” (sleep midpoint > 05:30) and found that late sleepers exhibited a shorter sleep duration, consumed more calories at dinner and after 20:00, consumed more fast food and full-calorie soda, and had a higher BMI compared to normal sleepers. In a group of healthy inpatients, individuals who ate during late-night/early morning (23:00-05:00) consumed more calories per day and gained more weight than non-nighttime eaters.29 In an outpatient weight-loss effectiveness study, participants who were late eaters (lunch after 15:00) lost less total weight and displayed slower weight loss than early eaters (lunch before 15:00).30 Animal studies have also shown a relationship between meal timing and weight gain: Circadian Locomotor Output Cycles Kaput (CLOCK) mutant mice exhibited an attenuated diurnal feeding rhythm and were hyper-phagic and obese.31,32 Moreover, mice exposed to light at night consumed more food during the light phase and had increased body mass compared with mice in a standard light/dark cycle, despite equivalent levels of caloric intake and total daily activity.33
The aforementioned lines of evidence suggest that sleep loss potentiates weight gain; however, few controlled experiments have manipulated sleep duration and objectively measured weight gain and ad libitum caloric intake. To address this deficiency, we experimentally tested the hypothesis that SR produces weight gain in a large sample of diverse healthy adults using a control condition. We also explored how SR affects possible contributors to weight gain and tested the hypotheses that SR increases daily caloric intake, increases meal frequency, and delays meal timing.
METHODS
The studies were approved by the Institutional Review Board of the University of Pennsylvania and all subjects were compensated for their participation.
Subjects and Protocol
Two-hundred twenty-five healthy individuals, aged 22-50 y, were recruited in response to study advertisements. They reported habitual nightly sleep durations between 6.5 h and 8.5 h, habitual bedtimes between 22:00 and 00:00, and habitual morning awakenings between 06:00 and 09:00. They had no evidence of habitual napping, no sleep disturbances (i.e., no complaints of insomnia, daytime sleepiness, or other sleep-wake disturbances), and an absence of extreme morningness or extreme eveningness, as assessed by questionnaire.34 They were free of acute and chronic medical and psychological conditions, as established by interviews, clinical history, questionnaires, physical examinations, and blood (including a fasting blood glucose test) and urine tests. Subjects were monitored at home with actigraphy, sleep-wake diaries, and time-stamped call-ins to assess bedtime and wake time during the week prior to the in-laboratory phase and the week after the laboratory phase.
Subjects were nonsmokers and had a BMI ranging between 19-30. They did not participate in shift work, transmeridian travel, or irregular sleep/wake routines in the 60 days prior to the study. Sleep disorders were excluded by a night of laboratory polysomnography and oximetry measurements. Subjects were not permitted to use caffeine, alcohol, tobacco, and medications (except oral contraceptives) in the week before the laboratory experiment, as verified by blood and urine screenings.
Subjects participated in one of five protocols in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania. Subjects were studied for 12, 14, or 18 consecutive days continuously with daily clinical checks of vital signs and symptoms by nurses (with an independent physician on call). Subjects were randomized as a group (n = 4 to 5 per group) to either the SR or control condition. In all five protocols, the SR condition consisted of two initial baseline nights of 10 h or 12 h TIB per night (22:00-08:00/10:00) followed by 5 nights of sleep restricted to 4 h TIB per night (04:00-08:00). A subset of subjects experienced 2 nights of recovery sleep (12 h TIB, 22:00-10:00) following sleep restriction. SR consisting of 4 h TIB for 5 consecutive nights was selected because this degree of sleep loss produces cumulative neurobehavioral deficits in most healthy adults35–39 and is within the range of sleep loss that occurs as a result of lifestyle factors.1,2,40,41
The protocol days for the SR condition are labeled as follows throughout the manuscript: baseline (BL, the day following the first night of baseline sleep with a 22:00 bedtime); extended wakefulness (EW, the day following the second night of baseline sleep with a 04:00 bedtime); sleep restriction days 1-4 (SR1-4, days following SR with a 04:00 bedtime); sleep restriction day 5 (SR5, the fifth day following sleep restriction with a 22:00 bedtime) and recovery days 1-2 (R1-2, days following recovery sleep with a 22:00 bedtime. The control condition involved the same procedures as the SR condition in each protocol, except that subjects were allowed 10 h TIB every night (22:00-08:00) during the in-laboratory stay.
During the in-laboratory phase of the study, subjects were not permitted to leave the laboratory. In both the SR and control conditions, subjects were ambulatory but were not allowed to exercise. Subjects were permitted to watch television, read, play video or board games, and perform other sedentary activities between test bouts (which were completed while sitting at a computer).
Subjects wore a wrist actigraph throughout the in-laboratory protocol. On certain protocol days, subjects wore ambulatory electroencephalography (EEG) and electrocardiography (ECG) recording equipment for 24 h intervals. The light levels in the laboratory were held constant at < 50 lux during scheduled wakefulness and < 1 lux during scheduled sleep periods. Ambient temperature was maintained between 22°-24°C. Subjects were behaviorally monitored by trained staff continuously throughout the protocol to ensure adherence.
Procedure and Measurements
Body Weight
Body weight was measured in N = 225 subjects (n = 198 sleep-restricted subjects and n = 27 control subjects). Of the control subjects (age 31.9 ± 8.4 y; BMI 25.0 ± 3.1 [mean ± standard deviation]), n = 12 (44%) were female and n = 17 (63%) were African American. Of the sleep-restricted subjects (age 31.3 ± 7.9 y; BMI 24.8 ± 3.3), n = 89 (45%) were female and n = 116 (59%) were African American. During a physical examination 6-7 days prior to the in-laboratory phase of the study, and upon admittance to and discharge from the in-laboratory protocol, nurses measured each subject's height and weight (while subjects wore minimal clothing and no shoes) at the Clinical and Translational Research Center (CTRC) at the Hospital of the University of Pennsylvania (HUP) using the same calibrated scale. All subjects were weighed during the physical examination between 10:00-12:00. The majority of subjects (n = 183, 81%) were weighed between 13:00-15:00 during admittance and discharge from the in-laboratory phase of the study. Of the remaining subjects, n = 26 (12%) were weighed between 09:00-12:00 during admittance and discharge, and n = 16 (7%) were weighed between 14:00-16:00 during admittance and discharge. In all cases, subjects were not fasted during the weigh-in days and they had access to a restroom for optional voiding before being weighed.
Caloric Intake
In order to determine contributors to weight gain, caloric in-take was measured in a subset of subjects (n = 31 sleep-restricted subjects: 52% female, 65% African American, age 34.4 ± 9.2 y, BMI 25.2 ± 3.7 and n = 6 control subjects: 33% female, 67% African American, age 34.0 ± 9.8 y, BMI 25.7 ± 3.1). All sleep-restricted subjects experienced 2 nights of baseline sleep followed by 5 nights of sleep restriction. Nineteen of the 31 sleep-restricted subjects experienced 2 nights of recovery sleep following SR. Control subjects experienced 10 h TIB per night for each night of the protocol.
Food/Drink Timing and Availability
Subjects selected their meals/snacks by choosing from various menu options, selecting additional food/drink available in the kitchen within the laboratory suite (which included a refrigerator, microwave, and toaster oven) and by making requests to the monitors and study coordinator. In order to ensure that subjects were provided sufficient time to eat each day, three 30- to 45-min opportunities were specified in the protocol during days with a 22:00 bedtime (09:00, 12:35, and 18:30) and one additional 30-min opportunity to eat was specified in the protocol during days with a 04:00 bedtime (00:30). In addition to these specified meal times, subjects were also allowed to consume food/drink at any time during the protocol other than when they were completing neurobehavioral tests. During a typical day, in addition to the meal times specified in the protocol, subjects could consume food/drink from 09:45-10:00, 11:05-12:00, 13:10-14:00, 14:30-16:00, 16:30-16:45, 17:30-18:00, 19:20-20:00, 20:30-22:00, 22:30-00:00, 01:15-02:00, and 02:30-03:50. Subjects were never told that they had to eat/drink and they were instructed to eat/drink whenever they wanted as long as it did not interfere with testing times. Subjects were also instructed that they could eat what they ordered or could select from other foods available in the laboratory kitchen and that they should eat as much (or as little) as they preferred. Subjects retrieved their own food/drink from the kitchen inside the laboratory suite whenever they wanted to eat/drink and could eat at a table in the common area or privately in their bedrooms.
Food/Drink Measurement
All food was weighed and recorded prior to being provided to subjects. To enhance the measurement accuracy of each food's weight, food was provided in individual containers (for example, a dinner consisting of chicken, peas, and rice was provided in three separate containers). Each day, a detailed description of the items and the amount consumed and intake time was recorded by trained monitors. Additionally, any food/ drink that was left over after each meal was weighed and recorded. The intake data were entered into The Food Processor SQL program (ESHA Research, Salem, OR), a validated42 professional nutrition analysis software and database program that provides components of food/drink intake including calories and macronutrients.
Data Analysis
Between-subjects analyses of variance (ANOVAs) (with study entry BMI, age, race, and gender as covariates) compared weight changes between control subjects and sleep-restricted subjects. Between-subjects ANOVAs (with study entry BMI and age as covariates) compared weight changes between gender and race groups. Repeated- measures ANOVAs compared caloric intake, macronutrients, and meal timing across protocol days. Only 19 sleep-restricted subjects were included in the analyses examining caloric intake during days following recovery sleep. Post hoc comparisons were performed with paired t-tests using the False Discovery Rate43 to account for multiple comparisons in order to examine differences between BL, SR, and R days. Effect sizes were calculated using Cohen's d44 (small, d = 0.2; medium, d = 0.5; large, d = 0.8). Correlation analysis between weight gain and caloric intake was performed using Spearman's rho.
RESULTS
Weight Change
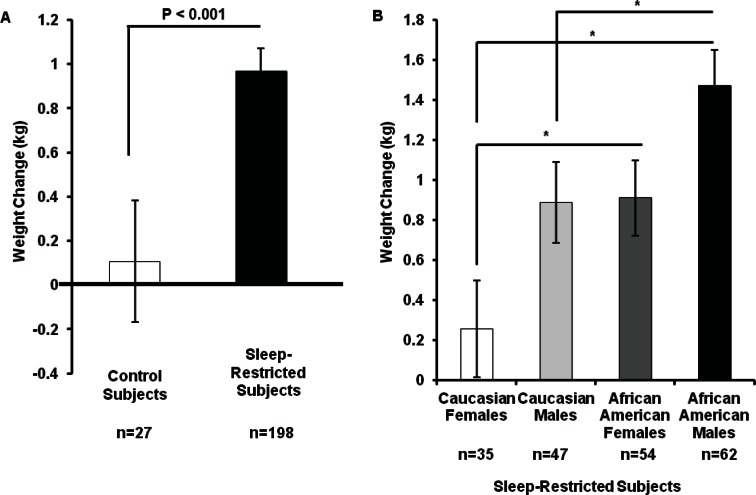
To examine if subjects were weight-stable at the start of the in-laboratory phase of the study, each subject's weight during admittance was compared with his/her weight during the physical examination (approximately a 1 week interval). The change in weight was not significantly different between control subjects (-0.04 ± 0.82 kg [mean ± standard deviation]) and sleep-restricted subjects (0.09 ± 0.95 kg; P > 0.4) and neither group's change in weight was different from zero (Ps > 0.20). During the in-laboratory protocol, sleep-restricted subjects (0.97 ± 1.43 kg) gained significantly more weight than control subjects (0.11 ± 1.85 kg; F (1, 223) = 7.52, P = 0.007, d = 0.51; Figure 1A). The change in weight during the protocol was not different from zero (P > 0.71) for control subjects but was significantly different from zero for sleep-restricted subjects (P < 0.001). The same pattern was observed when using weight change as a percentage of admittance body weight and BMI change as dependent variables. Sleep-restricted subjects gained a larger percentage of admittance body weight (1.4 ± 2.0%; F (1, 223) = 7.40, P < 0.007) and exhibited a greater increase in BMI (0.33 ± 0.49; F (1, 223) = 8.42, P < 0.004) than control subjects (percentage of admittance weight change: 0.2 ± 2.6%; BMI change: 0.03 ± 0.63). Sleep-restricted subjects whose caloric intake was monitored (n = 31) gained 0.52 ± 1.60 kg during the protocol and control subjects whose caloric intake was monitored (n = 6) lost 0.53 ± 1.16 kg during the protocol.
Figure 1.
Effect of sleep loss on weight gain. (A) Subjects were healthy adults aged 22-50 y with a body mass index ranging between 19-30. Sleep-restricted subjects gained significantly more weight than control subjects (d = 0.51). (B) Among sleep-restricted subjects, African Americans gained more weight than Caucasians (d = 0.37) and males gained more weight than females (d = 0.38). Data expressed as mean ± standard error of the mean, *P < 0.05.
Among sleep-restricted subjects there were significant main effects for gender and race; males gained more weight than females (F (1, 192) = 8.29, P = 0.004, d = 0.37) and African Americans gained more weight than Caucasians (F (1, 192) = 9.10, P = 0.003, d = 0.38). African American males showed the most weight gain, Caucasian females showed the least weight gain, and Caucasian males and African American females showed intermediate weight gain (post hoc analyses illustrated in Figure 1B). There were no sex or race differences in weight change in control subjects (Ps > 0.10). The same pattern was observed when using weight change as a percentage of admittance body weight and BMI change as dependent variables. Among sleep-restricted subjects, African Americans gained a larger percentage of admittance body weight (1.7 ± 2.2%; F (1, 223) = 9.85, P = 0.002) and exhibited a greater increase in BMI (0.40 ± 0.52; F (1, 223) = 9.18, P = 0.003) than Caucasians (percentage of admittance weight change: 0.94 ± 1.8%; BMI change: 0.22 ± 0.42) and males gained a larger percentage of admittance body weight (1.6 ± 2.0%; F (1, 223) = 5.38, P = 0.02) and exhibited a greater increase in BMI (0.38 ± 0.50; F (1, 223) = 4.96, P = 0.03) than females (percentage of admittance weight change: 1.11 ± 1.96%; BMI change: 0.26 ± 0.47).
Caloric Intake
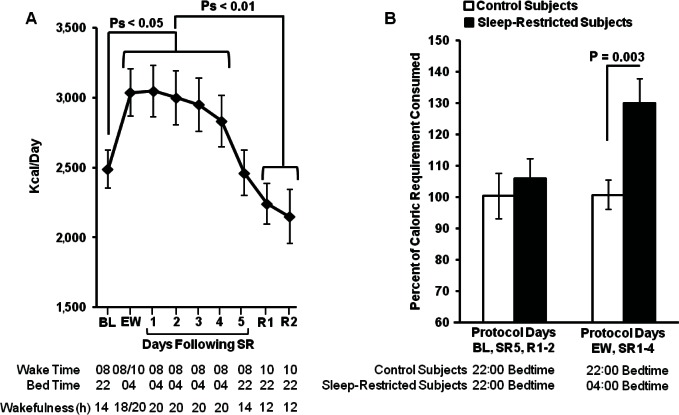
In control subjects, caloric intake did not vary significantly across protocol days (P = 0.09). By contrast, in sleep-restricted subjects, caloric intake varied significantly across BL and SR days (F (6, 180) = 7.49, P < 0.001) and across BL, SR, and R days (F (8, 144) = 6.79, P < 0.001). Subjects consumed more calories during days when bedtime was delayed until 04:00 (EW, SR1-4; Figure 2A) compared to BL (post hoc analyses, Ps < 0.05) and recovery days (post hoc analyses, Ps < 0.01). On days when bedtime and hours spent awake were comparable (BL and SR5), caloric intake did not significantly differ (P = 0.79).
Figure 2.
Caloric intake by day of protocol. (A) Sleep-restricted subjects consumed significantly more calories during days when bedtime was delayed to 04:00 (EW-SR4) compared to days when bedtime was 22:00 (BL, R1-2). Caloric intake did not differ between BL and SR5 (when waking hours were equivalent and bedtime was 22:00). Caloric intake did not differ between BL and each recovery day (R1-2). (B) Sleep-restricted subjects and control subjects did not differ in caloric intake during days when both groups had a 22:00 bedtime (BL, SR5, R1-2). Sleep-restricted subjects consumed more calories than control subjects during days when they had a 04:00 delayed bedtime (EW, SR1-4; d = 0.94). Data expressed as mean ± standard error of the mean. BL, baseline; EW, extended wakefulness; R, recovery; SR, sleep restriction.
In order to compare caloric intake between sleep-restricted subjects and control subjects as well as to examine the relationship between caloric intake and weight change, caloric intake was calculated as a percentage: actual daily caloric intake / estimated daily caloric intake required for weight maintenance. Each subject's daily caloric intake required for weight maintenance was estimated using the Harris-Benedict equation45 for basal metabolic rate [(men = 66.4730 + (13.7516 * weight (kg)) + (5.0033 * height (cm)) – (6.755 * age (y)); women = 655.0955 + (9.5634 * weight (kg)) + (1.850 * height (cm)) – (4.676 * age (y))] multiplied by 1.4, which corresponds to a sedentary lifestyle representative of a laboratory study in which activity is limited.46
Average caloric intake was not significantly different between control and sleep-restricted subjects during days with a 22:00 bedtime (BL, SR5, R1-2; P = 0.58). However, sleep-restricted subjects consumed significantly more calories than control subjects during days when they had a delayed bedtime (EW, SR1-4; F (1, 31.03) = 3.26, d = 0.94, P = 0.003; see Figure 2B). In sleep-restricted subjects, average caloric intake during days with a delayed bedtime (EW-SR4) was significantly positively correlated with weight change (Spearman's rho = 0.64, P < 0.001).
Macronutrients
In sleep-restricted subjects, the number of grams consumed from each nutrient varied significantly across BL and SR days (protein: F (6, 180) = 5.74, P < 0.001; carbohydrates: F (6, 180) = 6.78, P < 0.001); fat: F (6, 180) = 4.70, P < 0.001) and across BL, SR, and R days (protein: F (8, 144) = 6.18, P < 0.001; carbohydrates: F (8, 144) = 5.23, P < 0.001); fat: F (8, 144) = 5.17, P < 0.001; Table 1). Consistent with increases in total caloric intake, sleep-restricted subjects consumed more grams of each nutrient during the first four delayed bedtime days (EW, SR 1-3) compared to baseline (post hoc analyses, Ps < 0.05) whereas on the fourth day following sleep restriction (SR4), when overall caloric intake remained greater than BL intake, only fat consumption was significantly greater than BL (P < 0.05; Table 1). Macronutrient consumption was not significantly different between BL and SR5 protocol days (Ps > 0.40). Macronutrient consumption during each recovery day was not significantly different from BL or SR5 intake (Ps > 0.10) but was lower than intake during delayed bedtime days (EW, SR1-4; post hoc analyses, Ps < 0.05; Table 1).
Table 1.
Mean ± standard deviation macronutrient content and meal patterns of caloric intake by protocol day
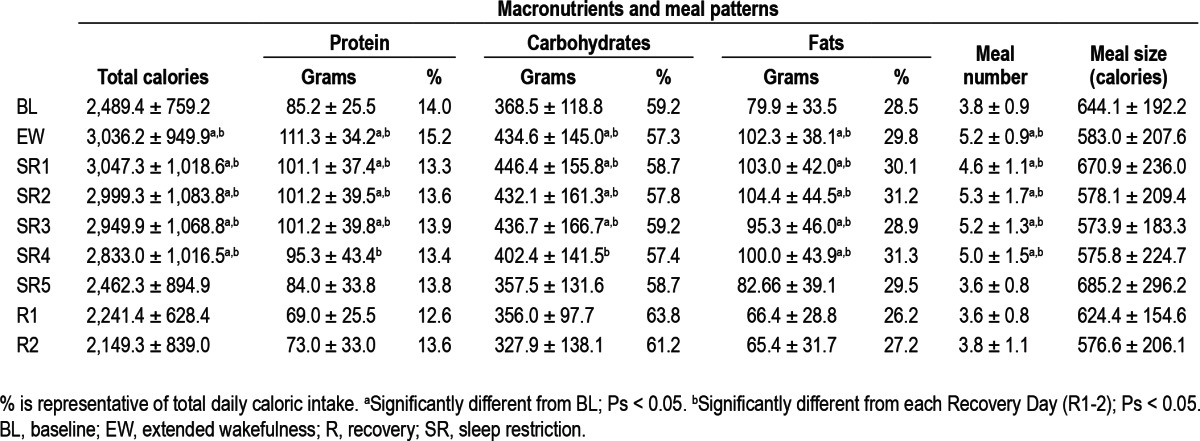
To control for changes in total caloric intake across protocol days, the percentage of daily caloric intake derived from protein, carbohydrates, and fat were also calculated and compared (Table 1). There were no significant differences in the percentage of calories from protein, carbohydrates, or fat across all protocol days.
Meal Patterns
The US Department of Labor considers 30 min sufficient for a ‘meal period’47; therefore, meals were considered as discrete episodes if there was a minimum of 30 min between intake bouts. The total number of meals varied across BL and SR days (F (6, 180) = 16.41, P < 0.001) and across BL, SR, and R days (F (8, 144) = 12.45, P < 0.001; Table 1). Compared with baseline, subjects consumed more meals during days when bedtime was delayed (EW, SR1-4; post hoc analyses, Ps < 0.01). The number of meals consumed during the fifth day following sleep restriction (SR5) did not differ from those consumed during BL (Ps > 0.10). Compared with each R day, subjects consumed more meals during days when bedtime was delayed (EW, SR1-4; post hoc analyses, Ps < 0.01). The number of meals consumed during each R day did not differ from the number of meals consumed during BL and SR5 (Ps > 0.30).
When examining average meal size, overall ANOVAs comparing meal size across BL and SR days (F (6, 180) = 3.16, P = 0.006) and across BL, SR, and R days (F (8, 144) = 2.86, P < 0.06) were significant (Table 1). However, post hoc analyses comparing EW, and SR1-5 days to BL and post hoc analyses comparing BL, EW, and SR1-5 days to each R day were not significant (Ps > 0.05; Table 1).
Meal Timing
Daily caloric intake was calculated for three time intervals: 08:00-14:59, 15:00-21:59 and 22:00-03:59 for BL and SR days. The first two time intervals were created by dividing the common waking hours across BL and SR protocol days into two equal 7-h intervals. The third time interval equaled the 6 h of wakefulness that occurred during delayed bedtime days (EW, SR1-SR4) but not during the baseline and SR5 protocol days. Recovery days were not included in these analyses due to a delayed wake time (10:00) that differed from the other protocol days.
Total caloric intake during 08:00-14:59 was lower during days following SR (SR1-5) compared to days following BL sleep (BL and EW; F (1, 30) = 4.23, P = 0.047; Figure 3A); however, calories consumed during 15:00-21:59 did not significantly differ between conditions (P = 0.096; Figure 3A). Caloric intake during 22:00-03:59 varied across days (F (4, 120) = 3.48, P = 0.01): this overall difference was due to a significant reduction in calories consumed between the first and second nights with a delayed bedtime (EW and SR1; post hoc analyses, P < 0.05, Table 2). Across all nights with a delayed bedtime, subjects chose to consume calories during each hour of the late-night interval as follows: 22:00-22:59: n = 19 (61%), 23:00-23:59: n = 13 (42%), 00:00-00:59: n = 25 (81%), 01:00-01:59: n = 23 (74%), 02:00-02:59: n = 25 (81%), and 03:00-03:59: n = 13 (42%); thus, intake was not limited to only the specified meal time (00:30), but occurred throughout the late-night interval. Notably, a majority of subjects voluntarily chose to consume calories during the late-night interval on each night: EW: n = 31 (100%), SR1: n = 26 (84%), SR2: n = 28 (90%), SR3: n = 29 (94%), SR4: n = 31 (100%).
Figure 3.
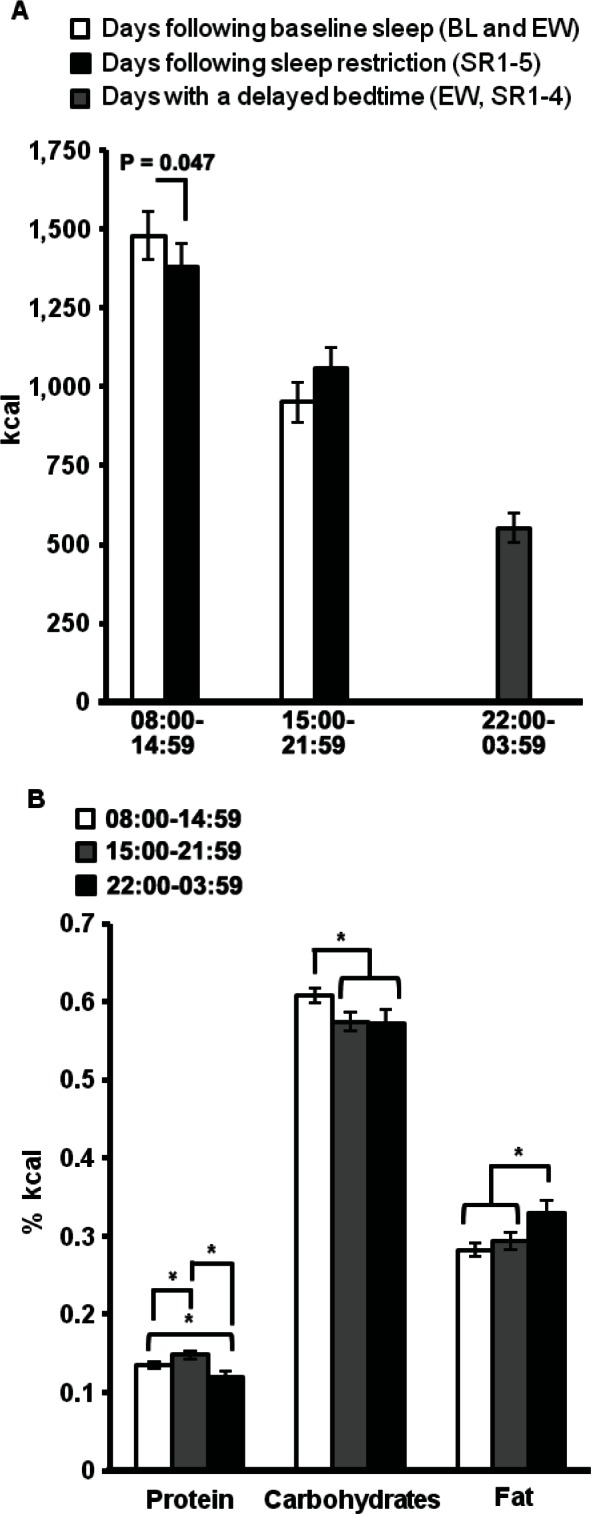
Effect of sleep loss on meal timing. (A) Subjects consumed significantly fewer calories from 08:00-14:59 on days following sleep restriction (SR1-5) compared to days following baseline sleep (BL and EW). During days with a delayed bedtime (EW, SR1-4), subjects consumed on average of 552.9 calories from 22:00-03:59. (B) The amount of calories derived from protein was significantly greater during 15:00-21:59 and was significantly reduced during 22:00-03:59 compared to the other two time intervals. Compared to the other two time intervals, the amount of calories derived from carbohydrates was significantly greater during 08:00-14:59 and the amount of calories derived from fat was significantly greater during 22:00-03:59. See text for explanation of time intervals. Data expressed as mean ± standard error of the mean, *P < 0.05. BL, baseline; EW, extended wakefulness; SR, sleep restriction.
Table 2.
Mean ± standard deviation timing of caloric intake by protocol day
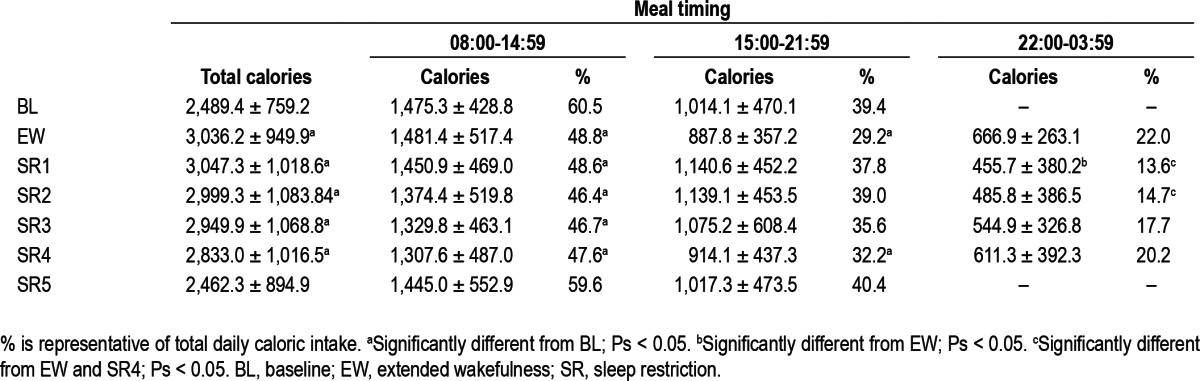
The percentage of daily caloric intake during each of the three time intervals was calculated to control for total caloric intake changes across protocol days. The percentage of calories consumed during the first two time intervals differed significantly across BL and SR days (08:00-14:59: F (6, 180) = 11.21, P < 0.001; 15:00-21:59: F (6, 180) = 4.53, P < 0.001; Table 2). Subjects consumed a significantly lower percentage of calories from 08:00-14:59 during delayed bedtime days compared to the day following baseline sleep (post hoc analyses, Ps < 0.05), and a lower percentage of calories from 15:00-21:59 during EW and SR4 days compared to the BL day (post hoc analyses Ps < 0.05). The percentage of calories consumed during the late-night time interval varied across SR days (22:00-03:59: F (4, 120) = 6.27, P < 0.001; Table 2). The percentage of calories consumed from 22:00-03:59 was greater during the first and fifth night of SR (EW and SR4) compared to the second and third nights of SR (SR1 and 2; post hoc analyses, Ps < 0.05, Table 2).
Macronutrients
The number of grams and the percentage of calories derived from protein, carbohydrate, and fat during each time interval were calculated and compared across protocol days. There were no significant differences in the macronutrient content (grams or percentage of calories) consumed during each time interval across protocol days (Ps > 0.05). The macronutrient content during each time interval was averaged across all days and also compared. The macronutrient content of each time interval differed significantly (protein: (F (2, 60) = 8.12, P = 0.001; carbohydrates: F (2, 60) = 3.48, P = 0.04; fat: F (2, 60) = 7.29, P = 0.001; Figure 3B). The amount of calories derived from protein was significantly greater during 15:00-21:59 and was significantly reduced during 22:00-03:59 compared to the other two time intervals (Ps < 0.05). Compared to the other two time intervals, the amount of calories derived from carbohydrates was significantly greater during 08:00-14:59 (Ps < 0.05) and the amount of calories derived from fat was significantly greater during 22:00-03:59 (Ps < 0.05).
DISCUSSION
In the largest sample of healthy adults studied to date under controlled laboratory conditions, sleep-restricted subjects gained more weight than control subjects. Among sleep-restricted subjects, African Americans gained more weight than Caucasians and males gained more weight than females. Among sleep-restricted subjects, caloric intake during days with a delayed bedtime was positively associated with weight gain. Sleep-restricted subjects consumed an excessive amount of calories beyond daily caloric requirements during days with a delayed bedtime compared with control subjects who consumed an adequate amount of calories during corresponding days. Thus, increases in caloric intake in sleep-restricted subjects were not due to novelty of the laboratory setting or other environmental factors.
In sleep-restricted subjects, daily caloric intake was increased during days when their bedtime was delayed until 04:00 (EW, SR1-4) compared to days when their bedtime was 22:00 (BL, SR5, R1-2) and this increase was associated with greater intake of all three macronutrients and greater meal frequency. Compared to days following BL sleep (BL and EW), the amount of calories consumed from 08:00-14:59 was reduced (by 96.8 calories), the amount of calories consumed from 15:00-21:59 was not significantly changed, and 552.9 additional calories were consumed from 22:00-03:59 during sleep restriction. Thus, the overall increase in caloric intake on days with a delayed bedtime was exclusively due to intake during the late-night period of additional wakefulness.
Our experimental weight gain findings support the relationship between short sleep duration and increased BMI observed in epidemiological studies16–19 and those studies indicating that males may be more susceptible to weight gain resulting from sleep loss.20,21 Sleep-restricted African Americans were particularly vulnerable to weight gain—this finding is important considering African Americans are more likely to habitually sleep less than 6 h per night.2,48 We are currently examining the behavioral and physiological mechanisms underlying differences in weight gain following sleep restriction between males and females and between African Americans and Caucasians.
Consistent with previous studies examining ad libitum food access during sleep restriction,15,22 our subjects increased caloric intake during days with a delayed bedtime. This caloric intake increase occurred during the EW day that did not follow sleep restriction but consisted of a 20-h day with a 04:00 bedtime, and notably did not occur during the SR5 day that followed sleep restriction but consisted of only 14 h of wakefulness and a 22:00 bedtime. The increase in caloric intake on EW but not on SR5 suggests that a delayed bedtime and/or hours of wakefulness may be better predictors of caloric intake than sleep duration the preceding night. Future studies are needed to determine the amount of additional calories burned with a delayed bedtime to compare increased caloric intake and the increased energy expenditure required from extended wakefulness. Jung and colleagues49 measured energy expenditure in a total sleep deprivation paradigm and found that approximately 134 additional calories were needed for an 8-h extended wake time; however, their subjects were confined to bed rest, so this finding likely is an underestimation of energy requirements under normal activity conditions. The positive correlation between weight gain and caloric intake during sleep restriction in our study suggests that subjects consumed more calories than necessary to compensate for the additional energy requirement of extended wakefulness.
In the current study, meal times were specified in the protocol to ensure that subjects had adequate time to eat; however, subjects were told they could eat/drink whenever they were not testing. Subjects were never presented with food/drink or told that they had to eat at a specific time; rather, food and drinks were always available for them to retrieve from the laboratory kitchen. Based on this ad libitum design, we were able to examine meal patterns (the number of meals subjects consumed throughout the day and the size of each meal they consumed) as well as meal timing (when subjects chose to consume calories). We observed an increase in the number of meals consumed during days with a delayed bedtime without a change in average meal size. This is an important finding because meal patterns are indicative of physiological mechanisms underlying caloric intake; the factors that control meal onset (meal number) are distinct from those that control meal termination (meal size).50 Processes that promote meal termination and limit meal size include gastrointestinal signals such as gastric distention and cholecystokinin.50 Postprandial signals (which affect the interval to the next meal) and meal initiation signals (which influence the start of meals) regulate meal number.50 Based on our findings, it is more likely that sleep restriction and a delayed bedtime affected postprandial and meal initiation signals rather than satiation signals. For example, ghrelin, a meal initiation signal, increases meal number without affecting the size of meals and studies have shown that ghrelin levels are increased during SR.12 Future studies should focus on how ghrelin is modulated by extended wakefulness/sleep loss and examine other post-prandial signals such as hypothalamic dopamine51 and amylin.52
Contrary to previous findings from a 14-day SR protocol,16 we did not observe an increase in carbohydrate intake during days with a delayed bedtime. The proportion of calories derived from protein, carbohydrates, and fat were consistent across all protocol days. Therefore, subjects did not overconsume a specific macronutrient at the expense of the other two macro-nutrients during sleep restriction. Our study only consisted of 5 nights of SR; therefore, we may have observed changes in macronutrient intake with a longer SR protocol. On protocol day SR4, subjects consumed significantly more grams of fat compared to the baseline day whereas grams of protein and carbohydrates consumed were not different between these 2 days. This increase in fat consumption is consistent with a SR experiment in adults which found an increase in fat consumption22 and a study showing that adolescents who were short sleepers (less than 8 h per night) consumed more fat compared to their peers who slept 8 h or more per night.24
Recent research has highlighted the critical contribution of meal timing to weight regulation.26 We observed a shift in the timing of caloric intake during days with a delayed bedtime. Subjects consumed additional calories during the late-night period when they remained awake and then consumed fewer calories the morning following SR. Thus, the proportion of calories consumed was altered such that subjects consumed the majority of calories in early evening/late-night hours rather than in morning/early afternoon hours. Previous experiments have shown that mice gained more weight when consuming calories during a period when they were normally asleep compared to mice who were fed on a normal schedule, even when the same amount of calories were consumed.33 Therefore, future studies should vary schedules of SR in humans to determine whether bedtime affects the timing of caloric intake and weight gain. In addition, studies examining brain activation and the neuroendocrine mechanisms underlying the relationship between sleep duration and energy balance, should focus on this late-night period, when additional calories are consumed.
We also observed an increase in the proportion of calories from fat during late-night hours; this increase may be particularly contributory to weight gain. Baron and colleagues found that the percentage of fat consumed after 22:00 was associated with greater total caloric intake and a higher BMI among individuals.53 In a laboratory controlled study, patients with night eating syndrome exhibited a delay in carbohydrate and fat in-take compared to healthy control subjects54 and epidemiological studies have shown that patients with night eating syndrome are at greater risk for obesity55,56 and weight gain.29,57 Recent studies examining brain activation in the morning following sleep loss showed that neuronal activity in response to food stimuli was greater after restricted sleep compared to after habitual sleep58 and that total sleep deprivation was associated with increased activation of the right anterior cingulate cortex, an area involved in reward and anticipation, in response to food images.59 Future studies focusing on sleep loss and brain activation should examine subjects during the late-night period of extended wakefulness as this is the time when neuronal activity related to reward may be associated with increased fat consumption.
Limitations
Our study has several limitations. First, energy expenditure is an important factor that might contribute to weight gain. Subjects in our study were not allowed to exercise during the protocol; therefore, activity levels were limited. Because caloric intake was ad libitum, subjects did not fast during the protocol and therefore we could not assess resting metabolic rate, which may be affected by sleep loss. Second, although caloric intake was ad libitum, subjects were only allowed to consume food and drink provided by hospital and laboratory staff; foods that contained caffeine (including chocolate) were prohibited. In addition, although there were approximately 10 opportunities to eat during a typical protocol day, subjects were not allowed to eat/drink during neurobehavioral testing that occurred throughout the day. Therefore, subjects may have desired to eat certain foods that were unavailable to them or may have wanted to eat during certain times when they were not allowed to do so due to testing; both factors may have reduced total caloric intake and subsequent weight gain. Third, it should be noted that timing of caloric intake and voiding varied across subjects prior to weight measurements. Fourth, our subjects were healthy, were between the ages of 22-50 y, and had BMIs between the range of 19-30. The results may therefore not generalize to other groups, including obese individuals, adolescents or the elderly. Finally, the sample size of subjects with caloric intake information was too small to make comparisons between race and gender—thus, we cannot determine whether caloric intake underlies the race and gender weight gain differences.
CONCLUSIONS
Previous epidemiological studies indicate a relationship between short sleep duration and weight gain. The current study examined behavioral mediators of this relationship by objectively measuring weight, caloric intake, and meal timing in controlled laboratory protocols involving 5 nights of sleep restricted to 4 h TIB per night. Sleep-restricted subjects gained more weight compared to controls and showed significant gender and race differences in weight gain. Chronically sleep-restricted subjects with late bedtimes may be more susceptible to weight gain and obesity due to overall greater caloric intake as well as increased consumption during late-night hours. Such caloric intake during late-night hours may be particularly contributory to weight gain as these calories appear to be greater in fat compared to calories consumed during morning, afternoon, and evening hours.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Goel has received compensation for serving as a consultant on NASA and NSBRI grants, for serving as a NIH study section member, and for lectures delivered at the University of Pittsburgh Medical Center, Drexel University College of Medicine, the World Federation of Sleep Research & Sleep Medicine Societies meeting, and the Society for Light Treatment and Biological Rhythms meeting. Dr. Dinges is compensated for serving on a scientific advisory council for Mars, Inc. Dr. Dinges is compensated by the Associated Professional Sleep Societies, LLC, for serving as Editor in Chief of SLEEP and Dr. Goel is an Associate Editor for SLEEP. Dr. Goel and Dr. Dinges recuse themselves from all decisions related to SLEEP manuscripts on which they have a conflict of interest. Ms. Spaeth has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the subjects who participated in the experiments and the faculty and staff of the Division of Sleep and Chronobiology who helped acquire the data. Work for this study was performed at the Division of Sleep and Chronobiology, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA. This research was supported by NIH grants R01 NR004281 (to Dr. Dinges) and F31 AG044102 (to Ms. Spaeth); the Department of the Navy, Office of Naval Research Award No. N00014-11-1-0361 (to Dr. Goel); Clinical and Translational Research Center (CTRC) grant UL1TR000003; and National Space Biomedical Research Institute through NASA award NCC 9-58 (to Dr. Dinges).
REFERENCES
- 1.Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33:149–59. doi: 10.1093/sleep/33.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975-2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelhans BM, Janssen I, Cursio JF, et al. Sleep duration and weight change in midlife women: the SWAN Sleep Study. Obesity (Silver Spring) 2013;21:77–84. doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath. 2012;16:829–33. doi: 10.1007/s11325-011-0583-0. [DOI] [PubMed] [Google Scholar]
- 6.Bo S, Ciccone G, Durazzo M, et al. Contributors to the obesity and hyper-glycemia epidemics. A prospective study in a population-based cohort. Int J Obes (Lond) 2011;35:1442–9. doi: 10.1038/ijo.2011.5. [DOI] [PubMed] [Google Scholar]
- 7.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–7. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer KA, Wall MM, Larson NI, Laska MN, Neumark-Sztainer D. Sleep duration and BMI in a sample of young adults. Obesity (Silver Spring) 2012;20:1279–87. doi: 10.1038/oby.2011.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 13.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 15.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. ObesFacts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 20.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 22.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shechter A, O'Keeffe M, Roberts AL, Zammit GK, Roychoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–9. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents' fat and carbohydrate consumption. Sleep. 2010;33:1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath G, Roach GD, Dorrian J, Ferguson SA, Darwent D, Sargent C. The effect of sleep restriction on snacking behaviour during a week of simulated shiftwork. Accid Anal Prev. 2012;45:62–7. doi: 10.1016/j.aap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garaulet M, Ordovas JM, Madrid JA. The chronobiology, etiology and pathophysiology of obesity. Int J Obes (Lond) 2010;34:1667–83. doi: 10.1038/ijo.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 29.Gluck ME, Venti CA, Salbe AD, Krakoff J. Nighttime eating: commonly observed and related to weight gain in an inpatient food intake study. Am J Clin Nutr. 2008;88:900–5. doi: 10.1093/ajcn/88.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paschos GK, Ibrahim S, Song WL, et al. Obesity in mice with adipocytespecific deletion of clock component Arntl. Nat Med. 2012;18:1768–77. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–9. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 35.Banks S, Van Dongen HPA, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 37.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 38.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:1–13. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 40.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 41.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hise ME, Sullivan DK, Jacobsen DJ, Johnson SL, Donnelly JE. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr. 2002;75:263–7. doi: 10.1093/ajcn/75.2.263. [DOI] [PubMed] [Google Scholar]
- 43.Storey JD. A direct approach to false discovery rates. J Roy Stat Soc B. 2002;64:479–98. [Google Scholar]
- 44.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 45.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4:370–3. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black AE. Physical activity levels from a meta-analysis of doubly labeled water studies for validating energy intake as measured by dietary assessment. Nutr Rev. 1996;54:170–4. doi: 10.1111/j.1753-4887.1996.tb03924.x. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Department of Labor. Fact Sheet #22: Hours Worked Under the Fair Labor Standards Act (FLSA) 2008. Available from: http://www.dol.gov/whd/regs/compliance/whdfs22.htm#.UO3qbW_Ac3p.
- 48.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 49.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meguid MM, Fetissov SO, Varma M, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–57. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 52.Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–71. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Baron KG, Reid KJ, Horn LV, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite. 2013;60:246–51. doi: 10.1016/j.appet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goel N, Stunkard AJ, Rogers NL, et al. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythm. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tholin S, Lindroos A, Tynelius P, et al. Prevalence of night eating in obese and nonobese twins. Obesity. 2009;17:1050–5. doi: 10.1038/oby.2008.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundgren JD, Allison KC, Crow S, et al. Prevalence of the night eating syndrome in a psychiatric population. Am J Psychiatry. 2006;163:156–8. doi: 10.1176/appi.ajp.163.1.156. [DOI] [PubMed] [Google Scholar]
- 57.Andersen GS, Stunkard AJ, Sorensen TI, Petersen L, Heitmann BL. Night eating and weight change in middle-aged men and women. Int J Obes Relat Metab Disord. 2004;28:1338–43. doi: 10.1038/sj.ijo.0802731. [DOI] [PubMed] [Google Scholar]
- 58.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benedict C, Brooks SJ, O'Daly OG, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]